Abstract
Objective
This study determined whether adding a self-regulatory intervention (SRI) focused on self-monitoring of spontaneous physical activity and sedentary behavior to a standard weight loss intervention improved maintenance of lost weight.
Design and Methods
Older (65–79 yrs), obese (BMI=30–40 kg/m2) adults (n=48) were randomized to a five-month weight loss intervention involving a hypocaloric diet (DIET) and aerobic exercise (EX) with or without the SRI to promote spontaneous physical activity and decrease sedentary behavior (SRI+DIET+EX compared to DIET+EX). Following the weight loss phase, both groups transitioned to self-selected diet and exercise behavior during a 5-month follow-up. Throughout the 10-months, the SRI+DIET+EX group utilized real-time accelerometer feedback for self-monitoring.
Results
There was an overall group by time effect of the SRI (P < 0.01); DIET+EX lost less weight and regained more weight than SRI+DIET+EX. The average weight regain during follow-up was 1.3 kg less in the SRI+DIET+EX group. Individuals in this group maintained ~10% lower weight than baseline compared to those in the DIET+EX group whom maintained ~5% lower weight than baseline.
Conclusions
Addition of a self-regulatory intervention, designed to increase spontaneous physical activity and decrease sedentary behavior, to a standard weight loss intervention enhances successful maintenance of lost weight.
Keywords: Obesity, older adult, weight loss maintenance, self-monitoring, sedentary behavior, physical activity
Introduction
The chronic disease and decreased mobility associated with obesity underscore the need to successfully treat the increasing prevalence of this condition in older adults.(1) Obesity treatment guidelines state that caloric restriction combined with moderate-intensity aerobic exercise should be the primary therapy for achieving weight loss in any age group.(2) However, while this approach produces short-term weight loss, weight regain is common,(3–6) highlighting the need to identify treatments that are effective for sustaining weight loss.
Difficulty in maintaining lost weight is in part due to the ‘energy gap’ caused by adaptive thermogenesis and the drive to defend existing energy stores.(7–10) One key adaptation is a reduction in both resting(11–14) and non-resting energy expenditure, including spontaneous physical activity (SPA), defined as energy expenditure resulting from movement-related activities including postural shifts and daily activities, but excluding structured exercise.(12, 15–20) SPA is a learned/conditioned component of energy expenditure with large individual differences shaped by personal attributes, social influence, occupational demands, and environmental factors.(21) A reduction in SPA increases sedentary behavior, a trend that is compounded with aging.(22, 23) Reduced SPA and greater sedentary behavior contribute to several adverse health outcomes, including obesity and aging-related functional decline, independent of exercise behavior.(24–26) Our prior work shows that reductions in SPA with weight loss are predictive of weight regain.(27) Yet, to date, there is very little research that tests strategies that can be adopted and sustained to prevent declines in SPA that occur with weight loss in older adults.
Self-regulation, particularly self-monitoring, is a powerful behavioral strategy to facilitate behavior change during weight loss.(28) However, the use of this strategy to raise awareness of energy expended through everyday activities to maintain or increase SPA, and to promote more effective self-regulation of SPA, has not been prospectively tested for weight loss maintenance. Thus, the purpose of this study was to determine whether adding a self-regulatory intervention (SRI), focused on self-monitoring of SPA, to a standard weight loss intervention results in less body weight regain following weight loss than a comparable intervention lacking this component.
Methods
Study Design
This study was a two-arm, 10-month pilot study in 48 older, obese adults. Participants were randomized to an intervention involving a hypocaloric diet and aerobic exercise (DIET+EX, n=24) or to the same weight loss intervention with the addition of a self-regulatory intervention (SRI) for promoting SPA (SRI+DIET+EX, n=24). Both groups underwent a controlled diet and four days/week of aerobic exercise for five months as described below. Following post-weight loss research assessments, both groups transitioned to a self-selected program of diet and exercise behavior during a five-month follow-up. Throughout the total 10-month period, the SRI+DIET+EX group was provided with an intervention component designed to promote/maintain a SPA level that was equal to or greater than each individual’s baseline level (described below). Research data were collected at baseline, after the five-month weight loss phase (five-month time point), and after the five-month follow-up phase (10–month time point).
