Abstract
CD13 is a multifunctional cell surface molecule that regulates inflammatory and angiogenic mechanisms in vitro, but its contribution to these processes in vivo or potential roles in stem cell biology remains unexplored. We investigated the impact of loss of CD13 on a model of ischemic skeletal muscle injury that involves angiogenesis, inflammation and stem cell mobilization. Consistent with its role as an inflammatory adhesion molecule, lack of CD13 altered myeloid trafficking in the injured muscle, resulting in cytokine profiles skewed toward a pro-healing environment. Despite this healing-favorable context, CD13KO animals showed significantly impaired limb perfusion with increased necrosis, fibrosis and lipid accumulation. Capillary density was correspondingly decreased, implicating CD13 in skeletal muscle angiogenesis. The number of CD45−/Sca1−/α7-integrin+/β1-integrin+ satellite cells was markedly diminished in injured CD13KO muscles and adhesion of isolated CD13KO satellite cells was impaired while their differentiation was accelerated. Bone marrow transplantation studies showed contributions from both host and donor cells to wound healing. Importantly, CD13 was co-expressed with Pax7 on isolated muscle-resident satellite cells. Finally, phosphorylated-FAK and ERK levels were reduced in injured CD13KO muscles, consistent with CD13 regulating satellite cell adhesion, potentially contributing to the maintenance and renewal of the satellite stem cell pool and facilitating skeletal muscle regeneration.
Introduction
Healing in response to ischemic injury universally involves the processes of inflammation and angiogenesis [1–3]. During inflammation, monocytes use adhesion molecules as addresses to traffic to and populate the injured muscle. Once at the site of injury they differentiate to macrophages and participate in the healing process by clearing the necrotic tissue [4–6], facilitating angiogenesis [5],and promoting muscle regeneration [7]. The critical role of myeloid cells in post-ischemic healing is illustrated by studies in which systemic depletion of these cells showed markedly impaired wound healing and perfusion recovery [8, 9]. Similarly, new vessel formation or angiogenesis is driven by tissue hypoxia and cytokines elicited by infiltrating inflammatory cells where nascent vessels increase capillary density, perfuse the hypoxic tissue and restore oxygen and nutrient supply routes [10]. We have previously demonstrated that the myeloid cell marker CD13 is an angiogenic regulator as well as an inflammatory adhesion molecule that forms a homotypic complex containing both monocytic and endothelial CD13 in vitro, and thus could contribute to wound healing in vivo on many levels.
While ischemic injury triggers similar responses, different organs also rely on tissue-specific mechanisms for optimal repair, many involving populations of resident regenerative/stem cells [11–13]. Pertinent to this study, healing of skeletal muscle injury is highly dependent on a well-characterized population of quiescent resident stem cells, the satellite cells. In response to trauma these become activated, proliferate and form new multinucleated myofibers or fuse to damaged myofibers to contribute substantially to muscle regeneration [14]. A second critical property of satellite cells is their ability to self-renew and thus maintain a pool of quiescent regenerative cells. Interestingly, in addition to its role as a myeloid marker, CD13 has been identified as a marker of human adult stem cells isolated from many tissues [15–20]. However, potential functional roles for CD13 in these cells have not been investigated.
We designed the current study to determine the contribution of CD13 in the wound healing response to severe peripheral ischemia in vivo, a model of occlusive peripheral artery disease which is highly dependent upon inflammatory cell trafficking, neovessel formation and stem cell function [21].
Materials and Methods
Additional methods and details included in Supplemental Methods online
Surgery
Surgical grade anesthesia was induced by intraperitoneal injection of Ketamine (100mg/kg) and Xylazine (10mg/kg). The right femoral artery was ligated proximal to the deep femoral artery and distal to saphenous artery. The deep femoral artery, superficial branches and bifurcation of the popliteal artery were cauterized, and the femoral artery was completely removed between the two ligatures avoiding injury of the femoral vein and nerve to preclude influence of inflammation and edema on arteriogenesis and angiogenesis. Postoperative analgesia was provided with buprenorphine (0.05mg/kg).
Laser-Doppler perfusion imaging
Noninvasive measurements of superficial hindlimb perfusion were obtained before and 0, 3, 7, 14, and 21 days after ligation using a Laser Doppler perfusion imager (model LDI2-IR, Moor Instruments, Wilmington, DE) that was modified for high resolution and depth of penetration (2mm) with and 830nm wavelength infrared 2.5mW laser diode, 100µm beam diameter, and 15kHz bandwidth. At each time point, an average of 4 measurements per animal were made on anesthetized (1.5% isoflurane on an isothermal heating pad). To avoid the influence of light and temperature, the results were expressed as a ratio of perfusion in the right (ischemic) versus left (nonischemic (NI)) limb [21].
In Vivo Assessment of Limb Function and Ischemic Damage
Semiquantitative assessment of impaired use of the ischemic limb (ambulation score) was performed using the following criterion: 3= most severe, unable to use the foot, dragging foot; 2=no dragging, but no plantar flexion (ability to flex the ankle); 1= positive plantar flexion; and 0= able to flex toes to grasp cage in response to gentle traction on the tail [22]. Semiquantitative measurement of the ischemic damage (necrosis score) was also assessed (1 to 5 = one to five fingernails damaged, 6 to 10 = one to five fingers fully damaged, 11= total paw damage).
Muscle satellite cell isolation and culture
Single muscle fibers were isolated from post-ischemic mice at d3. Gastrocnemius and tibialis muscles were digested in 2mg/ml collagenase II (Worthington, Lakewood, NJ), 1% P/S, no FBS in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) for 2hr at 37°C with mild agitation on orbital shaker (50–60rpm). After aspirating collagenase solution, triturate with a wide-bored pipette in growth medium (Ham’s F10, 20% FBS, 1% penicillin/streptomycin and 5ng/ml bFGF) to release single fibers. For isolation of primary satellite cells, freshly isolated fibers were stripped of their basal lamina using 19-gauge needle and syringe, filtered and plated for 2–4hrs until attached [23, 24]. Debris was aspirated from the plate and media gently replaced. All cultures were incubated at 37°C, with 5% CO2 and atmospheric O2 concentrations in growth medium on Matrigel (BD Biosciences, 1:400 dilutions) coated tissue culture plate. All primary cell experiments used cells at passage 2–3 to avoid effects of long-term culture.
Satellite cell migration from parent myofibers
To compare satellite cell migration from parent myofibers and colony formation ability [24] post-ischemic WT and CD13KO myofiber samples of equal numbers (200–220) were plated in 60mm tissue culture dish. After 1 week cells were fixed and stained with 0.5% crystal violet for colony visualization. Image was taken with Nikon SLR D40 camera.
Clonal Analysis
Single cell colony assay: Isolated mouse satellite cells were diluted (20 cells/ml) and plated 100µl/well in 96-well plates, such that approximately each well received one cell so resultant colonies originated from a single cell. Cultures were grown in growth medium for 8 days or with a switch to differentiation medium (DMEM, P/S, 2% Horse Serum) for a further 4 days on Matrigel (BD Biosciences, 1:300 dilution in minimal volume for coverage) [23]. At the appropriate time points, cells were fixed in 4% paraformaldehyde for 15 minutes, washed twice, stained with 0.04% trypan blue for visualization, and washed three times. Images were acquired at 20× magnification (2× objective) using a Nikon T-BPA camera attached to the Nikon Eclipse TE2000-U. The software used was SPOT version 4.1. Cells were seeded in 48 wells for each of WT and CD13KO and quantitated as indicated.
Western blotting
At d3 post-surgery, gastrocnemius muscles or isolated primary muscle satellite cells were lysed in ice-cold buffer (1% NP40 lysis buffer with protease and phosphatase inhibitors). Equal amount of protein from each group were separated by SDS-PAGE and transferred on to PVDF membrane and incubated with respective primary antibodies; CD13 SL-13 for mouse CD13 (ProMab Biotechnologies, Richmond, CA); 452 for human CD13 (Dr. Meenhard Herlyn, Philadelphia, PA); TGFβ (R&D); TNFα, IL-6, MCP-1 and PDGF (Abcam); pFAK 397 and pERK (cell signaling); Pax7 (Abcam); tubulin (Sigma); GAPDH (SIGMA) followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected with the use of a chemiluminescence reagent kit (Thermoscientific). The band intensities were quantified with the NIH Image J program.
