Summary
To study the role of c-Src kinase in prooxidant-induced stimulation of TLR4, we used LPS-EK and MPLA as TLR4 specific agonists and positive controls, and SIN-1 and PPC as prooxidant sources. We used HEK-Blue mTLR4 cell line that is stably transfected with mouse TLR4 and that expresses optimized SEAP reporter under the control of a promoter inducible by NF-κB transcription factor. The level of SEAP released due to TLR4 stimulation was a measure of NF-κB activation. Treatment with either the prooxidants or LPS-EK increased SEAP release and TNF-α production in these cells. These treatments also increased intracellular ROS accumulation with an enhanced production of nitric oxide and TBARS to confirm oxidant stress in these cells. Pretreatment with c-Src kinase inhibitors, PP2 and CA-pY, which act by different mechanisms, decreased these parameters. Pretreatment with SSG, a c-Src activator, enhanced the effects promoted by LPS-EK and prooxidants, and rescued cells from PP2- and Ca-pY-induced effects. Curiously, prooxidants but not TLR4 agonist increased the ratio of TNFα to IL-10 released suggesting that prooxidants can initiate and maintain an imbalance of TNFα production over IL-10. To different degrees, both prooxidant and TLR4 agonist increased formation of c-Src complexes with TLR4 and IκB-α as coimmunoprecipitates. Both prooxidant and TLR4 agonist increased c-Src phosphorylation of Tyr-42 residue in IκB-α, but prooxidant-induced effect was more robust and much longer lasting. Taken together, these studies provide a mechanism whereby c-Src assumes a central role in prooxidant-induced NF-κB activation in TLR4 signaling. Prooxidant-induced activation of TLR4 through c-Src/NFκB/IκB-α coupling provides a basis for a molecular dissection of the initiation and maintenance of sterile inflammation that may serve as a “pathophysiologic primer” for many diseases.
Keywords: Toll-like receptor 4, c-Src, IκB-α, NF-κB, pathophysiologic primer, prooxidant, sterile inflammation
INTRODUCTION
Oxidative/nitrosative stress (ONS), a pervasive condition of increased levels of reactive oxygen/nitrogen species (ROS/RNS) in cells or tissues, is now acknowledged to be a prominent feature of many acute and chronic diseases and even of the normal aging process [1]. As a “privilege” of aerobic organisms, ONS can be induced by exogenous oxidants as well as ROS/RNS generated during normal cellular processes [2]. Within cells, ROS can act as secondary messengers in intracellular signaling cascade to induce a dysregulated phenotype, but the mechanism(s) is not clear. ONS can activate many signaling pathways in different cell types, but the changes in cell signaling that may result in decreased or enhanced responsiveness following exposure to oxidants is not fully understood.
A number of tyrosine protein kinases and phosphatases play a role in oxidant-induced cell signaling [3, 4]. c-Src is the leading member of the Src family of non-receptor tyrosine kinases (SFKs) that are expressed in many cells and tissues [5, 6], and are involved in diverse signal transduction pathways [7, 8]. All members of the SFKs possess similar domain arrangements consisting of src homology 3 (SH3), SH2 and kinase (SH1) domains with a common myristoylated and/or palmitoylated membrane–anchoring N-terminal region known as the SH4 domain [9, 10] and a unique domain [11]. Regulation of c-Src activity is crucial for its biological functions. Under basal conditions, 90-95% of c-Src is in a dormant state in the cell [12], but growth factors, including inflammatory cytokines and bacterial LPS [13] can rapidly activate it by phosphorylation. An important mechanism for inactivation of c-Src is dephosphorylation of pTyr416 on c-Src by a member of non-receptor tyrosine phosphatases (PTPases). The potential candidates of PTPase implicated in dephosphorylation of pTyr416 on c-Src include cytoplasmic PTP1B, SHP1 (Src homology 2 domain-containing tyrosine phosphatase 1) and SHP2 [14, 15]. c-Src is sensitive to cellular redox stress [16, 17], but its role in prooxidant-induced inflammatory process is not known.
Stimulation of Toll-like receptors (TLRs) plays a critical role in innate immune responses [18] and subsequent development of adaptive immunity [19, 20]. All mammalian TLRs have similar structural organization consisting of an ectodomain, a transmembrane domain and a cytoplasmic domain with an intracellular Toll/Interleukin 1 receptor (TIR) domain that is critical for signal transduction [19]. Toll-like receptor 4 (TLR4), a member of TLR superfamily, is a pattern recognition receptor that is expressed mainly on immune cells and is involved in sterile inflammatory responses. TLR4 with an extracellular protein MD-2, is a native signaling receptor for LPS [21], but also serves as an important sensor for oxidant stress [22]. The receptor comprises a tri-molecular signaling complex of CD14 (as a TLR4 co-receptor), TIR domain and TLR4 itself [23, 24, 25]. TLR4 signaling cascade is initiated by the co-receptor CD14, following interaction of LPS with LPS binding protein (LBP). The receptor signaling is enhanced by its mono-dimerization followed by recruitment of adaptor proteins and kinases to the intracellular TIR domain of the receptor [26, 27]. The cytosolic adapter proteins including myeloid differentiation primary response protein 88 (MyD88), TIR adaptor protein (TIRAP), and tumor necrotic factor receptor-associated factor 6 (TRAF6) [28] initiate the proximal events of TLR4-mediated intracellular signaling. Association of TLR4 with MyD88 [29] can recruit other adapter proteins that leads to the activation of transforming growth factor-β-activated protein kinase 1 (TAK-1), which in turn results in NF-κB and AP-1 activation [30, 31].
Recently, we have shown that exogenous prooxidants act through TLR4 to activate NF-κB [32]. NF-κB is activated by diverse signals and its activation regulates the promoter regions of a variety of genes. In unstimulated cells, NF-κB is sequestered in the cytoplasm in an inactive form by interacting with inhibitory NF-κB (IκB) proteins. The key pathway in the regulation of NFκB activation is its nuclear translocation after release from the inhibitory kappa B alpha (IκBα) subunit to which it is bound in the cytosol [33]. Regulation of NFκB activation is usually achieved by phosphorylation of IκBα on Serine 32 and Serine 36 residues [pSer32/pSer36] mediated by IκBα kinase. NFκB activation is a primary regulator of stress response in vivo [34]. Under ONS, we propose a novel pathway that involves tyrosine phosphorylation [pTyr] of IκBα at the Tyr42 residue [17, 35], a site that is present only in IκBα, and that favors enhanced formation of [pTyr42]-IκBα by c-Src over [pSer32/pSer36]-IκBα. Stimulation of TLR4 appears to mediate both rapid and delayed activation of NFκB. Phosphorylation of IκBα at Tyr42 would activate NFκB for a long time, which delays its ubiquitin-dependent degradation [36, 37].
The role of c-Src in the molecular mechanisms that may underlie prooxidant-induced NF-κB activation through TLR4 signaling is still poorly understood. In the present study, we sought to establish a primary role for c-Src in prooxidant-induced TLR4 signaling through the NFκB activation pathway. With transfection of c-Src siRNA that blocked LPS-induced effects with high efficiency [38, 39], we have hypothesized that c-Src amongst the SFKs plays a dominant role in NF-κB activation induced by exogenous prooxidants through TLR4 signaling. Here we present evidence that integrates exposure to prooxidants with c-Src/TLR4-coupled signaling to increased c-Src/IκBα-induced effects in the NF-κB activation pathway (as compared with that induced by LPS-EK, a specific agonist for TLR4). We hypothesize that the product of the oxidant-induced c-Src/TLR4/NF-κB-coupled pathway has a potential to initiate and maintain long-lasting “sterile” inflammatory processes that may serve as a primer for different disease states.
