Abstract
Aims
To determine whether endogenous GLUT1 induction and the increased glucose utilization that accompanies pressure overload hypertrophy (POH) are required to maintain cardiac function during hemodynamic stress, and to test the hypothesis that lack of GLUT1 will accelerate the transition to heart failure.
Methods and Results
To determine the contribution of endogenous GLUT1 to the cardiac adaptation to POH, male mice with cardiomyocyte-restricted deletion of the GLUT1 gene (G1KO) and their littermate controls (Cont) were subjected to transverse aortic constriction (TAC). GLUT1 deficiency reduced glycolysis and glucose oxidation by 50%, which was associated with a reciprocal increase in fatty acid oxidation (FAO) relative to controls. Four weeks after TAC, glycolysis increased and FAO decreased by 50% in controls, but were unchanged in G1KO hearts relative to shams. G1KO and controls exhibited equivalent degrees of cardiac hypertrophy, fibrosis, and capillary density loss after TAC. Following TAC, in vivo left ventricular developed pressure was reduced in G1KO hearts relative to controls, but +dP/dt was equivalently reduced in Cont and G1KO mice following TAC. Mitochondrial function was equivalently impaired following TAC in both Cont and G1KO hearts.
Conclusions
GLUT1 deficiency in cardiomyocytes alters myocardial substrate utilization, but does not substantially exacerbate pressure-overload induced contractile dysfunction or accelerate the progression to heart failure.
Keywords: Cardiac hypertrophy, glucose transport and cardiac metabolism
1. INTRODUCTION
Heart failure is a leading cause of death worldwide. Although, under physiological conditions, oxidative metabolism of fatty acids (FA) accounts for more than 50% of myocardial ATP production [1]; during pathological circumstances, such as ischemia and cardiac hypertrophy, glucose import and utilization are increased [2]. An important regulatory mechanism in cardiac glucose utilization is the modulation of glucose uptake, which is largely mediated by two transporters, glucose transporter 1 (GLUT1, SLC2A1) and glucose transporter 4 (GLUT4, SLC2A4) [3]. GLUT1 is an insulin-independent glucose transporter mainly responsible for basal cardiac glucose uptake in quiescent myocytes. Despite developmental downregulation of GLUT1 soon after birth, GLUT1 expression is reinduced in the heart in several pathophysiological states, including pressure overload hypertrophy (POH) [4, 5].
Increased glucose utilization that accompanies POH is believed to be an adaptive response that may retard the transition to heart failure. The role of glucose transport in the preservation of cardiac function is further supported by studies showing that lifelong transgenic overexpression of GLUT1 in the heart prolongs survival and increases cardiac function after aortic constriction [6, 7]. In addition, cardiomyocyte-restricted GLUT4 knockout mice have modest cardiac hypertrophy with preserved contractile function, but poor response to ischemia/reperfusion injury [8]. It has also been reported that, as cardiac hypertrophy progresses to heart failure, GLUT1 mRNA expression is reduced in human hearts, suggesting that decreased GLUT1-mediated glucose uptake and utilization could play a role in the transition to heart failure [9]. However, whether endogenous expression of GLUT1 is required for the initial adaptation to POH is still incompletely understood. The purpose of the present study was to investigate the role of endogenous GLUT1 in the initial stages of compensated cardiac hypertrophy, and whether GLUT1 deficiency in the heart can exacerbate pressure-overload induced contractile dysfunction and accelerate the transition to heart failure.
To determine if endogenous GLUT1 expression plays a role in the initial functional and metabolic adaptation to pressure overload hypertrophy, we generated mice with deletion of the GLUT1 gene in cardiomyocytes. Our data shows that cardiomyocyte-restricted GLUT1 deficiency does not exacerbate pressure overload-induced LV remodeling and mitochondrial dysfunction, and only marginally impairs contractile function, suggesting that GLUT1 is not essential for the cardiac adaptations to POH.
2. METHODS
An expanded Methods section is provided in the Online Data Supplement.
2.1. Animals
Generation of Mice with the Floxed GLUT1 Allele
Mice with floxed GLUT1 alleles were generated as previously described [10]. For cardiomyocyte-specific deletion of the GLUT1 gene, GLUT1 floxed mice were crossed with C57BL6 mice expressing cre-recombinase under control of the alpha-myosin heavy chain promoter (G1KO). G1KO mice were backcrossed to the C57BL6 background for more than 10 generations. Age-matched wild-type littermates (GLUT1 floxed mice, lacking the alpha-myosin heavy chain cre-recombinase) were used as controls. Mice were fed standard chow (see supplementary materials for composition of chow) and housed in temperature-controlled facilities with a 12-h light and 12-h dark cycle (lights on at 06:00). Only male mice were utilized for the experiments. Animals were studied in the random fed state during the day (between 07:00 to 13:00) using protocols that were approved by the Institutional Animal Care and Use Committee of the University of Utah.