Murine bone marrow transplantation model
Recipient mice were treated with a suspension of 800mg/l sulfamethoxazole and 160mg/l trimethoprim (SMZ) for 1 week prior to irradiation and 2 weeks after bone marrow transplantation [26]. Tibias and femurs of 7 week WT and CD13KO donor mice were flushed with PBS to obtain bone marrow cells, triturated to form single-cell suspensions. Mononuclear cells were isolated by density centrifugation over Histopaque-1083 (Sigma), yielding an average 2×107 cells per animal. 6 week old WT and CD13KO recipient mice were lethally irradiated and intravenously infused with approximately 5×106 donor bone marrow cells in 200ml per animal in three groups; WT recipient-WT Donor n=7, WT recipient-CD13KO Donor n=9, CD13KO recipient-WT Donor n=8. 6 weeks post-transplantation, mice were checked by flow cytometry for reconstitution and underwent FAL surgery. Hindlimb perfusion was measured at 0, 3, 7, 14, and 21 days after ligation using Doppler. Transplanted mice were also assessed using the Digigait™ apparatus (Mouse Specifics Inc., Boston, MA, USA) at d20 post-ligation, which provides numerous spatial and temporal indices of gait dynamics. Detailed methods for Digigait are described in Supplementary Materials.
Statistical Analysis
The data were represented as mean±S.E.M of the indicated number of measurements. Statistical significance was calculated by two-tailed unpaired t test for two data sets. Two-way ANOVA was used to compare values between groups over time. Differences were considered significant at p<0.05.
RESULTS
CD13 expression is increased in response to peripheral artery occlusion and contributes to perfusion and functional recovery in occluded limbs
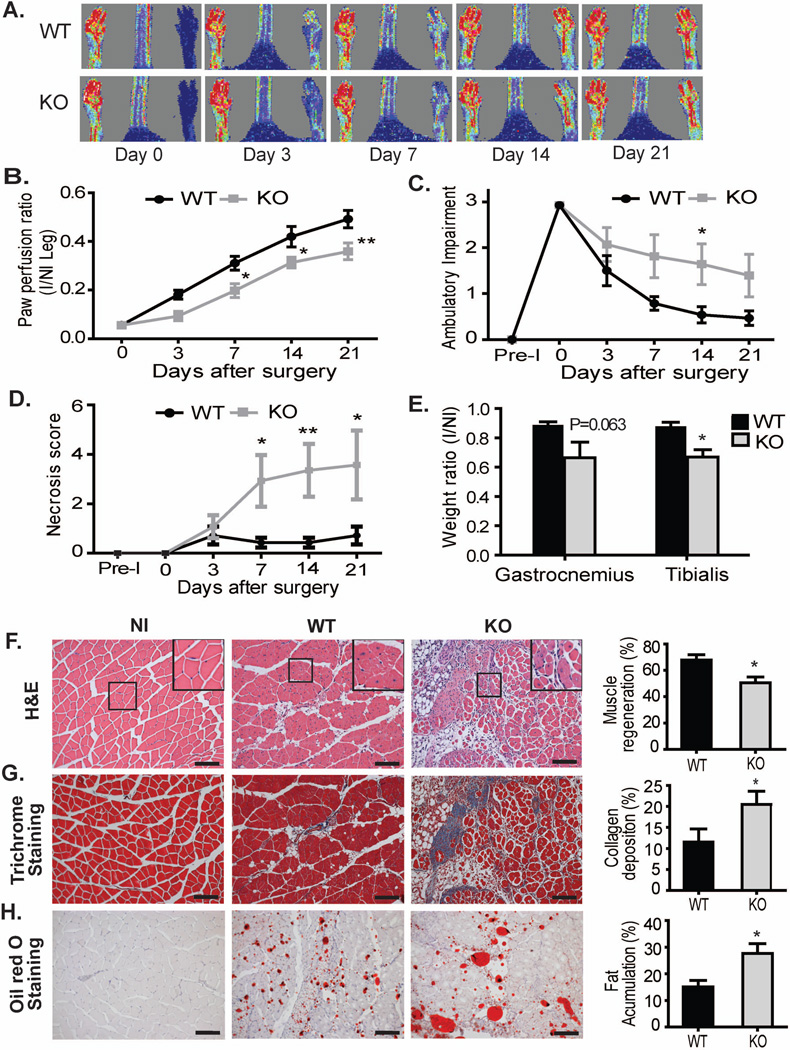
While we have previously shown that CD13 is an inflammatory adhesion molecule in vitro [25, 27] and a regulator of angiogenesis [28–30], its role in the healing muscle in vivo has not been examined. To address this issue, we chose a modification of the model of occlusive peripheral artery disease, permanent femoral artery ligation (FAL), where the artery is clamped, blocking blood flow but retaining the collateral arteries. Conventional FAL induces two distinct vascular processes, angiogenesis (formation of new vessels) and arteriogenesis (strengthening and remodeling of existing collateral arteries) [21]. To focus the current study on the processes of inflammatory infiltration and the angiogenic vascular response, we surgically removed the femoral artery and its collateral branches, thus precluding arteriogenesis [10]. We initially determined that CD13 expression in the wounded area was temporally upregulated following surgery of wild type animals, peaking between 3d and 7d post-injury and decreasing thereafter in a pattern consistent with its expression on infiltrating inflammatory cells and angiogenic vasculature (Supplemental Fig S1A). Quantitative analysis of the gastrocnemius muscles of the murine hindlimb shows that CD13 protein levels are upregulated by over 3-fold (Supplemental Fig S1B). Analysis of perfusion in ischemic limbs and in particular, the paw and digits, by Dopplar imaging showed a significant and prolonged delay in recovery of blood flow over 21d post-injury in the CD13KO as compared to wild type animals (Figs 1A, B). In agreement with this result, we found a higher degree of paw necrosis and reduced ambulatory capacity (impaired limb function) in the CD13KO animals (Figs 1C and D, criteria as outlined in Methods). Finally, recovery of muscle mass in the gastrocnemius and tibialis muscles was also impaired in CD13KO animals (Fig 1E).
Fig 1. CD13 plays a protective role in skeletal muscle regeneration after ischemic injury.
A) Representative color-coded images of WT and CD13KO mice on day 0, 3, 7, 14, and 21 after surgery assessed by laser Doppler imaging. Red is highest velocity, green intermediate, and blue, lowest velocity. B) Cumulative results for WT and CD13KO mice (n=8 each) are shown graphically as ratios of blood flow in ischemic limb (I) to that in the non-ischemic limb (NI) at each time point. C–E) Functional assessments of ischemic muscles. Cumulative results are shown graphically as C) the ambulatory impairment score; D) ischemic tissue damage score; and E) injured muscle weight ratio (ischemic/non-ischemic) on day 21. (n=8 in each group and values are shown as mean±SEM *P<0.05; assessment criteria in Methods). F) Hematoxylin and eosin (H&E) staining of gastrocnemius muscle regeneration was confirmed by the presence of multiple, centrally located myocyte nuclei. A significant reduction in muscle regeneration (average) was observed in CD13 null mice when compared with WT at day 21. G) Masson’s trichrome stain was used to measure the area of fibrosis. CD13 null mice showed significant increase of interstitial fibrosis (blue) in the ischemic limb. H) Lipid adjacent to regenerated myofibers was detected with Oil red O staining shows significantly more fat accumulation in KO mice. All data were quantified by ImagePro Plus. Values are shown as mean±SEM (*P<0.05) (n=8 per group; 20× objective; Bar=100µm).
Regenerating muscle was clearly evident upon histologic analysis of wild type animals at 21d post-surgery as illustrated by numerous myofibers with centrally located nuclei (gastrocnemius- Fig 1F and tibialis-supplemental Fig S2A). In contrast, CD13KO muscles displayed marked metaplasia with loss of myofibers, significant increases in collagen deposition (Figs 1F, G and S2A, B), an increase in Oil red O-positive lipid accumulation (Figs 1H and S2C) and decreased muscle regeneration characteristic of impaired muscle recovery [21], suggesting that CD13 promotes wound healing in this model of ischemic injury.