MATERIALS AND METHODS
Chemicals and materials
HEK-Blue selection medium, selection antibiotic Zeocin, Quanti-Blue detection reagent (alkaline phosphatase detection medium), synthetic mono-phosphoryl lipid A (MPLA), LPS-EK from E. coli K12 (LPS-EK Ultrapure), polyclonal rat IgG used as pAb neutralization control, were obtained from InvivoGen (San Diego, CA). Linsidomine chloride (SIN-1) SIN-1, an effective nitric oxide (NO) donor, was obtained from Cedarlane Inc (Burlington, NC). Ebselen was from Enzo Life Sciences (Farmingdale, NY). Src family inhibitor (PP2) was purchased from Cayman Chemical (Ann Arbor, MI). Low endotoxin, azide-free (LEAF) affinity purified rat IgG2a, -isotype anti-mouse TLR4 (CD284)/MD-2 complex pAb for neutralization of TLR4 and ELISA kits for human-specific TNF-α and IL-10 were purchased from BioLegend (San Diego, CA). CellROX® Deep Red reagent and NucBlue® Live ReadyProbes™ reagent were obtained from Invitrogen. (5Z)-7-Oxozeaenol (a potent and selective transforming growth factor-β-activated kinase 1 (TAK1) mitogen-activated protein kinase kinase kinase (MAPKKK) inhibitor), Caffeic acid-pYEEEIE (Ca-pY) (phosphopeptide ligand inhibitor for Src SH2 domain), UO126 (a potent inhibitor of mitogen-activated protein kinase kinase (MAPKK) family members MEK 1 and MEK 2), UO124 (an inactive analogue of U0126), PP3 (a negative control for PP2), Parameter™Total Nitric oxide and Nitrate/Nitrite, and Parameter™ TBARS kits were purchased from R & D Systems Inc (Minneapolis, MN). Sodium stibogluconate (a protein tyrosine phosphatase inhibitor; in effect, a c-Src activator) was obtained from EMD Millipore (Bellerica, MA). FACE™ c-Src kit was obtained from Active Motif, Inc. (Carlsbad, CA).
Preparation of Potassium peroxychromate (PPC)
PPC, used in the study as a source of ROS [40], is not available for purchase from any commercial vendor. We prepared it in the laboratory according to a published protocol [41]. We characterized the product by elemental and infrared analyses, and the purity was determined to be ≥ 98 %.
Cell lines and culture
Cells were derived from human embryonic kidney-293 (HEK-293) cell line. HEK-Blue mTLR4 and HEK-Blue-Null1-v cells were purchased from InvivoGen (San Diego, CA). HEK-Blue null-1v-1v1-v is the parental cell line of HEK-Blue mTLR4, but does not express mTLR4. HEK-Blue mTLR4 cells are stably transfected to express mTLR4 gene at high levels with MD-2 and CD14 co-receptor genes involved in TLR4 recognition and presentation. In addition, the cells stably express an optimized secreted alkaline phosphatase (SEAP) reporter gene under the control of a promoter inducible by NF-κB and activator protein-1 (AP-1) transcription factors. HEK-Blue™ Null v1 cells also express the SEAP reporter gene under the control of the IFN-β minimal promoter fused to NF-κB and AP-1 binding sites. We used the level of SEAP protein released into the culture media to quantify the extent of TLR4 stimulation, which also represents the levels of NF-κB activation.
Cells were grown in a 37 °C, 100 % humidified incubator in Dulbecco’s Modified Eagle’s Medium (DMEM, 4.5 g of glucose/L) without pyruvate but supplemented with 2 mM L-glutamine, 10 % (v/v) fetal bovine serum (FBS), 50 units/ml penicillin, 50 μg/ml streptomycin and 100 μg/ml Normocin™. HEK-Blue mTLR4 and Null1-v were maintained in growth medium supplemented with HEK-Blue selection reagent.
Quanti-Blue SEAP reporter assay
HEK-Blue-Null1-v and HEK-Blue mTLR4 cells were plated in 96-well plates and grown to 70 % confluence. Cells were then treated with various inhibitors for 30 min followed by overnight (~ 16 h) stimulation with MPLA, PPC or SIN-1 (a source of peroxynitrite), in the continued presence of the inhibitor and/or stimulator, where applicable. In addition, we carried out a dose-response effect of SIN-1 on SEAP release to provide a rationale for selecting the SIN-1 the concentration we used in all subsequent studies. Then, 20 μl aliquots of cell culture medium were removed and transferred to new 96-well plates containing 180 μl of pre-warmed Quanti-Blue detection reagent per well as per manufacturer’s instructions. Color was allowed to develop for 1 h, and absorbance was read at 650 nm in a Bio-Tek® microplate reader (Burlington, VT).
Immunoprecipitation
HEK-Blue mTLR4 cells were plated in 6-well plates at a density of 5×105 cells/plate. Cells were allowed to reach 70 % confluence and then treated with PBS (as control), LPS-EK, MPLA and PPC for 4 h. Cells were then lysed in mammalian cell protein extraction lysis buffer (Mammalian cell-PE-LB™) (G Biosciences, St. Louis, MO) supplemented with appropriate protease inhibitors. Cell lysates were centrifuged at 16,200-x g at 4 °C for 10 min to remove cell debris. The resultant supernatant was precleared by adding rabbit IgG together with Protein A/G Plus-agarose (Santa Cruz) followed by centrifugation at 600-x g for 5 min at 4° C. Total protein (250 μg) was incubated with either polyclonal TLR4 (Santa Cruz) or polyclonal IκB-α (ECM Biosciences) antibody together with Protein A/G Plus-agarose for 2 h at 4° C. Beads with the immunoprecipitates were collected by centrifugation, washed 4x in the lysis buffer, resuspended again in the loading buffer containing 0.5 % mercaptoethanol and then heated at 100 °C for 10 min. Proteins samples were fractionated on a 4 – 12 % Bis-TRIS electrophoresis gel, transferred onto polyvinylidene diflouride (PVDF) membrane filter (0.45 μ) and blocked with 5 % milk in TRIS buffered saline with 0.1 % Tween-20 (TBS-T) for 1 h. Membranes were incubated with anti-c-Src (1:2000) as primary antibody. After three washes in TBS-T, membranes were incubated for 1 h with anti-rabbit IgG (1:3000) secondary antibody. After additional washes, membranes were developed by enhanced chemiluminescent (ECL) method (Pierce), and signals were visualized with the Fujifilm LAS-400 imaging system.
Western blot
HEK-Blue mTLR4 cells were plated in 6-well plates at a density of 5×105 cells/plate. Cells were allowed to reach 70 % confluence, treated with LPS-EK or PPC for the indicated time, and then lysed in mammalian cell protein extraction lysis buffer (Mammalian cell-PE-LB™) (G Biosciences, St. Louis, MO) supplemented with protease inhibitors. Cell lysates were centrifuged at 16,200-x g at 4 °C for 10 min. Aliquots of cell extracts containing 20-30 μg of total protein were mixed with the loading buffer in a total volume of 30 μl, and heated at 95 °C. Equal amounts of the denatured protein (contained in 15 μl) were loaded per lane, fractionated on a 4 – 12 % Bis-TRIS electrophoresis gel, and transferred onto PVDF membranes. After blocking membranes with 5 % BSA in TRIS buffered saline with 0.1 % Tween-20 (TBS-T), membranes were incubated overnight with rabbit anti- IκB-α (Tyr-42) (1:1000). Following three washes in TBS-T, membranes were incubated for 1 h with anti-rabbit IgG (1:5000) secondary antibody. After additional washes, membranes were developed by ECL method, and signals were visualized with the Fujifilm LAS-400 imaging system.
FACE™ c-Src in-cell Western analysis and activation assay
LPS-EK- or PPC-induced c-Src activation was assessed using fast-activated cell-based c-Src ELISA (FACE™ c-Src ELISA) kit according to the manufacturer’s instructions. Briefly, HEK-Blue mTLR4 cells seeded at a density of 2.5 × 104 cells/well in a poly-lysine coated 96-well plate, were serum starved overnight. Cells were then treated with various inhibitors for 30 min followed by stimulation with LPS-EK (1 μg/ml) or PPC (5 μM) for an additional 20 min. After washing with PBS, cells were fixed in 4 % formaldehyde in PBS for 15 min followed by quenching in wash buffer containing 1 % H2O2 and 0.1 % azide. After blocking for an hour in a buffer supplied with the kit, cells were incubated overnight with either phospho-c-Src (pTyr418) or total-c-Src antibody. After washing 3x, cells were incubated with secondary antibody (anti-rabbit HRP-conjugated IgG). Finally, the wells containing cells were allowed to develop and the absorbance was read at 450 nm in a BioTek microplate reader. To normalize for different treatments, cells were stained with crystal violet for 1 h followed by washing and dissolution of the cells in SDS. Finally, the absorbance was read at 595 nm in the microplate reader.