2.2. Surgical procedures and hemodynamic measurements
Transverse Aortic Constriction
Mice at 6-wk of age were anesthetized (single intraperitoneal injection of 400 mg chloral hydrate / kg body weight) and placed in the supine position on a heating pad (37 °C). A topical depilatory agent was applied to the chest, and the area was cleaned. Following a horizontal skin incision ~ 0.5–1 cm in length at the level of the suprasternal notch, a 2- to 3-mm longitudinal cut was made in the proximal portion of the sternum. A titanium clip calibrated to the diameter of a 30-G needle was placed between the innominate artery and the left common carotid artery. Sham surgery was performed in the same manner except no clip was placed. After aortic manipulation, the chest and overlying skin were closed [11].
Left Ventricle Catheterization
: Four weeks after TAC, cardiac catheterizations were performed as previously described [11]. Mice at 10-wk of age were anesthetized (single intraperitoneal injection of 400 mg chloral hydrate / kg body weight) and placed in the supine position on a heating pad (37 °C). A Millar Mikro-Tip catheter (1.4F; Millar Instruments, Houston TX) was then inserted into the left ventricle via the right carotid artery, and hemodynamic measurements were obtained using LabChart7 Pro software (ADInstruments, Colorado Springs, CO). Prior to euthanasia (4 weeks after TAC), body weights were taken. While mice were still sedated, hearts and lungs were excised and weighed. Tibiae were also removed and their lengths measured.
2.3. Isolation of cardiac myocytes and determination of 2-DG uptake
Cardiomyocytes were isolated from 10-week old G1KO mice and their wild type controls using collagenase digestion of Langendorff-perfused mouse hearts as previously described by our group [12]. This isolation procedure initially yields between 80 and 90% viable rod-shaped myocytes, the majority of which attach to laminin-coated wells and maintain their morphology for the duration of the protocol. Basal glucose uptake was measured using 2-deoxyglucose. See Online Supplement for detailed methods.
2.4. Histology
Myocardial fragments were stained by Masson's trichrome for visualization and quantification of fibrotic tissue. Light microscopy was performed using an Olympus TH4-100 inverted microscope that was connected to an Olympus Microfire Digital Camera (New York, NY). For quantification of fibrotic tissue, pictures from each heart section (thickness of 3 μm) were taken systematically to ensure that the entire extent of the tissue section had been covered. Five different hearts were used per group, and a total of 20 sections were analyzed using the Image Pro-plus software (Media Cybernetics, Silver Springs MD). The percentage of fibrotic tissue in each micrograph was measured and averaged per heart. The average value for each heart was then utilized for statistical analysis. Vascularization Index (number of vessels/mm2) was determined in endothelin-1 immunostained sections of the left ventricle of mice from all groups. The number of capillaries per area was determined in 50 random fields per group, following the “Forbidden Line Principle” [13].
2.5. Isolated working heart
Cardiac substrate metabolism, left ventricular developed pressure, cardiac output, cardiac power, oxygen consumption, and cardiac efficiency were measured in isolated working hearts obtained after 4 weeks of TAC as previously described [14]. Hearts were perfused with 5 mmol/L glucose and 0.4 mmol/L palmitate. No insulin was added to the perfusate. See Online Supplement for detailed methods.
2.6. Mitochondrial function
Mitochondrial oxygen consumption and ATP production rates were measured in ventricular muscle fibers from control and G1KO mice as described before, with palmitoyl carnitine or succinate (in the presence of rotenone) as substrate [15]. See Online Supplement for details.
2.7. RNA Extraction and Quantitative RT-PCR
Total RNA was isolated and reverse transcribed to cDNA, which was quantified by real-rime PCR as previously described and transcript levels normalized to ribosomal subunit 16S [16]. Primer sequences and accession numbers are listed in the Online Supplement.
2.8. Western Blot Analysis
For immunoblotting analysis, ~ 50 mg of frozen tissue was homogenized in 200 μl Lysis buffer (details in Online Supplement), using the Tissue Lyser II (Qiagen Inc., Valencia, CA). Tissue lysates were resolved on SDS-PAGE and transferred to PVDF membranes (Millipore Corp., Billerica, MA). Primary and secondary antibodies used are summarized in the table below:
| Antigen | Company | ~Size (kDa) | 2° |
|---|---|---|---|
| GLUT1 | Millipore (Billerica, MA) | 55 | Rabbit |
| GLUT4 | Millipore | 50 | Rabbit |
| Phospho AKT (Ser 473) | Cell Signaling (Danvers, MA) | 60 | Rabbit |
| Akt | Cell Signaling | 60 | Mouse |
| Phospho AMPK (Thr 172) | Cell Signaling | 62 | Rabbit |
| AMPKα | Cell Signaling | 62 | Mouse |
IRDye 800CW anti-Mouse (LICOR, Lincoln, NE) and Alexa fluor anti-Rabbit 680 (Invitrogen, Carlsbad, CA) were used as secondary antibodies and fluorescence was quantified using the LICOR Odyssey imager (Li-COR, Lincoln, NE).