CD13 is required for angiogenesis during healing
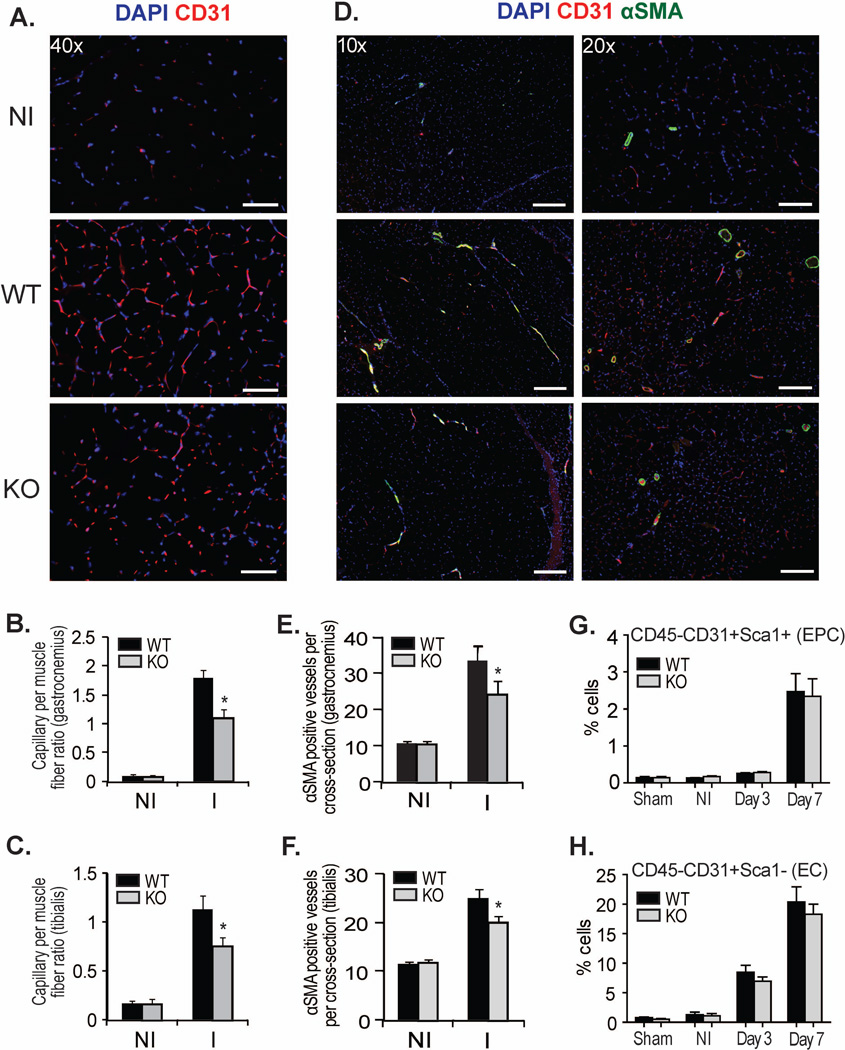
Since we and others have shown that CD13 is an angiogenic regulator [29–32], we began our investigation by analyzing the vascular response to peripheral ischemia in our wild type or CD13KO mice at 21d post-injury. Immunofluorescent analysis of CD31+ endothelial cell-lined luminal structures indicated a significant decrease in capillary density in the gastrocnemius and tibialis muscles of the CD13KO animals (Figs 2A–C). In addition, in the CD13KO animals these structures appeared more immature with fewer characteristic branches, confirming our earlier in vitro observations that CD13 is required for angiogenesis. Similarly, the number and diameter of more mature vessels covered with α-smooth muscle actin (αSMA) positive mural cells were also diminished (Figs 2D–F). Flow cytometric analysis of cells from d3-post injury collagenase-digested muscle showed that the progressive accumulation of Sca1+/CD31+ endothelial progenitor or total CD31+ endothelial cells in the wound was not significantly different between genotypes (Figs 2G,H), supporting our previous findings that CD13 regulates angiogenesis by controlling endothelial invasion but not proliferation [32]. Together, these data are consistent with an angiogenic basis for impaired healing in ischemic muscles.
Fig 2. Skeletal muscle vessel formation is impaired in CD13 null mice following ischemic injury.
A) Capillaries were visualized by immunofluorescent staining with CD31 (red) and nuclei with DAPI (blue); Objective 40× (Bar=50µm). B,C) Capillary density per fiber ratio was measured in gastrocnemius and tibialis muscles. D) Vessels detected by double staining of CD31 (red) and αSMA (green); Objective 10× (Bar=200µm) and 20× (Bar=100µm). E,F) αSMA positive vessels were measured per cross section in gastrocnemius and tibialis muscles harvested at 21 days after FAL. Capillaries and vessels were quantified by Image J software (NIH). Data represent the mean±SEM (n=8 mice per group) (*P<0.05). G,H) Endothelial progenitor cells (EPC) and mature endothelial cells (EC) were analyzed by flow cytometry at day 3 and day 7 compared to non-ischemic (NI) muscle (n=5 per group).
CD13 regulates the profile of infiltrating inflammatory cells and cytokine levels
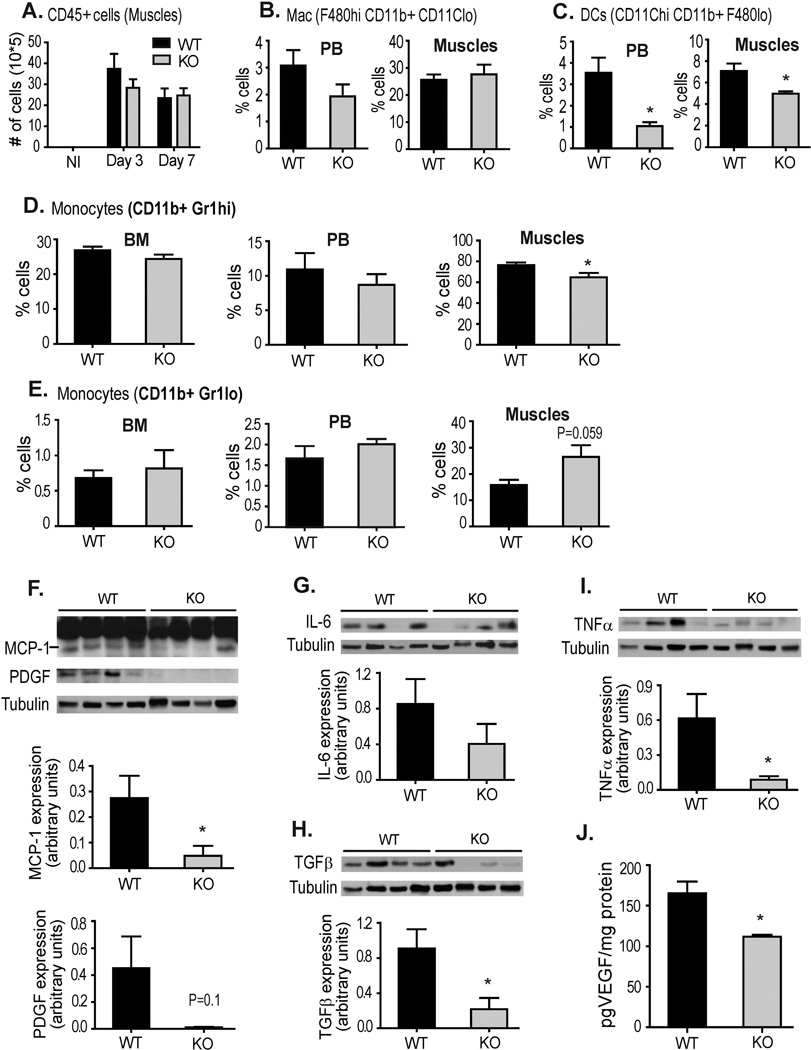
Tissue injury induces a strong inflammatory response that involves temporally-regulated phases of extravasation of functionally distinct myeloid cell subsets into the site of injury to orchestrate the removal of dead cells, attract additional cells and promote an environment optimal for wound healing [33]. CD13 can also function in vitro as an adhesion molecule to mediate the monocyte/endothelial interactions critical for inflammation and healing of injured tissue, an effect mediated by CD13 expressed on both the monocytes as well as endothelial cells [25, 34]. Flow cytometric analysis of cell suspensions isolated from wild type or CD13KO injured muscles (d3) showed equivalent numbers of infiltrating hematopoietic cells in ischemic peripheral tissues (Fig 3A, gating strategy-Supplemental Fig S3). Similarly, analysis of myeloid cell subsets in the peripheral blood and muscle tissue indicated that while percentages of tissue-resident and circulating macrophages are not significantly different (Fig 3B), the profiles of other populations were highly skewed in the CD13KO animals. Dendritic cells, which contribute to tissue damage by producing proinflammatory cytokines, chemokines, and other soluble inflammatory mediators (Fig 3C) from both peripheral blood and muscle were significantly decreased. Importantly, although the profiles of the inflammatory (Gr-1high, pro-inflammatory, Fig 3D upper) or resident (Gr-1low, pro-healing, Fig 3D lower) monocytes were equivalent in bone marrow and peripheral blood from both genotypes, the normally high ratio of inflammatory/reparative monocytes at d3 was decreased in muscles of CD13KO animals (WT= 4.6/1, KO=2.4/1). Therefore, this data indicates that the lack of CD13 alters the patterns of inflammatory cell trafficking in response to injury in a manner which would be expected to promote healing.