Quantitation of TNF-α and IL-10 levels
HEK-Blue mTLR4 cells were treated with various inhibitors for 30 min followed by stimulation overnight with LPS-EK or PPC for 16 h in the continued presence of the inhibitors. We quantified the levels of human TNF-α and IL-10 released into the conditioned media using TNF-α and IL-10 ELISA kits as per manufacturer’s instructions.
Measurement of intracellular ROS accumulation
HEK-Blue mTLR4 and HEK-Blue-Null1-v cells were treated with different specific inhibitors for 30 minutes followed by stimulation overnight with LPS-EK or PPC. The oxidative stress produced by treatment with LPS-EK or PPC was then determined using CellROX® Deep Red reagent as per manufacturer’s instructions. CellROX® Deep Red reagent is non-fluorescent while in a reduced state and becomes fluorescent upon oxidation by reactive oxygen species with emission maxima of ~665 nm that is measurable by fluorescent imaging. Cells were incubated with CellRox® (5 μM) and NucBlue live cell stain for 30 min at 37 °C followed by subsequent washes with PBS and fixation for 15 min with 4 % paraformaldehyde. After subsequent washes with PBS, images were acquired using fluorescence microscope (Axiovert 200M; Zeiss) at excitation and emission wavelengths: ~ 644/655 nm for CellRox® and 405/410-550 nm for NucBlue®. Images were analyzed using the Image J (V1.44, http://rsbweb.nih.gov/ij/) software. Finally, acquired images were merged and composite figures were obtained.
Immunofluorescence
HEK-Blue mTLR4 cells were seeded at a density of 2.5 × 104 cells/well in an 8-well Lab-Tek® II chamber slide (Nalge Nunc International, NY) and grown to 70 % confluence followed by overnight serum starvation. Cells were then stimulated with LPS-EK or PPC for 0 to 180 minutes. After fixation in 10 % formaldehyde in PBS for 20 min at room temperature (RT), cells were permeabilized with 0.2 % Triton X-100 in PBS for 5 min. After rinsing in PBS, cells were blocked in 5% BSA at room temperature (RT) for 1 h followed by incubation overnight at 4 °C with primary antibody (1: 100, rabbit polyclonal, IκB-α (Tyr -42), phospho-specific). After rinsing in PBS, cells were incubated with FITC conjugated goat anti-rabbit IgG for 1 h and NucBlue® live cell stain for 15 min. After subsequent washing with PBS, images were acquired using fluorescence microscope (Axiovert 200M; Zeiss) at excitation and emission wavelengths: ~ 495/519 nm for FITC and 405/410-550 nm for NucBlue®.
Measurement of total nitrate/nitrite and Thiobarbituric acid reactive substances (TBARS)
HEK-Blue mTLR4 cells were pre-incubated with Ca-pY or Eb for 30 min or SSG for 2 h, followed by stimulation with LPS-EK or PPC overnight in the continued presence of the inhibitors. Total levels of nitric oxide (NO) as nitrate/nitrite and lipid peroxides were determined in cell culture supernatants and cell lysates, respectively, in the same set of experiments after different treatments.
Culture supernatants were separated from cells, cleared by centrifugation and used to quantify total nitric oxide (NO) levels as nitrate/nitrite according to the manufacturer’s instructions in the Parameter™Total nitric oxide and Nitrate/Nitrite assay kit. NO is a gaseous free radical and biological mediator that regulates diverse activities in many cells. Due to its short half-life and lipid solubility, NO is not stored but is synthesized de novo and diffuses freely across plasma membranes. Because NO is rapidly metabolized to nitrite and nitrate, quantification of these nonvolatile and stable anions can be used to indirectly determine the amount of NO originally produced in the cell. Total NO levels in culture supernatants were determined based on enzymatic conversion of all nitrate to nitrite in each sample by NADH-dependent nitrate reductase. Griess reagent converts the nitrite into a purple-colored azo compound. The optical density (OD) of the azo compound is then quantified in a microplate reader set at 540 nm. Total nitrite level in each sample was corrected for background nitrite levels present in each sample determined prior to the sample treatment with nitrate reductase.
Cells from which the supernatants were used to assay total NO were collected, lysed and used to quantify malondialdehyde (MDA) as thiobarbituric acid reacting substances (TBARS) according to the instructions in the Parameter™TBARS assay kit. Briefly, ROS are the major initiators of lipid peroxidation, which are the byproducts that cause direct damage to cell membranes. As a well-defined mechanism of cellular damage, lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). As free MDA is typically low, samples require acid treatment of proteins and breakdown of peroxides by heat to facilitate color development in the TBARS reaction. The optical density (OD) of MDA-TBA adduct formed from the reaction of MDA in samples with TBA was measured with a microplate reader set at 532 nm. The TBARS levels in unknown samples were determined from MDA equivalence standard carried out simultaneously in the assay with unknown samples.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) was used to perform data analysis in all experiments. Data are presented as mean ± SEM from at least 3 – 7 independent experiments carried out in duplicate, where applicable, and analyzed by 1- or 2-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. Significance was assigned at p ≤ 0.05 %.
RESULTS
c-Src inhibitors decreased SEAP release in HEK-Blue mTLR4 cells
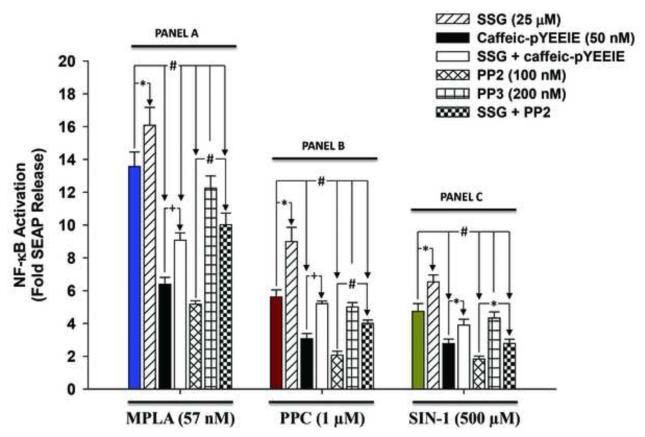
Treatment with 57 nM of MPLA (a synthetic TLR4-specific agonist) overnight (~ 16 h) induced SEAP release by a mean of 13.6 fold (Fig. 1, Panel A) over control cells under the same treatment conditions. Preincubation of cells with 25 μM of SSG (a tyrosine phosphatase inhibitor) for 2 h prior to MPLA stimulation increased SEAP release by 26 %. Cell pre-treatment with PP2 and Ca-pY for 30 min prior to MPLA stimulation decreased SEAP release by 53 % and 62 %, respectively.
Fig. 1. Effect of c-Src inhibitors on secreted embryonic alkaline phosphatase (SEAP) release in mono-phosphoryl lipid A (MPLA)- and pro-oxidants (PPC and SIN-1)-induced HEK-Blue mTLR4 activation.
Cells were pre-incubated with various inhibitors for 30 min followed by overnight (~ 16 h) incubation with MPLA, PPC, or SIN-1 in the continued presence of the inhibitors. SEAP release in the conditioned medium was determined using Quanti-Blue, and the absorbance was read at 650 nm. The color-coded column in each Panel (A to C) represents treatment with MPLA, PPC or SIN-1 alone, respectively. The data represent 5 independent experiments conducted in duplicate. +p ≤ 0.05, *p ≤ 0.01 and #p ≤ 0.001
Treatment with PPC (1 μM) overnight increased SEAP release by a mean of 5.6 fold (Fig.1, Panel B) compared to untreated control cells incubated under the same conditions. Moreover, preincubation with SSG 2 h prior to PPC treatment increased SEAP release by 60 %. Cell pre-treatment with PP2 or Ca-pY for 30 min prior to treatment with PPC decreased SEAP release by 45 % and 63 %, respectively.
Overnight treatment with 500 μM SIN-1 induced SEAP release by 4.74 fold (Fig. 1, Panel C) over untreated control cells. Pretreatment with SSG 2 h prior to SIN-1 treatment increased SEAP release by 37.7 %. Pre-treatment with PP2 and Ca-pY for 30 min prior to treatment with SIN-1 decreased SEAP release by 41 % and 61 %, respectively.