2.9. Statistical analysis
Data are presented as means±SEM. RT-PCR and western blot results are presented as fold change versus control sham. Significant differences were determined by t-Test in experiments when G1KO mice were compared with age-matched controls, and by ANOVA followed by Tukey multiple comparison test, when 4 groups (control sham, control TAC, G1KO sham and G1KO TAC) were compared. A probability value of p≤0.05 was considered significantly different. Statistical calculations were performed using the GraphPad Prism Software (La Jolla, CA, USA).
3. RESULTS
3.1. GLUT1 Deletion Decreases GLUT1 Expression and 2-DG Uptake in Cardiomyocytes
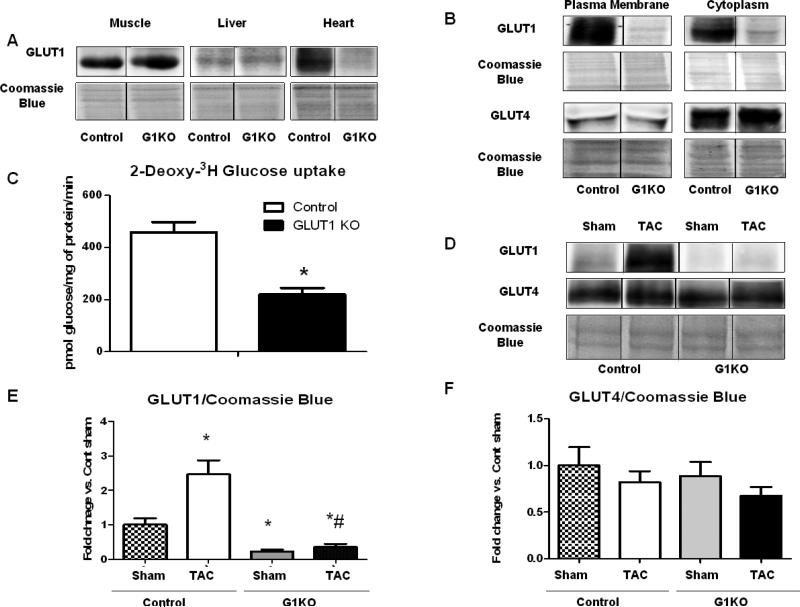
Western blots in multiple tissues confirmed the specificity of the conditional knock out. GLUT1 protein levels were maintained in muscle and liver of G1KO mice, but were reduced by > 60% in the hearts of G1KO mice relative to controls (Figure 1A). Moreover, GLUT1 deficiency in cardiomyocytes did not influence circulating concentrations of glucose (data not shown). GLUT1 content was also significantly reduced in cardiomyocytes isolated from G1KO hearts, while GLUT4 levels remained unchanged between groups in both the cytoplasmic and plasma membrane fractions (Figure 1B). Basal 2-DG uptake in cardiomyocytes isolated from 10-week old mice was reduced by 50% in G1KO mice relative to age-matched controls (Figure 1C). TAC increased GLUT1 protein content in whole heart homogenates in control mice, but GLUT1 protein remained low in G1KO mouse hearts (Figure 1D and E). No compensatory increase in GLUT4 protein levels was observed in G1KO mice, regardless of surgery (Figure 1D and F). mRNA expression of GLUT1 was significantly decreased in G1KO mice regardless of TAC surgery, but was unchanged in control TAC mice. mRNA expression of GLUT4 was variable, but was significantly reduced in G1KO relative to controls following TAC. GLUT8 mRNA levels were unchanged between control and G1KO mice, irrespective of genotype, and GLUT12 levels were similarly reduced in control and G1KO hearts following TAC, when compared to sham hearts (Online Figure 1).
Figure 1. GLUT1 deletion in cardiomyocytes.
A- GLUT1 protein levels in muscle, liver and hearts of control and G1KO mice. B- Cytoplasmic and sarcolemmal levels of GLUT1 and GLUT4 proteins in cardiomyocytes isolated from control and G1KO mice. C- Glucose uptake in isolated cardiomyocytes from 10-week old control and G1KO hearts. D- Western Blot for GLUT1 and GLUT4 in control and G1KO mice 4 weeks after TAC. E- Densitometric analysis of GLUT1 normalized by Coomassie Blue. F- Densitometric analysis of GLUT4 normalized by Coomassie Blue. Data are expressed as means±SEM. Significant differences were determined by Student's t-test or by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05, n=5 mice/group. (*) significantly different vs. control sham, (#) Significantly different vs. control TAC.