Fig 3. Inflammatory cells, cytokines and growth factor profiles are altered in the muscles of CD13KO mice in response to hindlimb ischemia.
A) Profile of total hematopoietic cells (CD45+) at d3 and d7 compared to non-ischemic muscle, B) Flow cytometric analysis of macrophages and C) DCs in the peripheral blood (PB) and muscle of WT and CD13KO mice. D) Infiltrating inflammatory monocytes- CD11b+ Gr-1hi, and E) reparative monocytes- CD11b+ Gr-1lo in the live CD45+ cell population were analyzed in the bone marrow (BM), Peripheral Blood (PB) and muscles isolated from WT and its knockout counterpart. Error bars represent mean±SEM for WT (n=5) and CD13KO (n=5) mice; *P<0.05. Gating strategy is shown in Supplemental Fig S3. F–I) Western blot analysis of protein expression levels of the indicated angiogenic and inflammatory factors in injured muscles of WT and CD13KO mice (n=4 per group) post-ischemic d3. Detected band sizes are: MCP1 22.5kDa, PDGF 19kDa, IL6 25kDa, TGFβ 25kDa and TNFα 22kDa. Band intensities were quantified with NIH Image J and are expressed relative to tubulin loading control (*P<0.05). J. VEGF levels were quantitated by ELISA assay.
Once in the wound, the infiltrating inflammatory cell subsets elicit specific patterns of cytokines required to orchestrate the subsequent wound healing process [35]. Logically, these altered cell profiles in the CD13KO animals would also result in changes in the relative levels of cytokines in the wound. Indeed, characterization of protein (Figs 3F–J) or mRNA expression (Supplemental Fig S4) of a panel of cytokines that are produced by these subsets showed that cytokine profiles are also distorted in a manner that corresponds to the profiles of infiltrating cells, with decreases in expression of the pro-inflammatory cytokines produced by inflammatory monocytes (TGFβ, MCP-1, TNFα and IL-6) and an increase in IL-10, a product of the reparative monocytic subpopulation. Decreases in the pro-angiogenic cytokines VEGF, PDGF and Ang1 may contribute to impaired angiogenesis as well (Fig 2). Remarkably, this phenotype (an increase in pro-healing and pro-angiogenic and decreased pro-inflammatory myeloid cells and cytokines) would be predicted to result in an environment beneficial for healing. This stark contrast to the compromised repair observed in the CD13 null animals prompted further investigation into potential underlying mechanisms in addition to impaired angiogenesis.
Muscle satellite cell numbers are decreased in CD13 null animals
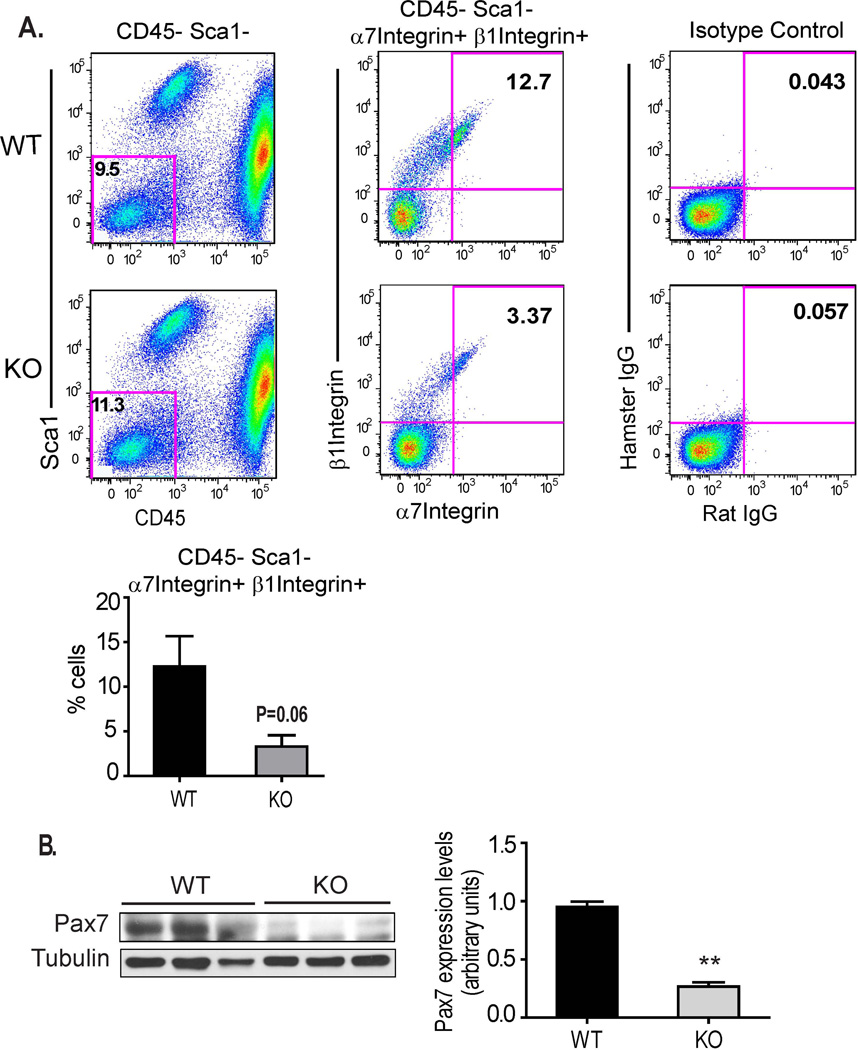
Skeletal muscle contains a well-characterized population of self-renewing regenerative cells known as satellite stem cells [36, 37] that supply a significant proportion of the cells that form the new myofibers critical to healing damaged peripheral muscles. A second critically important function of these satellite cells is to replenish this regenerative pool through a process of self-renewal via asymmetric division, resulting in a balance between differentiated myofibers and multipotent satellite cells [38, 39]. CD13 has been reported to be a marker of adult mesenchymal stem cells in numerous tissues and although its function on these cells is currently unknown, it is possible that the impaired muscle regeneration may involve stem cell CD13. Interestingly, flow cytometric analysis of cells isolated from collagenase-disrupted injured muscles at d3 showed that a lower percentage of the cells isolated from CD13KO muscles displayed the CD45−/Sca1−/α7-integrin+/β1-integrin+ muscle satellite cell phenotype as compared to wild type (Figs 4A, B). Accordingly, lower levels of Pax7 protein are found in CD13KO muscle lysates (Fig 4C).
Fig 4. The satellite pool is decreased in CD13 null skeletal muscles.