In all cases, cell pretreatment with SSG for 2 h prior to treatment for 30 min with PP2 or Ca-pY rescued cells from the inhibitory effect of both c-Src inhibitors on SEAP release. Pretreatment with PP3, an inactive congener of PP2, prior to MPLA, PPC or SIN-1 had no effect on SEAP release. Overall, our data confirm that pro-oxidants act through TLR4 signaling pathway. However, both PPC and SIN-1 increased SEAP release at quantitatively lower levels compared to MPLA, a pure TLR4-specific agonist, and used here as a positive control. Nonetheless, PPC produced a more robust SEAP release compared to SIN-1, which suggests a more enhanced NF-κB activation by PPC with its hydrolytic products.
TAK-1 inhibition deceased prooxidant–mediated SEAP release
Preincubation of cells with TAK-1 inhibitor or U0126 (a selective inhibitor of MEK 1 & MEK 2) for 30 min prior to MPLA stimulation (Fig. 2, Panel A) decreased SEAP release by 55.5 % and 27.5 %, respectively.
Fig. 2. Effect of TAK-1 inhibitors on secreted embryonic alkaline phosphatase (SEAP) release in mono-phosphoryl lipid A (MPLA)- and pro-oxidants (PPC and SIN-1)-induced HEK-Blue-mTLR4 activation.
Cells were pre-incubated with various inhibitors for 30 min followed by overnight incubation with MPLA, PPC, or SIN-1 in the continued presence of the inhibitors. SEAP release in the conditioned medium was determined using Quanti-Blue, and the absorbance was read at 650 nm. The color-coded column in each Panel (A to C) represents treatment with MPLA, PPC or SIN-1 alone, respectively. The data represent 5 independent experiments. +p ≤ 0.05, and #p ≤ 0.001.
Preincubation with TAK-1 inhibitor or U0126 for 30 min prior to stimulation with PPC (Fig. 2, Panel B) decreased SEAP release by 56 % and 27 %, respectively. Under the same pretreatment conditions, stimulation with SIN-1 (Fig. 2, Panel C) decreased SEAP release by 43 % and 34 %, respectively. UO124, an inactive analog of UO126, which was used as a negative control, had no effect on SEAP release in all cases (Fig. 2, Panels A, B & C).
SIN-1 induced SEAP release in HEK-Blue mTLR4 cells but not in the HEK-Blue null-1v cells
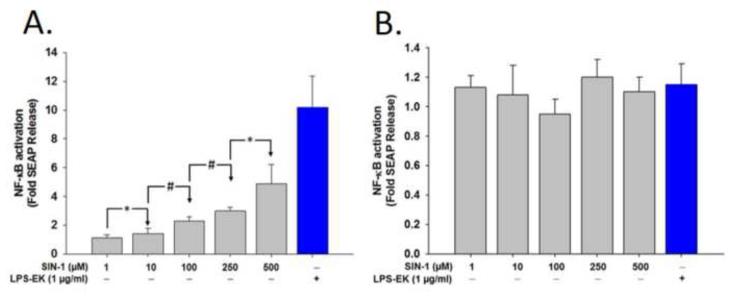
Overnight treatment of HEK-Blue mTLR4 cells with different concentrations of SIN-1 (1 - 500 μM) increased SEAP release in a SIN-1 concentration-dependent manner (Fig. 3A). There were no significant differences in SEAP release following treatment of HEK-Blue null-1v cells at all the SIN-1 concentrations we studied (Fig. 3B). Furthermore, the concentrations of SIN-1 had no significant effect on cell viability. On the basis of this data, we used SIN-1 at 500-μM in all subsequent experiments to maintain a robust SEAP release.
Fig. 3. A dose-dependent effect of SIN-1 on secreted embryonic alkaline phosphatase (SEAP) release.
Cells were treated with different concentrations of SIN-1 ranging from 1.0 to 500 μM for 16 h. SEAP release in the conditioned medium was determined using Quanti-Blue, and the absorbance was read at 650 nm. (A) Fold changes in SEAP release after treatment with different doses of SIN-1 in HEK-Blue mTLR4 cells and (B) Fold changes after treatment of HEK-Blue null-1v cells with the same concentrations of SIN-1 as in (A). Data represent the mean of four (4) independent experiments carried out in duplicate. *p = 0.05; #p ≤ 0.01
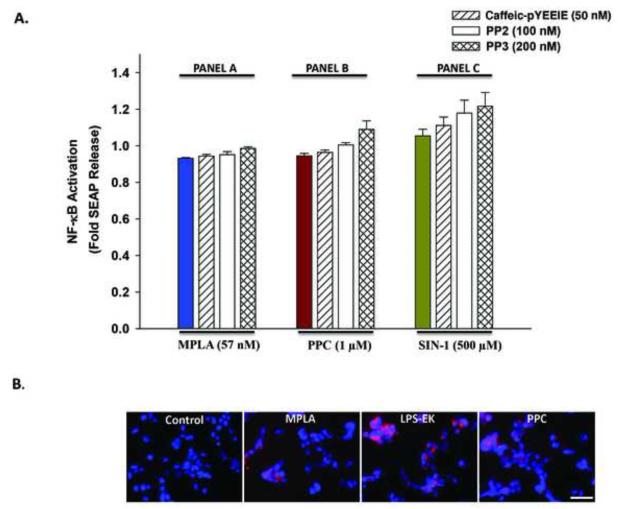
Prooxidants and LPS-specific agonist had no affect on TLR4 signaling in HEK-Blue null-1v cells
Neither MPLA (Fig. 4, Panel A) nor any of the pro-oxidants PPC (Fig. 4, Panel B) and SIN-1 (Fig. 4, Panel C) induced SEAP release from HEK-Blue null-1v cells. This confirmed the inactivity of the SEAP reporter gene insert in null cells. In addition, neither Ca-pY nor PP2 pretreatment influenced native SEAP release in these cells. The data confirm that TLR4 expression is necessary for manifestation of the effects of MPLA, PPC and SIN-1. Curiously, MPLA, LPS-EK and PPC induced intracellular ROS production to a very limited extent in HEK-Blue null-1v cells.
Fig. 4.
(A) The effect of LPS-EK, PPC and SIN-1 on secreted embryonic alkaline phosphatase (SEAP) release in HEK-Blue null 1-v cells. Cells were pre-treated with c-Src inhibitors for 30 min followed by incubation overnight with MPLA, PPC, or SIN-1 in the continued presence of the inhibitors. SEAP release in the conditioned medium was determined using Quanti-Blue, and the absorbance was read at 650 nm. The color-coded column in each panel represents treatment with MPLA (Panel A), PPC (Panel B) or SIN-1 (Panel C) alone, respectively. The data represent 5 independent experiments. (B) Effect of TLR4 agonists and PPC on accumulation of intracellular reactive oxygen species (ROS) in HEK-null 1-v cells. Cells were incubated overnight with MPLA, LPS-EK, or PPC. Then, cells were incubated with CellRox® and NucBlue live cell stain for 30 min at 37 °C followed by subsequent PBS washes and fixation with 4 % paraformaldehyde for 15 min. After subsequent washing with PBS, images were acquired using fluorescence microscope. Scale bar = 50 μm.
Prooxidant induced c-Src activation in HEK-Blue mTLR4 cells
Cell pretreatment with of LPS-EK (1 μg/ml) (Fig. 5A) or PPC (5 μM) (Fig. 5B) for 20 min induced c-Src activation almost by 3- and 2.5-folds, respectively. Preincubation with SSG (a tyrosine phosphatase inhibitor) 2 h prior to either LPS-EK or PPC treatment increased c-Src activation by 19 and 29 %, respectively, over induction by LPS-EK and PPC. Cell pretreatment with Ca-pY (c-Src inhibitor) or Ebselen (an ROS scavenger that also inhibits DNA binding by the p50 subunit of NF-κB transcription factor) for 30 min prior to stimulation with LPS-EK or PPC, significantly decreased c-Src activation. Again, pretreatment with SSG rescued cells from the inhibitory effect of Ca-pY. Preincubation of the cells with anti-TLR4 pAb followed by stimulation with either LPS-EK or PPC, inhibited c-Src activation induced by either agents by 47 % and 36 %, respectively. Preincubation with rat polyclonal IgG control had no effect on c-Src activation mediated by either LPS-EK or PPC. The data confirmed that both the ROS generated following PPC or LPS-EK treatment used c-Src activation to signal through TLR4.
Fig. 5. Comparative effects of LPS-EK and PPC on c-Src activation in HEK-Blue mTLR4 cells.