3.2. Conditional Deletion of GLUT1 does not Exacerbate Pathological Remodeling or the Hypertrophic Response After TAC
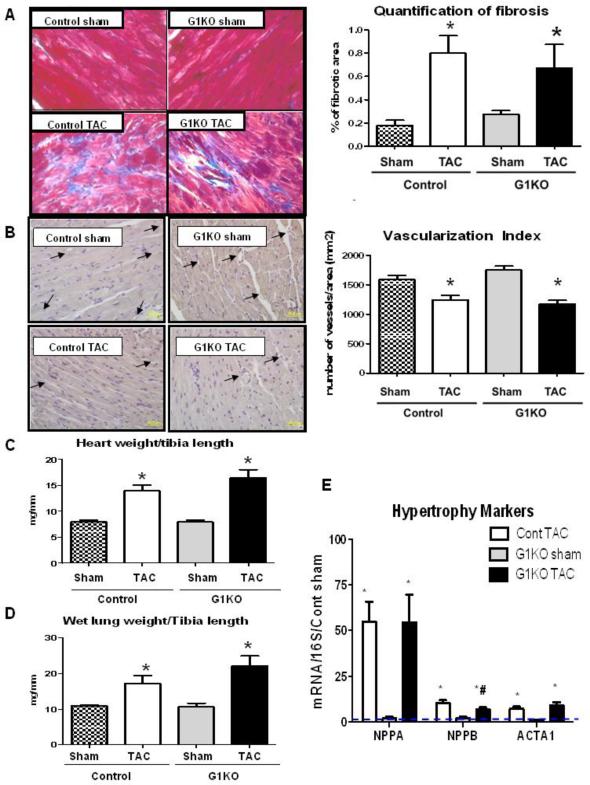
A cardioprotective role has been ascribed to, or correlated with increased GLUT1. We therefore sought to determine if lack of GLUT1 would worsen the pathological remodeling that follows POH. POH induced by 4 weeks of TAC equivalently increased cardiac fibrosis in control and G1KO mice (Figure 2A). Likewise, vascularization index evaluated in endothelin1-stained sections was equivalently reduced in control and G1KO hearts following TAC (Figure 2B). GLUT1 overexpression has also been associated with increased activation of pro-survival pathways, such as Akt in vitro [4]. However, we observed that Akt was similarly activated in response to TAC in both control and G1KO hearts, arguing against a role for GLUT1-mediated glucose uptake in Akt activation following TAC (Online Figure 2). GLUT1 ablation did not alter cardiac weight at base line, and did not exacerbate TAC-induced LV hypertrophy as measured by heart weight normalized to tibia length (Figure 2C). Relative to G1KO sham mice, body weight was significantly decreased in G1KO mice 4 weeks after TAC, and tibia length measurements were unchanged between groups (Online Table 1). Wet lung weight to tibia length ratios were equally increased in control and G1KO mice following TAC, indicating similar degrees of pulmonary edema (Figure 2D). Cardiac hypertrophy was accompanied by increased mRNA expression of the hypertrophy markers Nppa, Nppb and ACTA1, which were all similarly increased after TAC in control and G1KO mice (Figure 2E).
Figure 2. Lack of GLUT1 does not exaggerate pathological remodeling after TAC (5 hearts per group).
A- Masson's Trichrome staining of heart sections and quantification of percentage of fibrotic area. B- Endothelin 1 staining and quantification of vascularization. Arrows are highlighting the blood vessels. C- Heart weight to tibia length ratios. D- Lung weight to tibia length ratios. Data are expressed as means±SEM. E- mRNA expression of hypertrophy markers 4 weeks after TAC. Control (Cont) sham is represented as a dashed line. Data is represented as fold change vs. Cont Sham. Significant differences were determined by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05. (*) significantly different vs. sham, (#) Significantly different vs. control TAC.