A) Pseudo-colored plots of flow cytometric analysis for the CD45−/Sca1− population (left), CD45−/Sca1−/α7integrin+/β1integrin+ satellite cells (center) and isotype controls (right) in wild type and CD13KO muscle cell suspensions at d3 post-ischemia. The satellite cell pool is significantly reduced in CD13 null mice compared to WT (right, n=3 per group). B) Western blot of ischemic muscles for Pax7 (58kDa) protein levels. Muscles were lysed at d3 after ligation. Band intensities were quantified with NIH Image J. (n=6) (**P<0.01).
Adhesion of CD13KO satellite cells is impaired
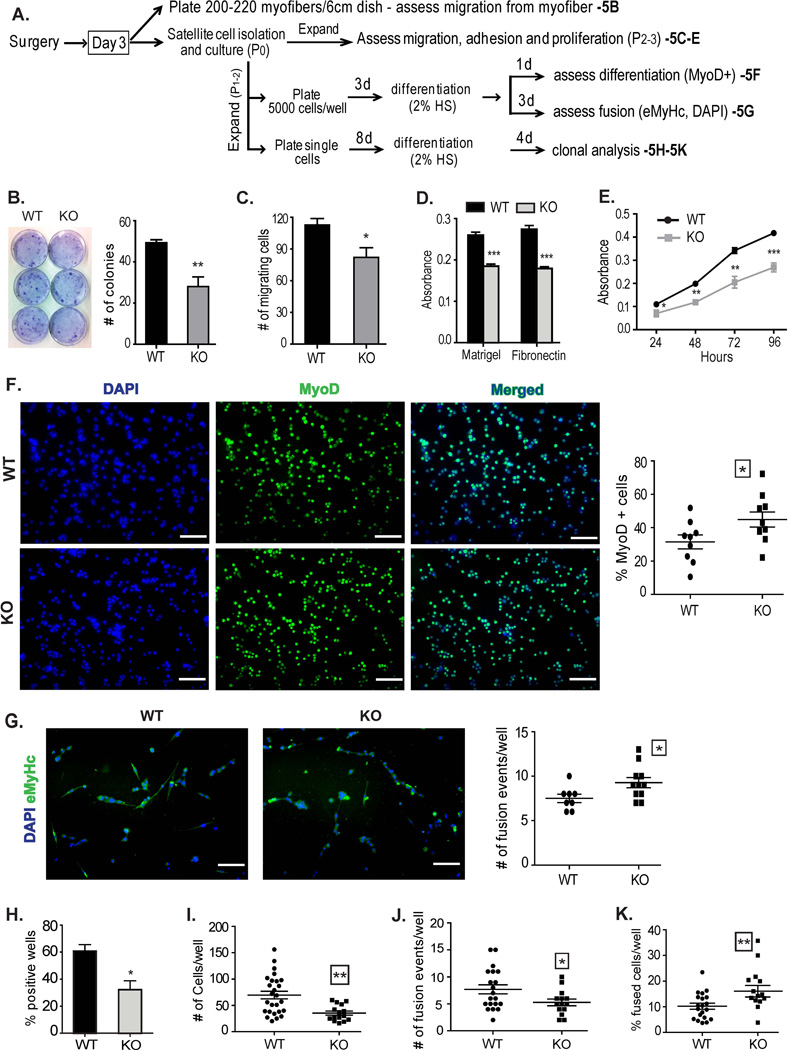
Proper adhesion to the niche is critically important for maintaining satellite cell pluripotency and impaired adhesion results in increased differentiation and compromised self-renewal, leading to depletion of the pool of renewable satellite cells [40–42]. Our previous studies demonstrating that CD13 mediates cell-cell adhesion during inflammation lead us to hypothesize that it may also play a role in adhesion of satellite cells to the extracellular matrix (ECM), which we investigated from various perspectives using a number of approaches (Fig 5A). Indeed, in vitro migration assays demonstrated that fewer satellite cells migrated away from CD13KO parent myofibers to form colonies (Fig 5B) in agreement with our flow cytometric data. Furthermore, isolated satellite cells showed reduced migration in Transwell assays (Fig 5C), adhered at significantly lower levels to both Matrigel and fibronectin matrices (Fig 5D) and proliferated more slowly (Fig 5E) in the absence of CD13. Similarly, in vitro cultures of CD13KO cells showed significantly more early-differentiating, MyoD+ cells at d1 post induction of differentiation (Fig 5F) and correspondingly more fusion events (two or more nuclei per myotube) at d3 as visualized by DAPI-stained nuclei in embryonic myosin heavy chain positive (eMHC+) fibers (Fig 5G). Consistent with this data, limiting dilution analysis of primary isolated satellite cells indicates that wild type tissues contained nearly twice as many cells capable of expansion (Fig 5H) and these colonies produced significantly more progeny in culture (Fig 5I). However, although fewer fusion events were seen in each CD13KO well (Fig 5J), a higher percentage of the CD13KO-derived cells had undergone fusion events when compared to wild type clones (Fig 5K). These data are consistent with CD13 functioning to regulate muscle satellite cell adhesion and its loss leads to impaired adhesion and increased differentiation which could potentially contribute to lower rates of self-renewal and a diminished satellite stem cell pool, in agreement with our in vivo results (Fig 4).
Fig 5. Lack of CD13 affects satellite cell anchorage and differentiation.
A) Outline of procedures for in vitro functional assessment. B) Primary satellite cell colonies migrating from equal numbers of myofibers after 2 weeks of culture visualized with 0.5% crystal violet. C) Transwell migration assay- equal numbers of primary satellite cells derived from d3 post-ischemic muscles of WT and CD13 null mice were plated on Matrigel coated Transwell filters and cells migrating through the filter visualized with DAPI (Supplemental Fig S5). Data represents the mean±S.E.M n=4/group of two independent experiments (*P<0.05). D) Colorimetric quantification of adhesion of primary satellite cells on different ECM substrates. Data represents the mean±S.E.M n=6/group of three independent experiments (*P<0.05). E) Proliferation kinetics of primary cultured satellite cells measured by MTT assay. Each bar is presented as mean ±S.E.M. (n=6 from two independent experiments, *P<0.05, **P<0.01, ***P<0.001). F) Pooled cultures of isolated stem cells were differentiated and stained for the early differentiation marker MyoD at d1 post-induction. CD13KO cultures contain more differentiated cells (20× objective; Bar=100µm). G) Cultures of isolated CD13KO stem cells display more fusion events as indicated by eMHC-positive myotubes containing two or more DAPI-stained nuclei at 3d post-induction (20× objective; Bar=100µm). H–K) Primary satellite cells (20 cells/ml) were plated in 96-well plates, such that each well received approximately one cell. Manual counts were made of: H) % wells containing cells, I) total number of cells per well, J) number of fusion events (myotubes containing two or more nuclei, black arrow bar), and K) calculated % differentiation/well (# of fusion events/ # of total cells). n=48 for each group and each bar is presented as mean S.E.M (*P<0.05, **P<0.01).
Impaired healing in the absence of CD13 is due to defects in both the infiltrating and resident cells
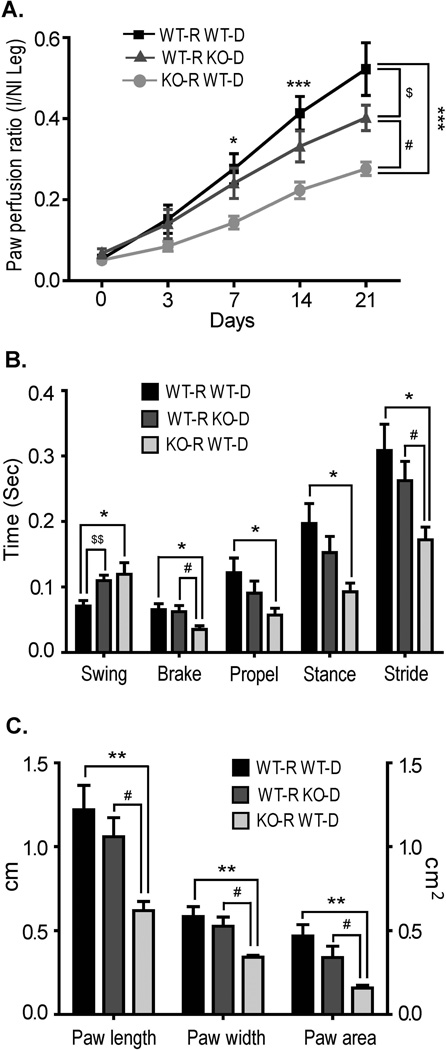
While we have concentrated on the effects of a lack of CD13 in satellite cell renewal, we also observed distorted inflammatory cell profiles in the CD13KO mice which could also contribute to the impaired healing. To assess the contribution of each of these populations in our model, we transplanted WT or CD13KO bone marrow into wild type recipients or wild type bone marrow into CD13KO recipients and performed surgery following reconstitution. Comparison of the rates of perfusion by Doppler (Fig 6A) and functional recovery using the Digigait apparatus (described in Methods; Figs 6B, C) indicated that CD13 expression on the circulating cells is necessary for optimal recovery. CD13KO bone marrow is unable to recapitulate the degree of perfusion achieved by an entirely wild type system, suggesting additional CD13-dependent effects due to impaired trafficking. However, healing in CD13KO recipient animals reconstituted with wild type marrow is clearly diminished when compared to wild type recipients, illustrating a critical, cell-intrinsic basis for the CD13KO defect that cannot be overcome by the presence of wild type inflammatory cells.