Participants
Forty-eight, obese, older women and men were screened and randomized to one of the two groups (n=24/group). After a phone screen, those eligible underwent a medical history, physical exam, and graded exercise stress test. Inclusion/exclusion criteria included: 1) age 65–79 yrs, 2) sedentary (<2 x/wk of structured exercise), 3) BMI=30–40 kg/m2, 4) weight-stable (± 5%) within the past year, 5) non-smoking for the past year, 6) not currently taking medications that affect body weight, 7) normal cognitive function, 8) no evidence of clinical depression, and no evidence of heart disease, cancer, liver or renal disease, chronic pulmonary disease, physical impairment that would prevent walking, uncontrolled hypertension, or any contraindications for either exercise or weight loss. The study was approved by the Wake Forest School of Medicine Institutional Review Board and all participants provided written, informed consent to participate.
Interventions
Both groups (DIET+EX and SRI+DIET+EX) took part in a five-month weight loss intervention via a hypocaloric diet and supervised exercise. Daily hypocaloric intake levels (−600 kcal/d deficit) were assigned for each person; the individual calorie level was derived by subtracting the participants’ required energy deficit from their estimated daily energy needs for weight maintenance. Weight maintenance energy needs were calculated from the direct measurement of resting energy expenditure, applying an activity factor based on daily activities (1.2 for sedentary).
Participants were provided two meals (lunch and supper) per day prepared by our Clinical Research Unit (CRU) kitchen. All meals were prepared individually after participants chose from a menu designed by the RD. No woman was provided with less than 1100 kcals/d and no man with less than 1300 kcals/d. The diet contained less than 30% calories from fat and at least 0.8 grams of protein per kg of ideal body weight per day. In addition, participants were provided with a daily calcium (1200 mg/d) and vitamin D (800 IU/d) supplement. They were provided menus to guide their food purchasing and preparation of breakfast meals and snacks that were consistent with the prescribed calorie level. They were asked to consume only the food given to them, or approved from the breakfast menu, and were asked to keep a daily food/drink log which were reviewed weekly to verify compliance. A compliance estimate was calculated which is an average percentage (over the course of the 20-week intervention) of the prescribed caloric intake (kcal/d) each participant reports on his/her food log. Body weight was measured weekly.
The exercise component involved treadmill walking four days/week in our exercise facility under the direction of an exercise physiologist. Blood pressure and heart rate (HR) were measured before each session and participants warmed-up by walking for 5 mins at a slow pace and then walked at an intensity of 65–70% of heart rate reserve (HRR). The duration of exercise progressed from 15–20 mins at 50% HRR the 1st week to 30 mins at 65–70% HRR by the end of the 6th week and thereafter. At least two HR readings were recorded each session to monitor compliance to the prescribed exercise intensity.
Participants assigned to the SRI+DIET+EX group underwent an additional intervention aimed at promoting SPA through self-monitoring. Each participant in this group was provided with an accelerometer and individual cognitive-behavioral counseling sessions (see below) to prevent the decline in SPA expected from the hypocaloric diet and structured exercise program. SPA was operationalized as minutes of light physical activity based on the accelerometer output. The interventionist assisted with goal setting of SPA minutes and tailored increasing the minutes in SPA to each individual participant. Thus, considerable attention was placed on the process of how to achieve increased SPA given the daily demands and environmental constraints faced by each individual. In consultation with the interventionist, participants were given personal SPA goals that were initially calculated to be at least 10% greater than baseline levels. Individuals whose baseline SPA was less than 10 minutes were counseled to increase levels to at least 10 minutes. SPA goals were increased throughout the intervention, with the overall goal being to increase total volume of light physical activity by 20% over baseline. This energy was able to be expended in a variety of ways and an individual’s SPA minutes resulted from a combination of several daily activities depending on individual habits and preferences.
During the first week, participants in the SRI intervention were given a Lifecorder Plus® tri-axial accelerometer (Suzuken, Co., LTD; http://www.new-lifestyles.com/) with instructions for wear and documentation of usage. They were asked to wear the accelerometers daily for the length of the 5-month intervention and the 5-month follow-up. The units are small devices worn on the hip. With this model, participants cannot be blinded to the output and, thus, are able to instantaneously view their SPA minutes throughout the day and to compare it to their daily SPA goal. Participants recorded SPA minutes (from the accelerometer) in a daily log to become more aware of the metabolic costs of different daily activities, and, if necessary, to devise ways to increase SPA. Daily documentation included participants’ personal SPA goal, actual accumulated daily minutes of SPA, adherence information (e.g., wearing the monitor, meeting the goal, etc.), and identifying daily barriers if participants did not meet their goals. The accelerometers were removed during the on-site, structured exercise sessions.