Cells were pre-incubated with various inhibitors for 30 min followed by stimulation with either LPS-EK (A) or PPC (B) for 20 min in continued presence of the inhibitors. c-Src activation in the cells were determined with FACE™ c-Src kit according to the manufacturer’s instructions. The data are expressed as % ratios of [pTyr418] c-Src (activated) to total c-Src present with respect to the effect of agonist treatment alone. Data represent 3 independent experiments carried out in duplicate with +p ≤ 0.05, *p ≤ 0.01 and #p ≤ 0.001.
Stimulation of HEK-Blue mTLR4 cells increased intracellular ROS production
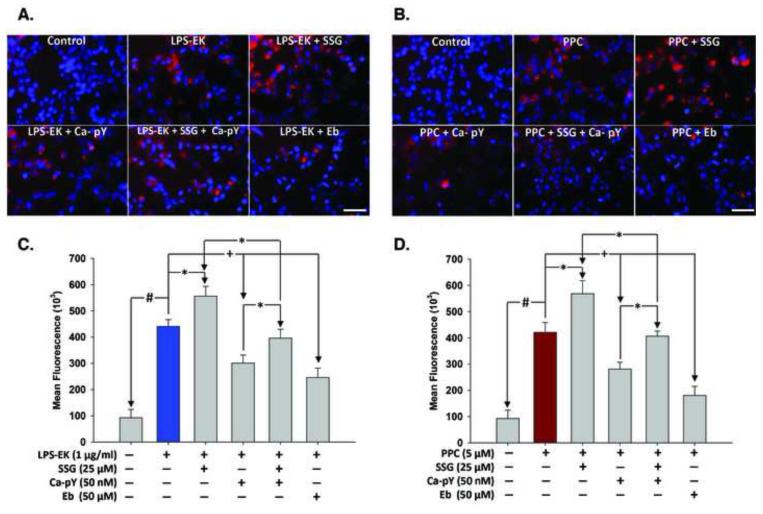
Overnight incubation of HEK-Blue mTLR4 cells with 1 μM LPS-EK increased intracellular ROS production by a mean of 4.7 fold over control cells under the same treatment conditions, but in the absence of LPS-EK (Figs. 6A & 6C). Pretreatment with SSG for 2 h prior to LPS-EK stimulation increased intracellular ROS production by 26 %. However, pretreatment with Ca-pY or Ebselen (Eb) for 30 min prior to LPS-EK stimulation decreased intracellular ROS production by 31 % and 44 %, respectively. Pretreatment with SSG for 2 h before Ca-pY rescued cells from the inhibitory effect of Ca-pY on intracellular ROS production.
Fig. 6. Immunofluorescence representation of levels of intracellular ROS produced following stimulation of HEK-Blue mTLR4 cells with LPS EK or PPC alone and/or in combination with different inhibitors.
Cells were pre-incubated with various inhibitors for 30 min followed by stimulation with LPS-EK (A) or PPC (B) overnight in continued presence of the inhibitors. Cells were then incubated with CellRox® and NucBlue live cell stain for 30 min at 37 °C. Cells were washed in PBS and fixed with 4 % paraformaldehyde for 15 min. After subsequent washes with PBS, images were acquired using fluorescence microscope. (Figs. 6A & 6B) are merged representative pictures of the immunofluorescence data with scale bar 50 μm. (Figs. 6C & 6D) are semi-quantitative histograms of (Figs. 6A & 6B) generated using ImageJ™ software. The data represent 4 independent experiments. pY = Caffeic-pYEEIE; Eb = Ebselen; +p ≤ 0.05; p ≤ 0.01; #p ≤ 0.001.
On one hand, overnight pretreatment with 1 μM of PPC (Figs. 6B & 6D) induced robust intracellular ROS production by a mean of 4.5 fold over untreated control cells under the same treatment conditions. Intracellular ROS production increased by 35 % following pretreatment with SSG 2 h prior to the addition of PPC. However, pre-treatment with Ca-pY or Eb for 30 min prior to PPC decreased intracellular ROS production by 33 % and 57 %, respectively. Pretreatment with SSG rescued cells from the inhibitory effect of Ca-pY on intracellular ROS production.
Prooxidant stimulation of HEK-Blue mTLR4 cells increased both production of nitric oxide and formation of lipid peroxides
Significant increase in NO (free radical) production occurred in HEK-Blue mTLR4 cells pretreated with Ca-pY, Eb or SSG (c-Src activator) followed by overnight incubation with LPS-EK or PPC (Fig. 7A & 7B) in the continued presence of SSG. Total NO levels quantified as nitrite/nitrate accumulation in the culture media and induced by either LPS-EK or PPC (Fig. 7A & 7B) did not significantly change following pretreatment with either Ca-pY or Eb.
Fig. 7. Effect of c-Src inhibitors used alone and in combination on Nitric oxide production in HEK-Blue mTLR4 cells stimulated with LPS-EK or PPC.
Cells were pre-incubated with Ca-pY or Eb for 30 min or SSG for 2 h, followed by stimulation with LPS-EK (A) or PPC (B) overnight in the continued presence of the inhibitors. Culture media was separated from cells, cleared by centrifugation and used to quantify total nitric oxide levels as nitrite/nitrate according to the manufacture’s instructions. The data represent 3 independent experiments conducted in duplicates. Ca-pY = Caffeic-pYEEIE; Eb = Ebselen; SSG = sodium stibogluconate; +p = 0.05.
The levels of TBARS, indicative of treatment-induced lipid peroxidation, increased significantly in all treatment groups compared with the control cells, with no differences between treatments (Fig. 8A & 8B).
Fig. 8. Effect of c-Src inhibitors used alone and in combination on lipid peroxides produced in HEK-Blue-mTLR4 cells stimulated with LPS-EK and PPC.
Cells were pre-incubated with Ca-pY or Eb for 30 min or SSG for 2 h, followed by stimulation with LPS-EK (A) or PPC (B) overnight in the continued presence of the inhibitors. Cells from which the supernatants were used to quantify total nitric oxide were scraped, lysed and prepared for use to quantify malondialdehyde (MDA) as thiobarbituric acid reacting substances (TBARs) according to the manufacturer’s instructions. The data represent 3 independent experiments carried out in duplicates. Ca-pY = Caffeic-pYEEIE; Eb = Ebselen; SSG = sodium stibogluconate. +p ≤ 0.01.
Effect of ROS and c-Src inhibition on TNF-α and IL-10 production in HEK-Blue-mTLR4 cells
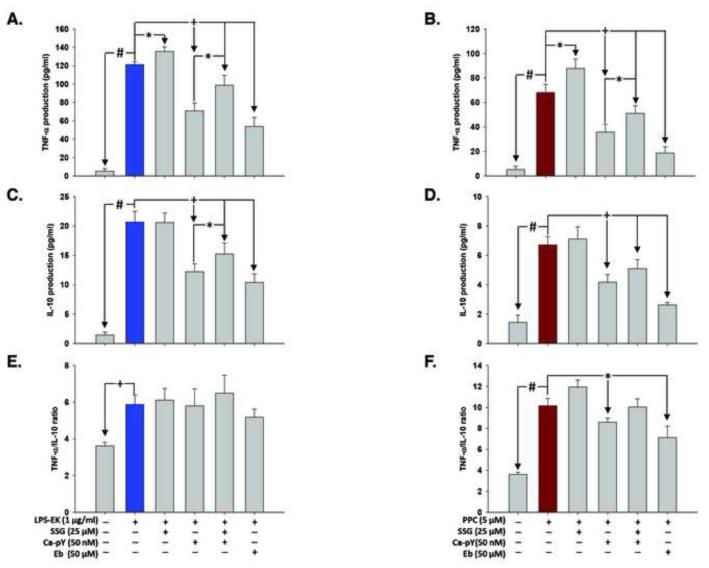
Treatment of cells overnight with LPS-EK (1 μM) induced TNF-α and IL-10 (Figs. 9A, 9C & 9E) production by 23.3- and 13.5-fold, respectively, over control cells under the same treatment conditions. Pretreatment with SSG for 2 h prior to LPS-EK stimulation increased TNF-α production by 12 %, with no perceptible change in IL-10 level. However, pre-treatment with Ca-pY or Eb for 30 min prior to LPS-EK stimulation decreased TNF-α production by 50.5 % and 55 %, respectively, and IL-10 production by 40 % and 49.6 %, respectively. However, pretreatment of cells with SSG rescued cells from the inhibitory effect of Ca-pY on TNF-α and IL-10 production.