Fig 6. Lack of CD13 in both the infiltrating and resident cells impairs healing.
A) Cumulative results of laser Doppler imaging analysis for mice transplanted with the bone marrow of the indicated genotype are shown as the ratio of blood flow in ischemic (I) limb to that in the non-ischemic limb (NI) over time. B) Gait dynamics of transplanted/injured mice. The horizontal axis represents the dependent variables acquired from the DigiGait Imaging System for the indicated groups. The graphic shows differences in swing, brake, propel, stance and stride duration of ischemic hindlimb among three groups at 20d post-ischemia. C) Gait dynamics of paw length, paw width and paw area. DigiGait-treadmill speed= 15 cm/s. Significance was determined by t test and indicated between groups by *P<0.05, **P<0.01, ***P<0.001 (WT-R WT-D vs. KO-R WT-D), $P<0.05, $$P<0.01 (WT-R WT-D vs. WT-R KO-D) and #P<0.05 (WT-R KO-D vs. KO-R WT-D). Groups WT-R WT-D, n=7; WT-R KO-D, n=9 and KO-R WT-D, n=8.
Signaling pathways downstream of adhesion are disrupted in the absence of CD13
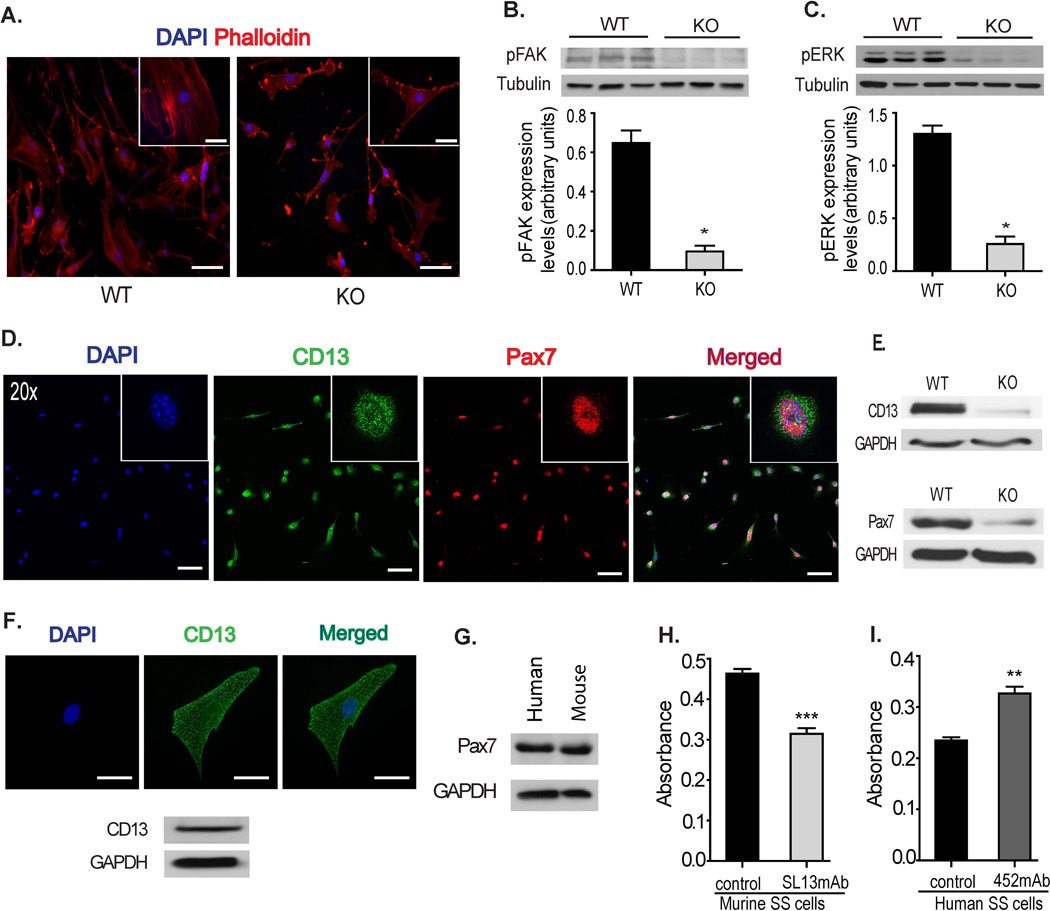
To determine the mechanism underlying CD13’s role as a regulator of satellite cell adhesion, we visualized actin filaments in satellite cell-derived primary myoblasts isolated from injured wild type and CD13KO muscles plated on Matrigel (Fig 7A). Analysis of phalloidin-stained cells showed remarkable alterations in overall cell and cytoskeletal morphology in CD13KO cells, where cells exhibited long thin protrusions with largely cortical or patches of actin instead of assembled stress fibers, suggesting disrupted adhesion. Cell adhesion to the ECM leads to phosphorylation of focal adhesion kinase (FAK), and inhibition or blocking of FAK phosphorylation has been shown to inhibit the development of focal adhesions and stress fibers [43]. To determine the status of FAK activation in the absence of CD13, protein lysates of d3-injured muscles were analyzed and demonstrated reduced levels of phospho-FAK (Fig 7B). Moreover, adhesion-dependent activation of FAK stimulates the MAP kinase pathway to further promote proper adhesion [44, 45]. A dramatic reduction in the levels of phosphorylated ERK in CD13KO muscles is consistent with CD13 participating in adhesion mechanisms in the injured muscle (Fig 7C). Co-immunostaining clearly demonstrated that CD13 is consistently expressed on Pax7+ isolated wild type murine muscle satellite cells (Fig 7D) and that satellite cells isolated from CD13KO muscles expressed lower levels of Pax7 protein (Fig 7E), consistent with enhanced differentiation. CD13 is also expressed in isolated human Pax7-positive satellite cells indicating a potential human relevance for our studies (Figs 7F, G). Finally, we have previously characterized two species-specific anti-CD13 mAbs as either blocking or enhancing CD13-dependent adhesion [25, 27, 34, 46]. In agreement with our genetic data, treatment of isolated wild type murine satellite cells with the CD13-blocking mAb SL13 significantly inhibits adhesion (Fig 7H), while treatment of human satellite cells with the adhesion-activating mAb 452 increases their adhesion to Matrigel (Fig 7I), further supporting a role for CD13 as a regulator of muscle satellite cell adhesion. Taken together, these data are consistent with a fundamental contribution of CD13 to muscle satellite cell function, leading to compromised healing in CD13KO muscles. Therefore, in addition to mediating cell-cell adhesion, CD13 plays a novel role in cell-ECM interactions that has important implications for the maintenance of satellite cell pluripotency and self-renewal.
Fig 7. Satellite cell defects in CD13KO mice are cell intrinsic.