Participants in the SRI+DIET+EX group met weekly for the first six-weeks with an interventionist to review progress. During these 10–15 minute sessions, accelerometers were downloaded, the SPA self-reported logs were collected, and participants received feedback based on a self-management model developed by Rejeski and colleagues.(29) This model assumes that the motive to change behavior is driven by the desire for specific outcomes along with both facilitative and inhibitory processes. The SRI intervention facilitated SPA behavior by clarifying the intended outcomes of increasing SPA and by providing specific, moderately-challenging goals that were evaluated frequently across the day using the accelerometer. During brief counseling sessions that were collaborative by design, feedback was provided and factors that inhibited successful self-management were addressed using Perri and colleagues approach to social problem solving.(30) If, during the first six-weeks of treatment, participants’ level of SPA activity dropped below established goals, additional counseling sessions were arranged. After the first six weeks, participants met bi-weekly with the SPA interventionist for the remainder of the five-month weight loss intervention and then monthly during the five-month follow-up period.
Follow-up phase
During the five-month follow-up phase, all participants followed a self-selected diet and exercise routine (e.g., no dietary counseling, diet, or formal exercise provided) and participants in both groups were asked to complete follow-up visits that occurred at five-week intervals. At these visits, participants were weighed and those in the SPA SRI group met briefly with the SPA interventionist to turn in accelerometers to download and change batteries, turn in SPA trackers, and briefly review progress.
Assessments
Height and weight were measured at baseline, after the five-month weight loss phase, and after the five-month follow-up phase (10-month time point) on the same scale, which is accurate to ±100g and calibrated weekly.
Percent body fat, lean mass, and adipose tissue mass were measured by dual energy absorptiometry (Hologic Delphi QDR) and maximal aerobic fitness was measured using expired gas analysis on a treadmill test to exhaustion.(31)
Resting energy expenditure (REE) was measured at each time point in the morning after a 10-hour fast by indirect calorimetry using the ventilated hood technique.(27)
Daily physical activity was assessed using a Kenz® Lifecorder EX® tri-axial accelerometer (Suzuken, Co., LTD; http://www.new-lifestyles.com/), which provides valid and reliable measures of activity duration and intensity.(32, 33) Participants in both groups wore the accelerometer for at least 10-hours for a period of seven days at each of the assessment time points and they were directed to keep an adherence diary giving details as to when the monitor was worn and taken off during the day. Participants wore the accelerometers during their structured exercise time at the five-month time point and were instructed to maintain their regular level of physical activity outside of structured exercise. They were asked to wear the monitor at all times, except while bathing and sleeping. Participants were blinded to the results of the data during these assessment periods; thus, they did not receive any performance feedback from the accelerometers or the staff.
Statistical analyses
Baseline descriptive statistics were calculated for each group and values are reported as mean ± standard deviation (SD) or as frequency in percentage. Absolute changes in all outcomes were calculated as baseline value subtracted from values at either the 5-month or 10-month time points. Observed means and group differences were analyzed using Student’s t-tests with means and standard errors being reported. Linear mixed effect model was performed to assess within-group and between-group differences of the changes of each outcome, after adjusting for age, gender and baseline measure. Least square means and standard errors were also estimated from the same model. All analyses were performed using SAS v.9.3 (SAS Institute, Cary, NC). A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Participant Characteristics at Baseline
A total of 46 (of the randomized 48) participants (DIET+EX: n=23; SRI+DIET+EX: n=23) completed the 5-month weight loss phase, and 41 participants completed the 5-month follow-up phase and returned for 10-month testing (DIET+EX: n=21; SRI+DIET+EX: n=20). Thus, there was 85% study retention over 10 months. Because the primary purpose of this pilot study was to examine whether the self-regulatory intervention enhanced weight loss maintenance, all analyses included only the 41 participants who completed the entire 10-month study. Table 1 shows baseline demographics and physical characteristics for these participants; there were no differences between study groups at baseline for any of the assessed variables. The participants who were lost to follow-up dropped out of the study due to life changes unrelated to the study interventions, including unanticipated illness, change in work schedules, new time constraints, relocation or family circumstances.
Table 1.