3.3. Impact of GLUT1 Deletion on Pressure Overload-Induced Contractile Dysfunction
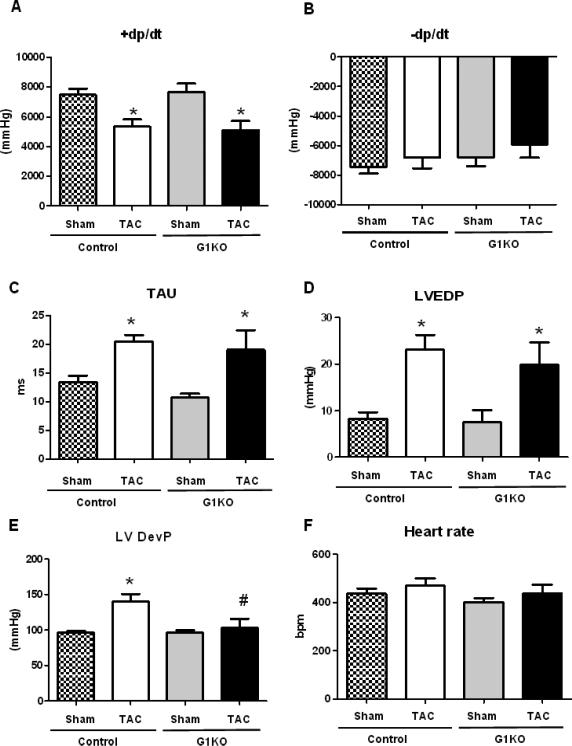
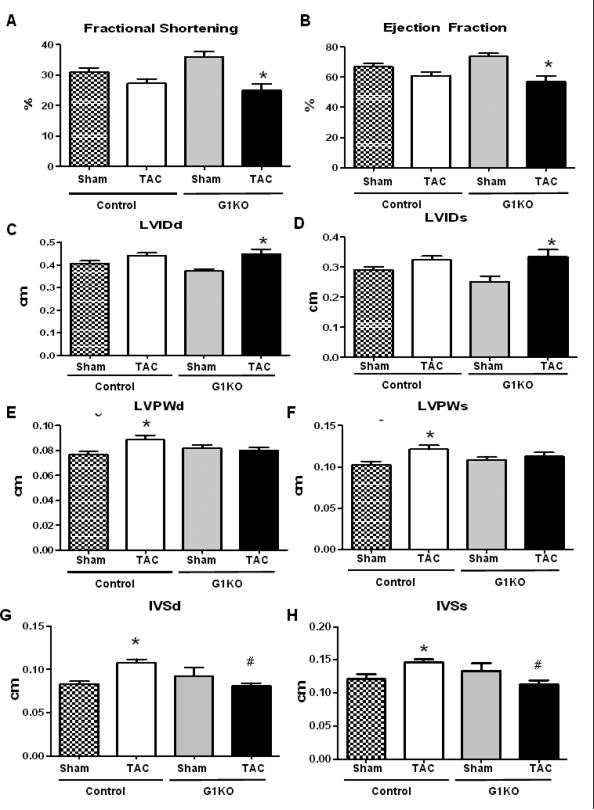
LV catheterization revealed an equivalent decrease in peak rates of ventricular contraction (+dP/dt) (Figure 3A), while –dP/dt were not significantly changed between groups (Figure 3B). The isovolumic relaxation constant (TAU) (Figure 3C) and left ventricle end diastolic pressure (LVEDP) (Figure 3D) were also similarly increased in control and G1KO mice after TAC, suggesting similar degrees of contractile dysfunction between control and G1KO hearts. In contrast, left ventricle developed pressure (LVDevP) was reduced in G1KO after TAC (Figure 3E). Heart rates were unchanged between groups (Figure 3F). By echocardiography, fractional shortening (FS) and ejection fraction (EF) were lower in G1KO mice after TAC, relative to G1KO sham mice (Figure 4 A and B). However, no significant differences in FS and EF were found between WT and G1KO after TAC. Left ventricle internal diameter (LVID) increased in G1KO mice after TAC relative to G1KO sham, but was not significantly different from control TAC hearts (Figure 4 C and D). In contrast, the thickness of the left ventricular wall (Figure 4 E and F) and the interventricular septum (Figure 4 G and H) were increased in control mice, but not in G1KO mice after TAC.
Figure 3. Left Ventricle Catheterization (6 hearts per group).
A- +dP/dt (rate of rise of left ventricular pressure). B- -dP/dt (rate of fall of left ventricular pressure). C- TAU (Isovolumic Relaxation Constant). D- LVEDP (Left Ventricular End Diastolic Pressure). E- LVDevP (Left Ventricular Developed Pressure). F- Heart rate. Data are expressed as means±SEM. Significant differences were determined by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05, n ≥6. (*) significantly different vs. sham, (#) significantly different vs. control TAC.
Figure 4. Echocardiography (6 hearts per group).
A- Fractional shortening. B- Ejection fraction. C- LVIDd (Left Ventricular Internal Diameter in Diastole). D- LVIDs (Left Ventricular Internal Diameter in Systole). E- LVPWd (Left Ventricular Posterior Wall Thickness in Diastole). F- LVPWs (Left Ventricular Posterior Wall Thickness in Systole). G- IVSd (Interventricular Septum Thickness in Diastole). H- IVSs (Interventricular Septum Thickness in Systole). Data are expressed as means±SEM. Significant differences were determined by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05, n =6. (*) significantly different vs. sham, (#) significantly different vs. control TAC.