Fig. 9. Effect of c-Src inhibition on the production of TNF-α and IL-10 following stimulation of HEK-Blue mTLR4 cells with LPS-EK or PPC.
Cells were pre-incubated with inhibitors for 30 min followed by stimulation with LPS-EK (A & C) or PPC (B & D) overnight in continued presence of the inhibitors. TNF-α and IL-10 levels in the conditioned medium were determined using their respective ELISA kits according to the manufacturer’s instructions. The ratios of TNF-α to IL-10 following treatments with LPS-EK (E) and PPC (F) were quantified. The data represent 4 independent experiments conducted in duplicate. +p ≤ 0.05, *p ≤ 0.01 and #p ≤ 0.001 (A & C) TNF-α and IL-10 production in LPS-EK- stimulated cells and (B & D) TNF-α and IL-10 production in PPC-stimulated cells.
Overnight incubation with PPC (1 μM) induced TNF-α and IL-10 production (Figs. 9B, 9D & 9F) by means of 13.5- and 4.7-fold respectively, over control cells treated under the same conditions. Preincubation with SSG 2 h prior to PPC treatment increased TNF-α production by 29 %. Cell pre-treatment with Ca-pY or Ebselen for 30 min prior to PPC treatment decreased TNF-α production by 49 % and 72 %, respectively, and IL-10 production by 39 % and 61 %, respectively.
However, when TNF-α and IL-10 concentration data are expressed as ratios of TNF-α to IL-10 for each corresponding treatment (Figs.9E & 9F), an imbalance in favor of TNF-α production following PPC treatment is apparent. The potential biological implications and significance of the data take on more clarity when compared with the TNFα/IL-10 ratios derived from LPS-EK treatment.
Prooxidant increased coimmunoprecipitation of TLR4 and IκBα with c-Src
LPS-EK, MPLA and PPC increased formation of complexes of c-Src with TLR4 (Fig. 10A) and IκBα (Fig. 10B) by immunoprecipitation. This provides further evidence for a role for c-Src that has not been documented before in IκBα/NF-κB-coupled activation and TLR4 signaling. The results of these studies also demonstrate that c-Src may serve as a target hub for understanding prooxidant-mediated signal transduction pathway for TLR4 activation.
Fig. 10. Immunoprecipitation complexes formed by c-Src interactions with TLR4 or with IκB-α in the NFκB activation pathway.
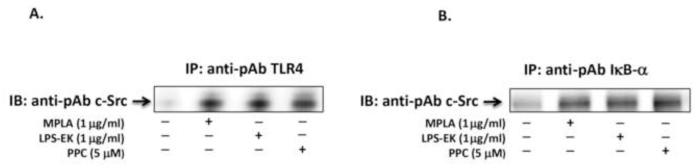
Cells were stimulated with MPLA, LPS-EK or PPC (A & B) for 4 h followed by immunoprecipitation with TLR4 (A) or with IκB-α (B). In both cases, coimmunoprecipitates were detected by Western blot with polyclonal anti-c-Src. Experiments were repeated three times with essentially the same results.
Prooxidant increased the phosphorylation in Tyr42 residue on c-Src/IκBα/NFκB coupled activation
Stimulation of TLR4 appears to mediate both rapid and delayed activation of NFκB. Treatment with LPS-EK (1 μg/ml) or PPC (5 μM) decreased the formation of IκBα in a time dependent manner (Figs. 11A & 11C for LPS-EK, and Figs. 11B & 11D for PPC). In addition, stimulation of TLR4 with LPS-EK did not appear to affect the level of [pTyr42] IκBα formed, whereas treatment with PPC significantly enhanced the production of [pTyr42] IκBα. These changes are also supported by time-course immunofluorescence data as depicted in Figs. 11E & 11F for LPS-EK and PPC treatments, respectively. Inhibition of c-Src by PP2 or Ca-pY treatment resulted in a significant reduction in IκBα Tyr phosphorylation and NFκB activation after prooxidant treatment (data not shown). These results indicate that c-Src-dependent Tyr phosphorylation of IκBα, and subsequent prolong activation of NFκB is controlled by cellular redox status.
Fig. 11. Effect of LPS-EK and PPC on [pTyr42] IκB-α formation and IκB-α degradation in HEK-Blue mTLR4 cells.
Cells were stimulated with LPS-EK (A) or PPC (B) for 0, 30, 60, 90, 120 and 180 min. I-κB-α (Y42) formation against IκB-α degradation was determined by Western blots. The time-course graphs (C & D) represent the optical density (OD) ratios of IκB-α (Y-42) and IκB-α immunoblot signals from (A & B) normalized to those of β-actin from the same test groups. The data represent 3 independent experiments with +p ≤ 0.05, *p ≤ 0.01 and #p ≤ 0.001 compared to time-matched corresponding treatments. For the immunofluorescence data (E & F), cells were incubated with FITC-conjugated secondary antibody and NucBlue live cell stain corresponding to treatments with LPS-EK (A) and PPC (B). Images were acquired using fluorescence microscope (scale bar = 50 μm).
DISCUSSION
In the present studies, we tested the hypothesis that c-Src plays a central role in prooxidant-induced NF-κB activation in the TLR4 signaling pathway. To test this hypothesis, we used HEK-Blue cells stably transfected with TLR4-CD14-MD2 complex that also express an optimized SEAP reporter gene under the control of a promoter inducible by NF-κB and AP-1 transcription factors. Cells were exposed to an oxidative stress-producing agents followed by measurement of SEAP release as a marker for NF-κB activation. A major finding of this study is that c-Src/IκBα-coupling plays a central role in prooxidant-induced NF-κB activation pathway through TLR4 signaling. The data to support this conclusion include the following findings: i) c-Src inhibitors significantly attenuated both prooxidant (PPC or SIN-1)- and TLR4 agonist (MPLA or LPS-EK)-induced NF-κB activation in HEK-Blue-mTLR4 cells ii) Neither TLR4 agonists and prooxidants, nor c-Src inhibitors had any effect on SEAP release in HEK Blue-null1 cells. iii) SSG, a phosphatase inhibitor (in effect a c-Src activator), not only augmented but also rescued cells from decreased SEAP release and intracellular oxidative stress induced by both TLR4 agonists and prooxidants. iv) Both prooxidant and TLR4 agonists induced formation of TLR4/c-Src and IκB-α/c-Src complexes as co-immunoprecipitates, and v) Prooxidant preferentially induced long-lasting phosphorylation of IκB-α at the Tyr42 residue. We have used three relatively complementary assays to confirm the role of c-Src activation in oxidant-mediated stimulation of TLR4. Our data appear to further confirm that activation of c-Src is an early chain of events that signal NFκB-dependent activation through TLR4 stimulation.
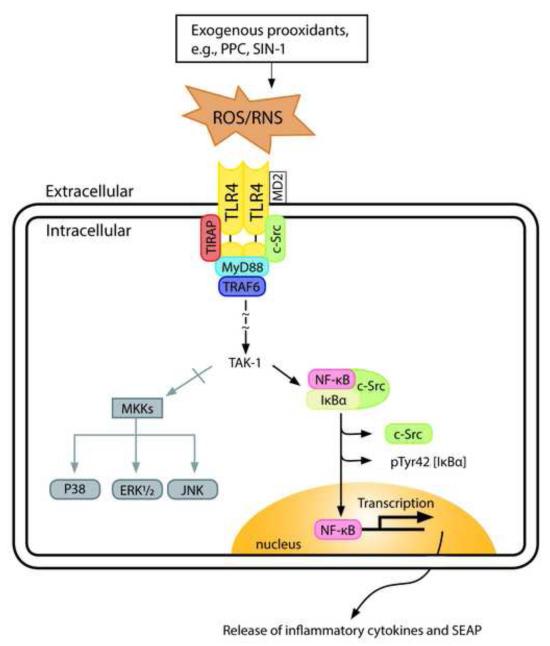
Based on the results of the present study, as well as previous work from our laboratory [32, 42] and others [43], we have proposed a schematic representation (Fig. 12) for prooxidant-induced signaling through the TLR4/c-Src/NF-κB-coupled activation. A novel aspect of our model here is the integrated role of c-Src in prooxidant-mediated TLR4 signaling through a switch in NF-κB activation pathway that favors phosphorylation of IκB-α at the Tyr42 residue. This pathway appears to present a unifying mechanism of disease processes induced by oxidative stress [44]. To examine the contribution of c-Src in TLR4 signaling pathway(s) through NF-κB activation, we used two c-Src inhibitors, Ca-pY and PP2, which act at different domains of c-Src. On one hand, PP2 appears to act as a non-selective inhibitor of SFKs by binding at the catalytic domain/ATP-binding site of the enzyme [45]. On the other hand, Ca-pY is a more selective inhibitor of the SH2 domain of c-Src that exhibits excellent cell membrane permeability and in vivo bioavailability [46].