Baseline characteristics of all participants who completed the entire study
| N (col %) or Mean±SD | SRI+DIET+EX (n=20) |
DIET+EX (n=21) |
|---|---|---|
| Age (yrs) | 69.5±3.4 | 70.6±3.8 |
| Gender #(%) | ||
| Female | 15 (75%) | 16 (76%) |
| Male | 5 (25%) | 5 (24%) |
| Race/ethnicity, #(%) | ||
| Non-Hispanic white | 18 (90%) | 17 (81%) |
| Non-white | 2 (10%) | 4 (19%) |
| Body Composition | ||
| Body weight (kg) | 88.6±12.5 | 90.2±9.5 |
| Body mass index (kg/m2) | 32.6±2.7 | 33.6±1.9 |
| Total fat mass (kg) | 37.4±6.6 | 38.3±5.8 |
| Total lean mass (kg) | 51.7±12.8 | 52.5±9.9 |
| % body fat | 42.5±7.6 | 42.4±6.6 |
| Maximal Aerobic Capacity (ml/kg/min) | 17.6±2.3 | 18.1±2.0 |
| Resting Energy Expenditure (kcal/d) | 1317±420 | 1399±246 |
| Daily Physical Activity | ||
| Steps per day | 4325±1976 | 4857±2080 |
| PAEE (kcal/d) | 141±70 | 168±81 |
| Light activity (mins/d) | 39.0±19.7 | 39.9±15.1 |
| Moderate+Vigorous activity (mins/d) | 10.9±6.4 | 14.7±13.1 |
Intervention Compliance
Exercise session attendance to the prescribed four day per week intervention during the 5-month weight loss phase averaged 91±8% and 90±16% for the DIET+EX and SRI+DIET+EX groups, respectively. The absolute energy expenditure during the exercise sessions averaged 234±60 kcal and 205±56 kcal for the DIET+EX and SRI+DIET+EX groups, respectively. Self-reported compliance to the dietary intervention (calculated as a percentage of daily caloric intake over or under what was prescribed) was also good with the DIET+EX group reporting an average compliance of 101.1±2.4% and the SRI+DIET+EX group reporting an average compliance of 99.8±4.3% to the prescribed diet.
For participants in the SRI+DIET+EX group, the average SPA goal during the 5-month weight loss phase was 27±13 minutes (range=10–59 minutes) and did NOT include structured exercise time. Of the 20 participants who completed the SRI+DIET+EX intervention, 17 (85%) provided accelerometer process data for the entire 10 months; the remaining three stopped providing accelerometry logs at 19, 21 and 31 weeks. Participants reported wearing the accelerometer at least 10 hrs/day for an average of 87±14% of the days and the daily SPA goal was met for 81±14% of the days. The average number of SPA minutes recorded (39±14 minutes) was higher than the average SPA goal (P < 0.0001). The most common reported barriers to full accelerometer compliance (10 hrs/day, every day for 10 months) were: device malfunction/need for battery change (13%), illness or health reason (9%), forgot to wear (7%), and too busy or time conflict (7%).
Intervention Effects on Body Weight
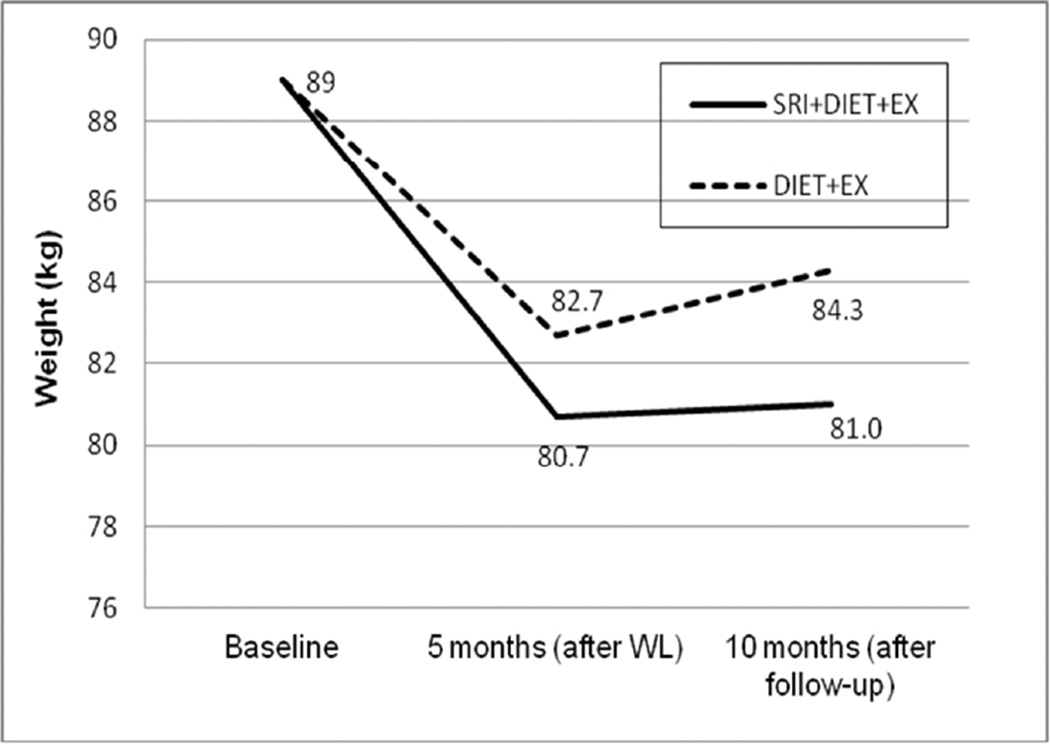
Table 2 shows unadjusted and adjusted (for baseline weight, age, and gender) body weight changes by group during each phase of the study. During the initial 5-month weight loss intervention, the absolute amount of lost weight tended to be greater, and the percentage of weight loss was significantly greater, in the SRI+DIET+EX compared to the DIET+EX group. Moreover, the average weight regain during follow-up was 1.3 kg less in the SRI+DIET+EX group. At the 10-month time point, body weight in both groups was still significantly (P < 0.001) lower than at baseline, but individuals in the SRI+DIET+EX group had maintained an approximate 10% lower weight than baseline, compared to those in the DIET+EX group whose weight was approximately 5% lower than baseline (Table 2; P < 0.01 between groups). Figure 1 shows body weight at each time point, adjusted for time, treatment, time×treatment interaction and baseline weight. There is an overall group by time effect of the SRI intervention (P=0.005).
Table 2.