3.4. Substrate Metabolism and Cardiac Efficiency in G1KO Hearts Following TAC
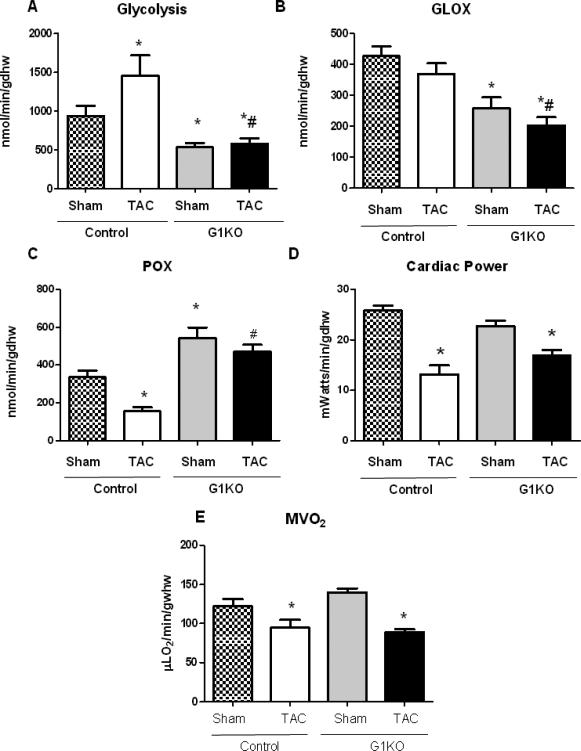
Substrate metabolism was determined in isolated working hearts, 4 weeks after sham or TAC surgery. Glycolysis and glucose oxidation were decreased in G1KO sham mice relative to control sham mice. After TAC, glycolysis rates increased in control hearts, but remained low in G1KO mice (Figure 5A). Glucose oxidation was unchanged in control TAC mice, but was significantly reduced in G1KO relative to control mice following TAC (Figure 5B). Palmitate oxidation rates were increased in non-stressed G1KO hearts relative to control and were maintained after TAC. In contrast, palmitate oxidation rates were reduced in control hearts following TAC (Figure 5C). Cardiac power (Figure 5D), and oxygen consumption (MVO2) (Figure 5E) were equivalently reduced in control and G1KO mouse hearts after TAC. We measured malonyl-CoA levels in the hearts of control and G1KO mice to address the question of whether reduced malonyl-CoA might play a role in the increase in palmitate oxidation observed in G1KO hearts. Malonyl-CoA levels were increased in G1KO sham hearts relative to control hearts and were reduced in G1KO hearts after TAC, relative to sham (Online Figure 3).
Figure 5. Cardiac substrate metabolism and function in isolated working hearts (6 hearts per group).
A- glycolysis, B- glucose oxidation (GLOX) C- palmitate oxidation (POX), D- cardiac power, E- oxygen consumption (MVO2), in isolated working hearts from control and G1KO mice 4 weeks after TAC or sham surgery. Hearts were perfused with 5 mmol/L glucose and 0.4 mmol/L palmitate; n=6 hearts per group for metabolism, MVO2 and efficiency and n=12 per group for cardiac function (pooled data from glucose and FA perfusions). Data are expressed as means±SEM. Significant differences were determined by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05. (*) significantly different vs. sham, (#) significantly different vs. control TAC.
3.5. GLUT1 Deficiency Does Not Exacerbate POH-induced Mitochondrial Dysfunction
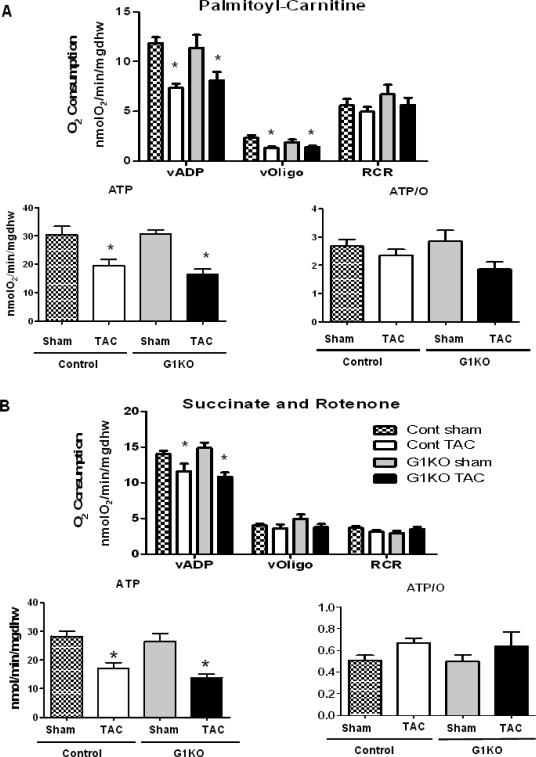
Mitochondrial function was assessed in control and G1KO mice 4 weeks after TAC or sham surgeries in saponinpermeabilized cardiac fibers. Palmitoyl-Carnitine (PC) or succinate (in the presence of rotenone) -supported maximal ADP-stimulated mitochondrial oxygen consumption (VADP) and ATP synthesis (Figures 6A and B) were similarly reduced in control and G1KO mice following TAC. AMPK phosphorylation was equivalently increased in control and G1KO mice following TAC (Online Figure 4A), and mRNA expression of PGC-1α was significantly reduced after TAC in both control and G1KO hearts (Online Figure 4B). Impaired mitochondrial function occurred independently of a decrease in mitochondrial number, as quantification of mtDNA (Online Figure 4C) and citrate synthase activity were found to be unchanged between groups (Online Figure 4D).