A) phalloidin-stained primary satellite cells isolated from injured muscles of CD13KO mice showed remarkable cytoskeletal disruption compared to cells from WT mice; Objective 20× (Bar=100µm) and 63× (Bar=30µm). B and C) Protein lysates of d3 post-ischemic injured muscles were probed for phospho-FAK (125kDa) and phospho-ERK (44/42kDa) with tubulin as the loading control. The band intensities were quantified with NIH Image J. Data represents mean±SEM (n=7/group, *P<0.05). D) Co-immunostaining of CD13 (green) and Pax7 (red) clearly confirmed that isolated primary satellite cells from wild type mice expressed both CD13 and Pax7; Objective 20× (Bar=100µm). E) Primary satellite cell lysates were probed for CD13 and Pax7 expression. CD13KO satellite cells expressed lower levels of Pax7 protein. GAPDH is shown as a loading control. F) Human satellite cells also express CD13 (green); Objective 63× (Bar=30µm) and G) Pax7 by immunoblot of human cell lysates. Mouse satellite cell lysate was used as a positive control. H) Colorimetric quantification of adhesion to matrigel of WT mouse satellite cells treated with the CD13 blocking mAb, SL13. Data represents the mean±S.E.M. n=8 from three independent experiments (***P<0.001), or I) human satellite cells treated with the CD13 activating mAb 452. Data represents the mean±S.E.M. n=9 from two independent experiments (**P<0.01).
Discussion
CD13 is a multifunctional cell surface peptidase expressed in a number of tissues where it acts in both enzyme-dependent and -independent manners to regulate disparate processes such as tumor angiogenesis [30–32, 47], endothelial filopodia formation [47] and dendritic cell antigen uptake and presentation [46] among others [48]. We have recently identified CD13 as a homotypic adhesion molecule that mediates in vitro monocyte adhesion to anti-CD13 activated, but not to classical, TNF-activated endothelial cells [25]. These studies suggest additional complexity in inflammatory trafficking processes and raise questions regarding the specific circumstances under which CD13 may participate in monocyte trafficking. These questions have prompted us to investigate if CD13 also regulates inflammation in vivo in a model of ischemic injury following peripheral femoral artery dissection of the hind limb. We find that CD13KO mice are impaired in their ability to repair the ischemic wound, resulting in significantly reduced perfusion and ambulation and increased paw necrosis. Cellular and molecular analysis of injured muscles showed deleterious effects of the lack of CD13 on angiogenesis with reductions in numbers of both neovessels and more mature vessels. In addition, inflammatory cell trafficking was indeed altered and resulted in skewed inflammatory profiles in CD13KO wounds, producing a pro-healing environment in contrast to the defective repair. However, we found that a lack of CD13 also apparently perturbs adhesion of the well-characterized satellite cell population to the niche, thus promoting differentiation, perhaps at the expense of renewal and potentially leading to depletion of the regenerative pool, thus adding cell-ECM adhesion to the list of pleiotropic functional effects of CD13 and implicating it in satellite stem cell function. A potential role for CD13 in satellite cell self-renewal is currently under investigation.
In contrast to general concepts of ischemic wound healing, skeletal muscle repair and regeneration has been shown to be particularly dependent on the population of normally quiescent cells that upon injury, become activated, differentiate and fuse to repair damaged myofibers and importantly, self-renew by asymmetric division (reviewed in refs [39, 49]). In addition to angiogenic defects, we see a striking decrease in the satellite cell population in CD13KO muscles post-injury, which may contribute substantially to impaired repair. Whether satellite cell number is limiting for repair is not clear; however a relationship between satellite number and function seems logical. If stem cells are abundant, the functional demand on each cell is modest and dispersed in response to injury. However, if this population is scarce, demand on these cells will be increased and they may soon be overwhelmed [38]. This notion is supported by studies showing that depletion or compromise of satellite cells by various methods universally impairs regeneration (reviewed in ref [39]). Pertinent to the current study, perturbation of a number of components of the Notch signaling pathway leads to increased satellite cell differentiation, decreased self-renewal and exhaustion of the pool leading to reduced muscle repair in response to injury [50, 51]. While potential interplay between Notch and CD13 is currently unknown, the Notch studies support the concept that a reduction in satellite stem cell numbers could be an underlying mechanism of altered repair in CD13KO mice.
Many factors have been shown to influence maintenance of the satellite cell population [14, 38, 39]. The composition of the microenvironment is essential to maintain an adequate supply of quiescent cells in the niche in case of injury, termed ‘niche addiction’ [52]. For example, the majority of progenitor cells in resting muscles reside within a few microns of capillaries and capillary density correlates with satellite cell numbers, linking endothelial cells and myogenesis [53]. Thus angiogenic defects observed in the CD13KO muscles may also contribute to a reduction in the satellite cell pool. Alternatively, trafficking defects presumably would not be a factor since satellite cells are tissue resident and unable to migrate through the endothelium [49]. Similarly, inflammatory monocyte/macrophage subsets have been postulated to differentially control maintenance of the niche and support wound healing through production of cytokine programs, which recruit additional immune cells to clear damaged tissue, promote neovascularization, stimulate precursor cell proliferation and renewal, and promote myocyte fusion and repair [7, 54]. We find that although the percentages of muscle-resident CD11b+ myeloid cells are comparable in wild type and CD13KO animals, the profiles of the pro- and anti-inflammatory monocytic subsets are clearly skewed in the null animals, which would accordingly result in alterations in the levels of cytokines produced. Indeed, cytokine protein and/or mRNA profiles in CD13KO injured muscles show reduced expression of a number of pro-inflammatory molecules such as IL-6, TNFa and MCP-1, consistent with fewer proinflammatory Gr1hi monocytes. Similarly, impaired trafficking could underlie the decrease in levels of VEGF, PDGF and Ang-1 and thus contribute to diminished angiogenesis. Finally, CD13KO muscle repair is impaired despite a decline in expression levels of the potent inhibitor of skeletal muscle cell differentiation and regeneration, TGFβ [14], although our studies in aging muscles have demonstrated that TGFβ and TNF levels that fall below physiological levels can also impair regeneration, and thus may contribute to the CD13 phenotype [55]. Overall, these findings support the concept that proper healing requires a balance and favorable conditions in one aspect may not be sufficient to overcome deleterious defects in others.
Alternatively, stem cell adhesion to the niche is critical to the maintenance of pluripotency in the embryonic brain, as was recently demonstrated to involve Id1 transcriptional control of adhesion of neural stem cells, where proliferation and self-renewal of Id1 null stem cells was reduced and differentiation enhanced [42]. This study illustrated that cell intrinsic differentiation mechanisms are kept in check by specific signals derived from the niche but that once released, cells proceed along default differentiation pathways. Similarly, we find that following injury, CD13KO muscle satellite cells proliferate and adhere at significantly lower rates than wild type cells, CD13KO injured muscles show clear decreases in the activation levels of FAK and ERK kinases critical to adhesion, and there are nearly 30% fewer CD45−/Sca1−/α7-integrin+/β1-integrin+ cells in the injured muscles lacking CD13, consistent with impaired adhesion and enhanced differentiation; perhaps leading to decreased self-renewal and depletion of the compartment, resulting in impaired regeneration. Interestingly, one of us has previously reported that pERK levels decline in aging muscles which contributes to the decreased activation of Notch and the failure to activate aged satellite cells in response to muscle attrition [56, 57], perhaps suggesting a role for CD13 in aging muscles as well. Alternatively, animals lacking the endothelial-expressed adhesion molecule E-selectin in the bone marrow vascular niche demonstrated increased hematopoietic cell quiescence and enhanced self-renewal [58], suggesting the niche microenvironment both positively and negatively regulates stem cell fate. Whether niche mechanisms and molecules are conserved between tissues or if they differ in developmental, homeostatic vs. injury responses is a fascinating area of future investigation.
Finally, taken together, this study may begin to speak to the diversity and potential heirarchical ranking of interdependent processes in regard to the complex response to injury. When addressed individually or in vitro, particular angiogenic responses, inflammatory cell profiles, cytokine ‘signatures’ or vascular addresses have been determined to either support or undermine the healing process. For example, high numbers of Ly6Clow monocytes accompanied by low levels of TNFa, IL-6 and TGFβ would be considered ‘pro-healing’ and predict improved repair. Interestingly, in the CD13KO animal, healing and muscle regeneration is unambiguously impaired despite the decidedly pro-healing cytokine environment provided by distorted ratios of regulatory monocytes, illustrating that a healthy progenitor cell pool may be more fundamental to repair and thus be a more effective therapeutic target for skeletal muscle injury. Dissection of the relative contribution of these interconnected healing processes may provide valuable insights leading to more focused and successful treatment programs.