Changes in body weight, energy expenditure, and daily physical activity during weight loss (baseline to 5 mos), during follow-up (5 mos to 10 mos) and after follow-up (baseline to 10 mos)
| Observed mean (SE) | Observed mean (SE) | ||||||
|---|---|---|---|---|---|---|---|
| SRI+ DIET+EX |
DIET+EX | p-value | SRI+ DIET+EX |
DIET+EX | p-value | ||
| Body weight (kg) | |||||||
| during weight loss | −8.8 (0.7) | −6.6 (0.9) | 0.07 | −8.9 (0.8)* | −6.5 (0.8)* | 0.06 | |
| during follow-up | 0.3 (0.6) | 1.6 (0.3) | 0.07 | 0.3 (0.5) | 1.6 (0.5) | 0.06 | |
| after follow-up | −8.5 (0.8) | −5.0 (0.9) | <0.01 | −8.6 (0.8)* | −5.0 (0.8)* | <0.01 | |
| % body weight | |||||||
| during weight loss | −10.0 (0.8) | −7.2 (1.0) | 0.03 | −9.6 (1.0)* | −6.8 (1.1)* | 0.04 | |
| during follow-up | 0.4 (0.8) | 1.9 (0.4) | 0.09 | 0.3 (0.6) | 1.7 (0.6) | 0.09 | |
| after follow-up | −9.7 (0.9) | −5.5 (1.0) | <0.01 | −9.3 (1.0)* | −5.1 (1.1)* | <0.01 | |
| Resting energy expenditure (kcal/kg) |
|||||||
| during weight loss | 1.1 (0.5) | −0.05 (0.5) | 0.36 | 1.0 (0.5)* | 0.1 (0.5) | 0.15 | |
| during follow-up | 0.6 (0.7) | −0.7 (0.5) | 0.24 | −0.7 (0.6) | −0.6 (0.6) | 0.16 | |
| after follow-up | 1.6 (0.6) | −0.7 (0.5) | 0.05 | 1.6 (0.5)* | −0.6 (0.5) | <0.01 | |
| Physical activity energy expenditure (kcal/kg) |
|||||||
| during weight loss | 1.3 (0.1) | 0.9 (0.2) | 0.31 | 1.3 (0.2)* | 1.1 (0.2)* | 0.46 | |
| during follow-up | −0.9 (0.1) | −0.6 (0.2) | 0.34 | −0.9 (0.2) | −0.6 (0.2) | 0.34 | |
| after follow-up | 0.3 (0.2) | 0.3 (0.2) | 0.81 | 0.4 (0.2)* | 0.5 (0.2)* | 0.71 | |
| Steps (per day) | |||||||
| during weight loss | 2831 (252) | 1788 (354) | 0.02 | 2988 (412)* | 2276 (432)* | 0.20 | |
| during follow-up | −2067 (344) | −166 (515) | 0.16 | −2093 (447) | −1289 (436) | 0.21 | |
| after follow-up | 639 (427) | 457 (496) | 0.78 | 895 (418)* | 986 (447)* | 0.87 | |
| Light activity (mins/day) | |||||||
| during weight loss | 9.8 (2.2) | 1.7 (2.5) | 0.02 | 12.6 (2.6)* | 6.6 (2.7)* | 0.08 | |
| during follow-up | −6.4 (3.2) | −0.7 (2.4) | 0.16 | −6.9 (2.0) | −1.2 (2.7) | 0.15 | |
| after follow-up | 1.4 (3.9) | 0.8 (2.8) | 0.90 | 5.7 (2.6)* | 5.4 (2.8) | 0.93 | |
| Mod+vig activity (mins/day) | |||||||
| during weight loss | 18.5 (1.7) | 14.7 (2.1) | 0.18 | 18.3 (2.7)* | 15.9 (2.8)* | 0.49 | |
| during follow-up | −14.1 (1.9) | −9.5 (3.3) | 0.23 | −14.0 (2.7) | −10.2 (2.6) | 0.32 | |
| after follow-up | 4.5 (1.8) | 4.1 (3.4) | 0.93 | 4.3 (2.7) | 5.7 (2.9) | 0.70 | |
P-values reflect between group differences;
indicates significant within group difference at P≤0.05
N=20 for SRI+DIET+EX and N=21 for DIET+EX
Adjusted models are adjusted for baseline variable, age, and gender
Figure 1.
Body weight at each time point by treatment group, adjusted for time, treatment, time × treatment interaction and baseline weight; there is an overall group by time effect (P=0.005) of the SRI intervention.
During the weight loss intervention, 45 of the 46 completers lost some weight (range= −15.4 to −0.5kg; one person gained 2.1kg). The majority of participants (34 of 46, 74%) lost at least 5% of their initial body weight; of the 12 participants who did not lose ≥5%, 9 (75%) were in the DIET+EX group. The range of weight changes during the 5-month follow-up phase was −9.3 to +4.2 kg. A total of 31 participants (76%) experienced some weight gain and 18 of those (44%) experienced a weight gain of 2kg or more. Of the 18 participants who gained ≥2kg, 12 (67%) were in the DIET+EX group.
Intervention Effects on Daily Physical Activity and Energy Expenditure