Figure 6. Mitochondrial function is equivalently reduced in control and G1KO mice after TAC (5 to 7 hearts per group).
Mitochondrial respiration, ATP synthesis rates, and ATP/O ratios were measured in saponinpermeabilized cardiac fibers. A- Palmitoyl–carnitine or B- Succinate (in the presence of Rotenone) were used as substrates. Significant differences were determined by ANOVA followed by Tukey multiple comparison test, using a significance level of p<0.05. Data are expressed as means±SEM. (*) significantly different vs. sham.
4. DISCUSSION
Increased glucose uptake and utilization that accompanies cardiac hypertrophy is believed to be cardioprotective; [17-20] however, the functional significance of this adaptation is not completely understood [21]. Studies of mice with lifelong cardiomyocyte-specific overexpression of GLUT1 suggest that chronic increases in basal glucose uptake and glycolysis confer increased tolerance to pressure overload and delays the progression to heart failure following ascending aortic constriction in adult mice [6]. It has also been reported that GLUT1 mRNA expression is reduced in human hearts as cardiac hypertrophy progresses to heart failure, suggesting that reduced GLUT1-mediated glucose uptake could play a role in the transition to heart failure [9]. Nevertheless, the question remains as to whether endogenous expression of GLUT1 is required for the initial adaptation to POH and if GLUT1 deficiency can accelerate the transition to heart failure in a model of POH. To address these questions we generated mice with conditional deletion of the GLUT1 gene specifically in cardiomyocytes and subjected them to TAC. Our data suggests that endogenous GLUT1 is not critical for the initial adaptation to POH, since lack thereof does not exacerbate POH-induced pathological remodeling and contractile dysfunction.
Decreased fatty acid oxidation (FAO) is a hallmark of the metabolic remodeling that occurs in pathological cardiac hypertrophy, in parallel with increased glucose utilization. This reduction in FAO has been hypothesized to contribute to contractile dysfunction in the transition from compensated LVH to cardiac failure [22]. Recent studies of mice with cardiac-specific deletion of acetyl CoA carboxylase 2 (ACC2) that develop a sustained increase in FAO demonstrated that maintaining high rates of FAO could improve cardiac function and energetics during the development of pressure-overload hypertrophy [23]. In the present study, the decrease in glucose utilization resulting from GLUT1 deletion in cardiomyocytes was accompanied by a compensatory increase in FAO, which was not of sufficient magnitude to prevent mitochondrial dysfunction or pathological remodeling in these hearts. Moreover, contractile dysfunction was only modestly impaired in G1KO mice subjected to TAC. The possibility exists that the increase in FAO observed in our model could have mitigated some of the adverse consequences of the absence of induction of GLUT1 following POH in GLUT1 deficient hearts. Increased glucose utilization can inhibit FAO by increasing malonyl-CoA with subsequent inhibition of carnitinepalmitoyltransferase (CPT) I, which controls the entry and oxidation of long-chain fatty acids in the mitochondria [24]. Thus, we hypothesized that allosteric mechanisms in the intact heart, that might involve reduced malonyl-CoA and increased activation of CPT-1, may regulate FAO in G1KO hearts given reduced palmitoyl-carnitine supported respirations observed in in vitro mitochondrial fiber preparations. Surprisingly, our data showed an increase in malonyl-CoA levels in G1KO sham hearts relative to controls, which was significantly reduced after TAC, suggesting a mechanism independent of malonyl-CoA levels.