Fig. 12. A simplified schematic representation of a putative mechanism for exogenous prooxidant-induced TLR4 signaling in c-Src-mediated NFκB activation.
TLR4 is stimulated in response to exogenous oxidants resulting in the activation of c-Src, which interacts with TLR4. The formation of TLR4/c-Src complex leads to the recruitment of different cytosolic adaptor proteins such as myeloid differentiation 88 protein (MyD88), toll-interleukin 1 receptor adaptor protein (TIRAP), TNF receptor-associated factor 6 (TRAF6), etc. resulting in c-Src/NFκB/IκBα coupled activation. This would result in c-Src phosphorylation of the Tyr42 residue in IκBα kinase to form [pTyr42] IκBα that delays ubiquitination and degradation by proteosome that leads to activation of NFκB for a longer time. Following release of the complex, p50/p65 eventually translocates to the nucleus to induce the expression of new genes.
Both Ca-pY and PP2 inhibited MPLA-, PPC- and SIN-1-induced c-Src activation as determined by fast-activated cell-based ELISA (FACE™). We used this novel high-throughput assay to quantify the activity of c-Src kinase after stimulation of TLR4 with LPS-specific agonists or prooxidants. The kit provided a new and sensitive in-cell Western analysis for quantifying [pTyr-418] c-Src, the activated form of c-Src, without preparing cell extracts or performing electrophoresis and membrane blotting. The assay is based on the simple premise that in cells c-Src is phosphorylated either on Tyr418 (active) or Tyr527 (inactive) residue. There is no documented report of unphosphorylated state of the enzyme [47].
It has previously been shown that tyrosine phosphorylation of the TIR domain of TLR4 is necessary for receptor signaling [48]. In the present study, we have demonstrated that c-Src coimmunoprecipitates with both TLR4 and IκBα kinase, which supports the premise that c-Src, interacts with segments of both proteins in the signaling pathway. Treatment with LPS can increase Tyr phosphorylation of many host proteins including the SFKs. For instance, in monocytes LPS activates SFK lyn-associated with CD14, a TLR4 co-receptor in LPS binding on the surface of these cells [49]. Consistent with this finding, we have shown here that LPS-EK induced activation of c-Src in HEK-Blue-mTLR4. Gong et al [50] reported that TLR4 signaling appears to be coupled to the activation of SFK in endothelial cells, which is consistent with our immunoprecipitation data (Fig. 11A & 11B). This research group did not examine the role of SFK in prooxidant-induced TLR4 stimulation.
Signaling by TLR4 involves interactions between its proline-rich domain within TRAF6 adaptor protein and the SH3 domain of c-Src [28]. More importantly, neutralization of TLR4 receptor resulted in decreased c-Src activation, which further supports active involvement of c-Src in TLR4 signaling. Not only the pharmacological inhibitor PP2 attenuated LPS-induced injury, but also prior knockdown of c-Src with c-Src siRNA significantly decreased LPS-mediated response [39, 50]. Increased production of ROS may contribute to enhanced Tyr phosphorylation by transient suppression of the activity of members of the protein tyrosine phosphatase (PTPase) family that promote a burst of protein tyrosine kinase (PTK) activity [51, 52]. However, Meng et al [53] demonstrated that multiple PTPases were oxidized and inactivated by ROS in vivo. SSG, a pentavalent antimony compound used in the treatment of Leishmaniasis infection [54], is a potent in vitro inhibitor of PTPases, including Src homology PTPase-1 (SHP-1) [55]. SSG not only augmented LPS-EK- and prooxidant-induced c-Src activation but also rescued cells from c-Src inhibition by PP2 and Ca-pY following LPS-EK or PPC treatment.
We used the level of SEAP release as a reliable quantitative marker for NF-κB and AP-1 activation in TLR4 signaling pathway. A change in SEAP activity in culture is a reflection of the intracellular concentrations of SEAP mRNA and protein. Therefore, SEAP levels in culture provide an extremely sensitive tool not only to quantify SEAP, but also to monitor the overall transcriptional and promoter activity in the HEK-Blue cells. In all cases, HEK-Blue mTLR4 cell pretreatment with SSG significantly increased MPLA-, PPC- and SIN-1-induced SEAP release, whereas pretreatment with PP2 or CA-pY decreased it (Figs. 1). SSG rescued TLR4 activation from PP2 and CA-pY-induced inhibition of SEAP release. TAK-1, a member of the MAPKKK family is thought to be a key bifurcation point in TLR4 signaling pathway, which induces NF-κB and AP-1 activation [55] and, therefore, plays a crucial role in regulating the genes that mediate inflammation. Blocking of TAK-1 [56] decreased MPLA-, PPC- and SIN-1-induced SEAP release, which suggests decreased AP-1 and NF-κB activation. U0126, MEK inhibitor in AP-1 signaling pathway [57], weakly inhibited TLR4 agonist- and prooxidant-induced SEAP release, whereas Bay 11-7082, an IKK inhibitor [58], completely abolished it [32]. This indicates that NF-κB activation may be a more dominant event downstream of the TLR4 signaling pathway. c-Src inhibitors (PP2 and Ca-pY) decreased SEAP release while SSG not only augmented but also rescued the cells from the inhibitory effect of c-Src inhibitors on SEAP release following TLR4 stimulation by MPLA or prooxidants (PPC and SIN-1).
A variety of signaling events including phosphorylation of NF-κB, hyperphosphorylation of inhibitory kappa kinases (IKKs), induction of IκB synthesis, and the processing of NF-κB precursors, provides potential mechanisms that may modulate the level and duration of NF-κB activity [59]. LPS-EK, MPLA and prooxidants are potent NF-κB activators. IκB-α, a cytosolic inhibitory protein of NF-κB, tightly controls the regulation of NF-κB activation [59]. However, two pathways for phosphorylation of IκB-α appear to be established in the activation of NF-κB, but what determines their use by different NF-κB activators is not clear. One pathway involves serine (Ser) phosphorylation of IκB-α at Ser32/Ser36 residues, the so-called canonical pathway, which induces rapid ubiquitin-dependent degradation of IκB-α by the proteosome [33, 59]. The other is Tyr phosphorylation at Tyr42 residue, which has the potential to directly couple NF-κB activation to c-Src, but without inducing rapid degradation of IκB-α itself [36, 60]. PPC, but not LPS-EK, appears to be able to induce robust Tyr phosphorylation of IκB-α indicating that the post TLR4 mechanism of NF-κB activation induced by PPC is qualitatively and quantitatively different from that induced by LPS-EK (Fig. 11). However, both PPC and LPS-EK induced IκB degradation, possibly by different enzymes. Degradation of IκB eventually leads to its dissociation from NF-κB dimers (p50/p65), thereby allowing translocation of the latter into the nucleus [61] to initiate gene transcription. Tyr phosphorylation of IκBα at the Tyr42 residue represents a proteolysis-independent mechanism of NF-κB activation that directly couples NF-κB to cellular tyrosine kinase [36]. Oxidative stress leads to NFκB activation through IκBα Tyr phosphorylation [17, 36]. The N-terminal Tyr42 residue is crucial for this pathway [62, 63]. Because Tyr-42 is not conserved in other IκB family members, this pathway is specific for IκBα. Subsequent dissociation of IκBα phosphorylated at the Tyr42 residue by c-Src appears to be mediated by interactions with phosphoinositide-3 (PI3) kinase, and not by degradation by the proteosome [64]. Otherwise, the IκB tyrosine kinase responsible has not yet been characterized. Nonetheless, in view of our present findings, the kinase appears to be an attractive target for intervention in oxidative stress states that may initiate and maintain the inflammatory cascade associated with many human diseases.