Changes in resting energy expenditure and blinded accelerometer measures of daily physical activity are also shown in Table 2. To account for changes in body weight, physical activity energy expenditure (PAEE) and resting energy expenditure (REE) are expressed per kg of weight. Most notably, physical activity increased in both groups during the weight loss phase and adjusted changes in minutes of light activity tended to be significantly (P=0.08) greater in the SRI+DIET+EX group compared to the DIET+EX group. In addition, 10-month changes in REE were also greater in SRI+DIET+EX; this group experienced an overall increase in REE per kg body weight, whereas REE per kg body weight decreased overall in the DIET+EX group.
Discussion
The primary finding of this study was that adding a self-monitoring intervention, designed to increase light physical activity in order to prevent potential declines in SPA, to a standard weight loss intervention resulted in a lower body weight over a period of ten months compared to a group without the self-regulatory intervention. Specifically, the SRI+DIET+EX group lost more weight during active treatment, and regained less weight (essentially no weight regain) during the short five-month follow-up, than the DIET+EX group. In addition, at the end of the weight loss intervention phase, the group that received the self-regulatory intervention tended to have higher levels of light physical activity and greater REE. These group differences in REE were sustained at ten months, suggesting that this self-monitoring intervention may have beneficial effects on both resting and non-resting energy expenditure.
Presently, there are observational data demonstrating that a high level of physical activity distinguishes those individuals who are successful at weight loss maintenance from those who are not.(34–36) However, long-term adherence to a high volume of exercise may be particularly difficult for older and obese persons who also may be more likely to compensate for the caloric expenditure of structured exercise by decreasing SPA, resulting in reduced total energy expenditure. Fortunately, evidence suggests that less intense activity is also beneficial for weight loss maintenance.(37) A few clinical trials show that less structured exercise of a lower intensity may be better for weight loss maintenance than higher-intensity exercise.(38, 39) Thus, we posit that incorporating more movement into daily activity (i.e., increasing SPA and reducing sedentary behavior) during and following weight loss may be more beneficial than promoting structured exercise in older, obese adults. There is a growing body of literature suggesting it is feasible to intervene on sedentary behavior, which is an independent predictor of health outcomes when considered in conjunction with structured exercise.(24, 26, 40)
The strengths of this study include the randomized controlled design, the carefully and successfully delivered interventions, and the excellent compliance to the daily self-monitoring and accelerometry use. Yet, there were limitations as well. First, the study was designed as a pilot to test the feasibility and potential efficacy of adding the SRI intervention to a conventional weight loss protocol and, therefore, the sample size is small. Next, although there were group differences in body weight at both follow-up time points, there was only a trend for physical activity to be greater in the SRI+DIET+EX group. Therefore, we cannot deduce with certainty that the body weight differences primarily result from increased SPA. The SRI also involved other behavioral strategies known to influence weight loss success, including goal setting and problem solving, that may have also contributed to the larger weight loss of the SRI+DIET+EX group.