The present study underscores important differences between GLUT1 versus GLUT4 mediated glucose uptake in the heart. Cardiac-selective GLUT4 deficiency leads to compensated ventricular hypertrophy with preserved cardiac contractile function [25]. GLUT4 deletion has also been shown to predispose the heart to profound ischemic injury [8]. Recent studies have reinforced the importance of GLUT4 for cardiac function in showing that cardiomyocyte GLUT4 deficiency is sufficient to induce profound alterations in cardiomyocyte Ca2+ and pH homeostasis, exacerbates cardiac fibrosis [26] and is associated with hypertrophy and contractile dysfunction [27]. In contrast, although contractile dysfunction was marginally worse in G1KO mice, the phenotypes observed are considerably more subtle than those observed in GLUT4 knockout hearts. Moreover, mitochondrial dysfunction and cardiac fibrosis were not exacerbated in our model, suggesting that endogenous GLUT1 does not play a critical role in the cardiac adaptations to POH. Future studies in mice with inducible KO of GLUT1 in cardiomyocytes that may attenuate the adaptive increase in FAO will be required to definitively confirm this assertion. An important caveat in our study is the short-term nature of our functional analyses (4-weeks post-TAC). We cannot rule out the possibility that lack of GLUT1 could exacerbate the transition to heart failure in a more severe long-term model of pressure overload hypertrophy.
5- CONCLUSIONS
In summary, we show for the first time that endogenous GLUT1 is dispensable for normal cardiac function. GLUT1 deficiency in cardiomyocytes alters myocardial substrate utilization by decreasing glucose utilization and increasing FAO and impairs metabolic flexibility following TAC. Despite this, left ventricular remodeling and POH-associated LV dysfunction were not exacerbated. Thus, GLUT1 induction is not sufficient for maintaining the structural or functional adaptations of the heart to POH and GLUT1 deficiency does not accelerate the transition to heart failure.
Supplementary Material
Highlights.
Endogenous GLUT1 is dispensable for normal cardiac function
Lack of GLUT1 impairs metabolic flexibility following transverse aortic constriction.
GLUT1 deficiency does not accelerate the transition to heart failure in POH.
GLUT1 is not critical for the initial adaptation to POH.
ACKNOWLDGEMENT
We thank Jeffrey Lei, Sylvia Hu and Li Wang for important technical help during the course of these studies.
7. SOURCES OF FUNDING:
This work was supported by the National Institutes of Health (NIH) [Grants - RO1DK092065, U01 HL087947 to E.D.A. and 5T32 HL007576 to R.O.P.]. R.O.P. was also supported by a postdoctoral fellowship from the American Heart Association (AHA) Western Affiliates, Dallas. A.R.W. was supported by a postdoctoral fellowship from the AHA Western Affiliates and an advanced postdoctoral fellowship from the Juvenile Diabetes Research Foundation (JDRF). C.R. was supported by a postdoctoral fellowship from the German Research Foundation (DFG). This study has no relationship with industry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. DISCLOUSERS:
None
REFERENCES
- 1.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 2.Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation. 1995;91:1824–33. doi: 10.1161/01.cir.91.6.1824. [DOI] [PubMed] [Google Scholar]
- 3.Abel ED. Glucose transport in the heart. Frontiers in bioscience : a journal and virtual library. 2004;9:201–15. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 4.Morissette MR, Howes AL, Zhang T, Heller Brown J. Upregulation of GLUT1 expression is necessary for hypertrophy and survival of neonatal rat cardiomyocytes. Journal of molecular and cellular cardiology. 2003;35:1217–27. doi: 10.1016/s0022-2828(03)00212-8. [DOI] [PubMed] [Google Scholar]
- 5.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, et al. Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. Journal of the American Heart Association. 2013;2:e000301. doi: 10.1161/JAHA.113.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–31. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 7.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–9. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 8.Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–6. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 9.Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, et al. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–9. doi: 10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 10.Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PloS one. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, et al. PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circulation research. 2011;109:783–93. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. The Journal of clinical investigation. 2002;109:629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundersen HJ, Christensen NJ. Intravenous insulin causing loss of intravascular water and albumin and increased adrenergic nervous activity in diabetics. Diabetes. 1977;26:551–7. doi: 10.2337/diab.26.6.551. [DOI] [PubMed] [Google Scholar]
- 14.Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 15.Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 16.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 17.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. The American journal of physiology. 1994;267:H742–50. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 18.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. The American journal of physiology. 1970;218:153–9. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 19.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–26. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, et al. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 1995;92:1274–83. doi: 10.1161/01.cir.92.5.1274. [DOI] [PubMed] [Google Scholar]
- 21.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circulation research. 1991;68:466–81. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 22.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovascular research. 2011;90:234–42. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolwicz SC, Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circulation research. 2012;111:728–38. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. The Journal of clinical investigation. 1977;60:265–70. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. The Journal of clinical investigation. 1999;104:1703–14. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huggins CE, Domenighetti AA, Ritchie ME, Khalil N, Favaloro JM, Proietto J, et al. Functional and metabolic remodelling in GLUT4-deficient hearts confers hyper-responsiveness to substrate intervention. Journal of molecular and cellular cardiology. 2008;44:270–80. doi: 10.1016/j.yjmcc.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J, Delbridge LM. Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation. Journal of molecular and cellular cardiology. 2010;48:663–72. doi: 10.1016/j.yjmcc.2009.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.