Supplementary Material
Acknowledgements
We would like to thank Dr. Kotaro Takeda for technical support in hindlimb ischemia, Dr. Kevin Claffey for use of his microscope, Dr. Anu Maharjan for helping with culturing human myoblast cells and Charan Devarakonda for confocal microscopy. In addition we thank the staff of the UCHC Gene Targeting and Transgenic Facility (GTTF) and the Histology Core Facility.
This work was supported by Public Health Service grants CA-106345 from the National Cancer Institute and HL-70694 from the National Heart, Lung and Blood Institute and the State of Connecticut Stem Cell Research Program grant #09-SCA-UCHC-009.
Footnotes
Author contributions:
developed study concept: MMR, LHS, G-HF, MEC
designed experiments: MMR, LHS, MEC
performed experiments: MMR, MG, JS
interpreted data: MMR, LHS, MEC, MG, JS
wrote manuscript: MMR, LHS
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest
References
- 1.Ingersoll MA, Platt AM, Potteaux S, et al. Monocyte trafficking in acute and chronic inflammation. Trends in Immunology. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller WA. Sorting the Signals from the Signals in the Noisy Environment of Inflammation. Sci Signal. 2011;4:pe23. doi: 10.1126/scisignal.2002051. [DOI] [PubMed] [Google Scholar]
- 3.McGettrick HM, Butler LM, Buckley CD, et al. Tissue stroma as a regulator of leukocyte recruitment in inflammation. Journal of Leukocyte Biology. 2012;91:385–400. doi: 10.1189/jlb.0911458. [DOI] [PubMed] [Google Scholar]
- 4.Auffray C, Fogg D, Garfa M, et al. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 5.van Amerongen MJ, Harmsen MC, van Rooijen N, et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 7.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M-G, Su Boo C, Sook Ko Y, et al. Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrology Dialysis Transplantation. 2010;25:2908–2921. doi: 10.1093/ndt/gfq183. [DOI] [PubMed] [Google Scholar]
- 9.Hirose N, Maeda H, Yamamoto M, et al. The Local Injection of Peritoneal Macrophages Induces Neovascularization in Rat Ischemic Hind Limb Muscles. Cell Transplantation. 2008;17:211–222. doi: 10.3727/000000008783906919. [DOI] [PubMed] [Google Scholar]
- 10.Heil M, Eitenmuller I, Schmitz-Rixen T, et al. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies LC, Locke M, Webb RD, et al. A multipotent neural crest-derived progenitor cell population is resident within the oral mucosa lamina propria. Stem cells and development. 2010;19:819–830. doi: 10.1089/scd.2009.0089. [DOI] [PubMed] [Google Scholar]
- 12.Jakob M, Hemeda H, Bruderek K, et al. Comparative functional cell biological analysis of mesenchymal stem cells of the head and neck region: Potential impact on wound healing, trauma, and infection. Head & neck. 2012 doi: 10.1002/hed.23196. [DOI] [PubMed] [Google Scholar]
- 13.Carlson S, Trial J, Soeller C, et al. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovascular Research. 2011;91:99–107. doi: 10.1093/cvr/cvr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. Journal of cellular physiology. 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 15.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 16.Covas DT, Piccinato CE, Orellana MD, et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340–344. doi: 10.1016/j.yexcr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Fan CG, Tang FW, Zhang QJ, et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14:311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 18.Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–509. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- 19.Seeberger KL, Dufour JM, Shapiro AM, et al. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 20.Trubiani O, Di Primio R, Traini T, et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–221. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- 21.Limbourg A, Korff T, Napp LC, et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 22.Stabile E, Burnett MS, Watkins C, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 23.Ross J, Benn A, Jonuschies J, et al. Defects in glycosylation impair satellite stem cell function and niche composition in the muscles of the dystrophic Large(myd) mouse. Stem Cells. 2012;30:2330–2341. doi: 10.1002/stem.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danoviz ME, Yablonka-Reuveni Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 2012;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mina-Osorio P, Winnicka B, O'Conor C, et al. CD13 is a novel mediator of monocytic/endothelial cell adhesion. J Leukoc Biol. 2008;84:448–459. doi: 10.1189/jlb.1107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid MC, Varner JA. Chapter 15. Methods to study myeloid cell roles in angiogenesis. Methods Enzymol. 2008;445:343–371. doi: 10.1016/S0076-6879(08)03015-2. [DOI] [PubMed] [Google Scholar]
- 27.Mina-Osorio P, Shapiro LH, Ortega E. CD13 in cell adhesion: aminopeptidase N (CD13) mediates homotypic aggregation of monocytic cells. J Leukoc Biol. 2006;79:719–730. doi: 10.1189/jlb.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagwat SV, Petrovic N, Okamoto Y, et al. The angiogenic regulator CD13/APN is a transcriptional target of Ras signaling pathways in endothelial morphogenesis. Blood. 2003;101:1818–1826. doi: 10.1182/blood-2002-05-1422. [DOI] [PubMed] [Google Scholar]
- 29.Petrovic N, Schacke W, Gahagan JR, et al. CD13/APN regulates endothelial invasion and filopodia formation. Blood. 2007;110:142–150. doi: 10.1182/blood-2006-02-002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasqualini R, Koivunen E, Kain R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer research. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 31.Rangel R, Sun Y, Guzman-Rojas L, et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc Natl Acad Sci U S A. 2007;104:4588–4593. doi: 10.1073/pnas.0611653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhagwat SV, Lahdenranta J, Giordano R, et al. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97:652–659. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller WA. Mechanisms of leukocyte transendothelial migration. Annual review of pathology. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winnicka B, O'Conor C, Schacke W, et al. CD13 is dispensable for normal hematopoiesis and myeloid cell functions in the mouse. J Leukoc Biol. 2010;88:347–359. doi: 10.1189/jlb.0210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White ES, Mantovani AR. Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. The Journal of pathology. 2013;229:141–144. doi: 10.1002/path.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell stem cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marthiens V, Kazanis I, Moss L, et al. Adhesion molecules in the stem cell niche--more than just staying in shape? J Cell Sci. 2010;123:1613–1622. doi: 10.1242/jcs.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niola F, Zhao X, Singh D, et al. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nature cell biology. 2012;14:477–487. doi: 10.1038/ncb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. The Journal of Cell Biology. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q, Kinch MS, Lin TH, et al. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. Journal of Biological Chemistry. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 45.Renshaw MW, Price LS, Schwartz MA. Focal Adhesion Kinase Mediates the Integrin Signaling Requirement for Growth Factor Activation of Map Kinase. The Journal of Cell Biology. 1999;147:611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh M, McAuliffe B, Subramani J, et al. CD13 Regulates Dendritic Cell Cross-Presentation and T Cell Responses by Inhibiting Receptor-Mediated Antigen Uptake. J Immunol. 2012;188:5489–5499. doi: 10.4049/jimmunol.1103490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrovic N, Bhagwat SV, Ratzan WJ, et al. CD13/APN transcription is induced by RAS/MAPK-mediated phosphorylation of Ets-2 in activated endothelial cells. J Biol Chem. 2003;278:49358–49368. doi: 10.1074/jbc.M308071200. [DOI] [PubMed] [Google Scholar]
- 48.Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tedesco FS, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. The Journal of clinical investigation. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conboy IM, Rando TA. The Regulation of Notch Signaling Controls Satellite Cell Activation and Cell Fate Determination in Postnatal Myogenesis. Developmental cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 51.Lin S, Shen H, Jin B, et al. Blockade of notch signaling in muscle stem cells causes muscular dystrophic phenotype and impaired muscle regeneration. Stem Cells. 2013 doi: 10.1002/stem.1319. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 52.Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christov C, Chretien F, Abou-Khalil R, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villalta SA, Nguyen HX, Deng B, et al. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Human molecular genetics. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson ME, Conboy MJ, Hsu M, et al. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson ME, Suetta C, Conboy MJ, et al. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlson ME, Hsu M, Conboy IM, et al. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.