Substantial research (28, 29) suggests that goal setting and other self-regulatory skills, particularly self-monitoring, are critical for successful behavior change. This study provides important feasibility and early efficacy data regarding the benefits of using a novel self-monitoring strategy within a broader conceptual model of behavior change to modify the likely reduction in SPA that occurs during periods of negative energy balance among older adults. Our approach focuses on a behavioral strategy to eliminate the compensatory reduction in non-exercise activity seen in older adults who are losing weight with a hypocaloric diet and structured exercise training. While these data are compelling, they are not definitive, and a full-scale randomized clinical trial is needed to examine the effects of a SPA self-regulation intervention compared to structured exercise for the maintenance of weight loss in this population. If our hypothesis is correct, and confirmed in a larger and longer randomized trial involving a state-of-the-art weight loss intervention, the findings would challenge the current standard of care (i.e., exclusive prescription of structured moderate-intensity exercise) for obesity therapy in older adults, leading to new obesity treatment guidelines for both initial weight loss and weight loss maintenance.
What is already known about this subject?
Current obesity treatment guidelines are not effective for long-term weight loss maintenance.
Weight loss results in a reduction in both resting energy expenditure and spontaneous physical activity, especially in older adults.
Self-monitoring of spontaneous physical activity has not been prospectively tested for weight loss maintenance.
What does this study add?
We show that addition of a self-regulatory intervention designed to promote an increase in spontaneous physical activity and a decrease in sedentary behavior to a standard weight loss intervention enhances successful maintenance of lost weight.
These results provide important feasibility and early efficacy data on the weight loss benefits of using a self-monitoring strategy that targets increases in spontaneous physical activity among older adults.
If confirmed, these findings would challenge the current standard of care (i.e., exclusive prescription of structured moderate-intensity exercise) for obesity therapy in older adults, leading to new obesity treatment guidelines and/or recommendations for both initial weight loss and weight loss maintenance.
Acknowledgements
We thank the volunteers for this study, as well as the study coordinators, dietitians, exercise physiologists and other research staff.
Grant Support: This study was supported by NIH grants R21HL097252 and R01 HL093713, and the WFU Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Footnotes
Competing interests: the authors have no competing interests
Author contributions were: Study conception and design (BN, WJR, XL); Intervention material development (WJR, JG); Data collection and intervention conduct (JG, KB); Data analyses and interpretation (BN, WJR, XL, JB); and Manuscript writing (BN, JG, WJR, XL). All authors had final approval of the submitted and published versions of the manuscript.
References
- 1.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11(9):671–685. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Obesity (Silver Spring) 2013 [Google Scholar]
- 3.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29(10):c1153–c1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 4.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29(10):1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 5.Lowe MR, Kral TV, Miller-Kovach K. Weight-loss maintenance 1, 2 and 5 years after successful completion of a weight-loss programme. Br J Nutr. 2008;99(4):925–930. doi: 10.1017/S0007114507862416. [DOI] [PubMed] [Google Scholar]
- 6.Barte JC, ter Bogt NC, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11(12):899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 7.Hill JO, Thompson H, Wyatt H. Weight maintenance: what's missing? J Am Diet Assoc. 2005;105(5 Suppl 1):S63–S66. doi: 10.1016/j.jada.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Cornier MA. Is your brain to blame for weight regain? Physiol Behav. 2011;104(4):608–612. doi: 10.1016/j.physbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 10.Tremblay A, Royer MM, Chaput JP, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond) 2013;37(6):759–764. doi: 10.1038/ijo.2012.124. [DOI] [PubMed] [Google Scholar]
- 11.Apfelbaum M. Adaptation to changes in caloric intake. Prog Fd Nutr Sci. 1978;2:543–559. [PubMed] [Google Scholar]
- 12.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 13.Roberts SB, Fuss P, Heyman MB, Dallal GE, Young VR. Effects of age on energy expenditure and substrate oxidation during experimental underfeeding in healthy men. J Gerontol A Biol Sci Med Sci. 1996;51(2):B158–B166. doi: 10.1093/gerona/51a.2.b158. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JL, Manore MM, Thomas JR. Effects of diet and diet-plus-exercise programs on resting metabolic rate: a meta-analysis. Int J Sport Nutr. 1996;6(1):41–61. doi: 10.1123/ijsn.6.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Gorsky RD, Calloway DH. Activity pattern changes with decreases in food energy intake. Hum Biol. 1983;55(3):577–586. [PubMed] [Google Scholar]
- 16.Weigle DS, Sande KJ, Iverius PH, Monsen ER, Brunzell JD. Weight loss leads to a marked decrease in non-resting energy expenditure in ambulatory human subjects. Metabolism. 1988;37:930–936. doi: 10.1016/0026-0495(88)90149-7. [DOI] [PubMed] [Google Scholar]
- 17.Martin CK, Heilbronn LK, de JL, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 18.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4(2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camps SG, Verhoef SP, Westerterp KR. Weight loss-induced reduction in physical activity recovers during weight maintenance. Am J Clin Nutr. 2013;98(4):917–923. doi: 10.3945/ajcn.113.062935. [DOI] [PubMed] [Google Scholar]
- 20.Bonomi AG, Soenen S, Goris AH, Westerterp KR. Weight-loss induced changes in physical activity and activity energy expenditure in overweight and obese subjects before and after energy restriction. PLoS One. 2013;8(3):e59641. doi: 10.1371/journal.pone.0059641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine JA, Kotz CM. NEAT--non-exercise activity thermogenesis--egocentric & geocentric environmental factors vs. biological regulation. Acta Physiol Scand. 2005;184(4):309–318. doi: 10.1111/j.1365-201X.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 22.Arnardottir NY, Koster A, Van Domelen DR, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: Age, Gene/Environment Susceptibility- Reykjavik Study. Age Ageing. 2013;42(2):222–229. doi: 10.1093/ageing/afs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis. 2012;9:E26. [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 25.Santos DA, Silva AM, Baptista F, et al. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp Gerontol. 2012;47(12):908–912. doi: 10.1016/j.exger.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45(8):1493–1500. doi: 10.1249/MSS.0b013e318288a1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc. 2008;40(10):1781–1788. doi: 10.1249/MSS.0b013e31817d8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rejeski WJ, Brawley LR, Jung RT. Self-management of health behavior in geriatric medicine. In: Halter JB, Ouslander JG, Tinetti ME, et al., editors. Hazzard's Geriatric Medicine and Gerontology. 6th. New York, NY: McGraw-Hill,Inc.; 2009. pp. 325–341. [Google Scholar]
- 30.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69(4):722–726. [PubMed] [Google Scholar]
- 31.Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89(4):1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClain JJ, Craig CL, Sisson SB, Tudor-Locke C. Comparison of Lifecorder EX and ActiGraph accelerometers under free-living conditions. Appl Physiol Nutr Metab. 2007;32(4):753–761. doi: 10.1139/H07-060. [DOI] [PubMed] [Google Scholar]
- 33.Abel M, Hannon J, Lillie T, Sell K, Anderson D, Conlin G. Comparison of Kenz Lifecorder versus actigraph physical activity output in free-living conditions. J Phys Act Health. 2009;6(Suppl 1):S141–S147. doi: 10.1123/jpah.6.s1.s141. [DOI] [PubMed] [Google Scholar]
- 34.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 35.Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab. 2007;3(7):518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550–1559. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Baak MA, van ME, Astrup AV, et al. Leisure-time activity is an important determinant of long-term weight maintenance after weight loss in the Sibutramine Trial on Obesity Reduction and Maintenance (STORM trial) Am J Clin Nutr. 2003;78(2):209–214. doi: 10.1093/ajcn/78.2.209. [DOI] [PubMed] [Google Scholar]
- 38.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281(4):335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 39.Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med. 2000;160(14):2177–2184. doi: 10.1001/archinte.160.14.2177. [DOI] [PubMed] [Google Scholar]
- 40.Fitzsimons CF, Kirk A, Baker G, Michie F, Kane C, Mutrie N. Using an individualised consultation and activPAL feedback to reduce sedentary time in older Scottish adults: Results of a feasibility and pilot study. Prev Med. 2013;57(5):718–720. doi: 10.1016/j.ypmed.2013.07.017. [DOI] [PubMed] [Google Scholar]