Interestingly, PPC-induced NF-κB activation appears to be mediated mainly by Tyr phosphorylation at the Tyr42 residue and subsequent degradation of IκBα. Both LPS-EK and prooxidant appear to rapidly induce IκB-α degradation (Fig. 11). However, prooxidant-induced Tyr42 phosphorylation of IκB-α occurs more slowly, which suggests a dual role for PPC in NF-κB activation. Phosphorylation of IκBα at Tyr42 residue would activate NFκB for a long time, which delays its ubiquitin-dependent degradation [36, 37]. Nonetheless, formation of [pTyr42] IκBα in c-Src/IκBα-NFκB-coupled activation can still lead to NFκB signaling without its immediate degradation by proteosome, but with a consequent long-term production of inflammatory mediators. Hence, we hypothesize that the product of c-Src/IκBα-NFκB-coupled activation plays a role of an insidious “pathophysiologic primer” that initiates changes in a well-honed homeostasis of the cell. Over time, the product of c-Src/IκBα-NFκB-coupled activation accumulates with a resultant dysregulation of NFκB activation and initiation of acute inflammation. As a result, chronic inflammation may ensue, the cell loses homeostatic control and a disease process can set in. Prolonged activation of NF-κB appears to be detrimental by eliciting signals that trigger chronic inflammation through enhanced release of cytokines including TNF-α, IL-1, and IL-6, leading to endoplasmic reticulum stress and cell death [66]. This pathway may therefore provide a potential mechanism of transition from acute inflammation to a chronic inflammatory phase. This pathway also provides a basis for a unifying hypothesis for a “pathophysiological primer” through oxidant-induced NF-κB activation that may play a role in initiating and maintaining inflammatory state in many disease states [45].
A major outcome of NF-κB activation is the production of various pro-inflammatory cytokines including TNF-α and chemokines [66]. There is a correlation between the extent of NF-κB activation and severity of inflammation in animal models of arthritis and allergic airway disease [67]. The association of NF-κB activation and inflammatory disease is not easy to interpret. This is because both pro- and anti-inflammatory mediators are produced during inflammation, and the balance between these factors is likely to dictate disease initiation and progression [68]. Consistent with this view, our present data (Fig. 9) show that stimulation of TLR4 signaling pathway by LPS-EK or prooxidants not only induced TNF-α but also increased IL-10, an anti-inflammatory cytokine. Adib-Conguy et al [69] reported that TLR4 mediates the production of TNF-α and IL-10 during systemic inflammation. TNF-α not only regulates the expression of a variety of pro-inflammatory cytokines including IL-1, IL-6, PDGF, TGF-β but also enhances chemotaxis of macrophages and neutrophils at the site of inflammation [70]. However, IL-10 exhibits multifaceted anti-inflammatory properties, including inhibition of the prototypic inflammatory transcription factor NF-κB, which could lead to suppression of cytokine production [71]. IL-10 may also increase during inflammation to obviate the cascade of pro-inflammatory cytokine produced [72]. We determined ratios of the expression levels of TNF-α to those of IL-10, which would represent the balance of biological effects between the two cytokines. Indeed, the severity of inflammation appears to depend on high ratio of TNF-α to IL-10 [73]. Interestingly, TNF-α/IL-10 ratios were consistently higher after PPC than after LPS-EK treatment. This suggests that prooxidants might induce a more persistent inflammatory response, possibly through persistent TNF-α production. TNFα has previously been shown as a physiological activator of NF-κB via Tyr phosphorylation of I-κBα at Tyr42 residue in bone marrow macrophages [74]. This supports the idea that prooxidants may not only initiate, but also help maintain inflammatory responses in vivo. As inflammation is a complex physiological process, we cannot extrapolate the role of NF-κB in actual inflammatory response from in vitro studies.
On one hand, the c-Src inhibitor (Ca-pY) decreased both LPS-EK- and PPC-induced TNF-α and IL-10 production. On the other hand, SSG augmented LPS-EK- and PPC-induced TNF-α production and rescued cells from the inhibitory effect of Ca-pY on TNF-α production following treatment with LPS-EK or PPC. Remarkably, SKFs have been shown to be involved in oxidant-induced priming of LPS signaling [75]. Nonetheless, some members of SFKs (lyn, fgr and hck) did not appear to be obligatory in LPS-induced TNF-α production as exhibited in triple knockout mice [76]. Ca-pY diminished LPS-EK- and PPC-mediated TNF-α and IL-10 production in HEK-Blue-mTLR4 cells in the present study, which was consistent with a previously reported abrogation of LPS induction of TNF-α production in RAW264.7 cells by PP2 [77]. Indeed, decomposing PPC has previously been used as a source of ROS to examine its effects on cellular functions [40, 78]. Among the various oxidation states of chromium (Cr) from (+II) to (+VI), the peroxychromate anion [CrO8]−3 consisting of a central chromium atom in the oxidation state of (+V) can decompose spontaneously in aqueous systems to release several reactive species including hydrogen peroxide, hydroxyl radical, singlet oxygen, and possibly superoxide. We chose to use Cr salt in (+VI) valency state based on its ability to generate a robust amount of prooxidants in biological systems, and not due to the clinical utility of Cr as a trace metal element per se. None of the other clinically relevant transition trace metals such as iron (Fe) or copper (Cu) in any of their respective valency states compares with Cr (+VI) because of its fidelity in generating reactive molecules in vivo and in vitro.
Curiously, MPLA, PPC and LPS-EK induced intracellular ROS accumulation in both HEK-Blue-mTLR4 and HEK-Blue null-1v cells. However, these agents did not induce NF-κB activation in HEK-Blue null-1v cells. In addition, it has recently been shown that superoxide anion can “penetrate” cell membranes through an anion chloride channel-3 (ClC3) or aquaporin channels to increase oxidative stress in the intracellular compartment [79]. Interestingly, Ca-pY and PP2 did not have a minimal effect on SEAP release induced by PPC, MPLA or LPS-EK. It is therefore conceivable that ROS that utilize TLR4 to gain entrance into the cell is able not only to produce oxidative stress per se, but also to engage in NF-κB activation and signaling as seen in the case of HEK Blue-mTLR4 cells. On the other hand, the ROS that may diffuse non-specifically through the chloride channel would produce oxidative stress without NF-κB activation as seen in HEK Blue-null1 cells. It is entirely conceivable that PPC, MPLA and LPS-EK generated ROS by activating NADPH oxidase (Nox 2) localized in the plasma membranes.
Although, we have focused mainly on the role of c-Src in the present study, we cannot at this time empirically rule out the contributions by the other members of SFKs [80]. The results we have reported here raise a number of appealing hypotheses. First, we showed that c-Src co-immunoprecipitated with TLR4 and IκBα in PPC- and LPS-EK-mediated stimulation of TLR4. Second, we showed that c-Src co-immunoprecipitated more robustly with IκBα after prooxidant PPC-induced TLR4 activation than with LPS-EK. Activation of TLR4 through NFκB activation appears to bifurcate into two divergent pathways. These may comprise of LPS-EK- and prooxidant-induced pathways that involve IκBα phosphorylation at Ser32/Ser36 residues and at the Tyr42 residue mediated by IκBα kinase and by c-Src, respectively.
In summary, as depicted in Fig. 12, our results have shown that prooxidant propagated TLR4 signaling through the c-Src/NFκB/IκBα kinase coupling in c-Src-mediated NFκB activation. This, in turn, could initiate long-lasting NFκB activation, which leads to [pTyr42] IκBα formation that may serve as a “pathophysiologic primer”. Thus, prooxidant activation of TLR4 produces a prolonged activation of NFκB with a potential to initiate and maintain disease processes in different tissues. We define “pathophysiologic primer” as a factor produced in the signaling pathways that may initiate and maintain plastic changes in different tissues with a potential outcome for diverse disease processes.
Highlights.
Oxidants and LPS-EK activate c-Src through Toll-like receptor 4 stimulation.
Inhibition of c-Src decreased both oxidant- and LPS-EK-induced TLR4 activation.
Oxidant induced robust and prolong c-Src phosphorylation of Tyr42 residue in IκBα.
Oxidant enhanced the coimmunoprecipitation of TLR4 and IκB-α with c-Src.
Phosphorylation of Tyr42 residue in IκBα by c-Src is a “pathophysiologic primer”.
Abbreviations
- ONS
oxidative/nitrosative stress
- TLR
toll-like receptor
- HEK
human embryonic kidney
- MD
myeloid of differentiation
- CA-pY
caffeic acid-pYEEIE
- CD
cluster of differentiation
- Eb
Ebselen
- MPLA
mono-phosphoryl lipid A
- LPS-EK (Ultrapure)
lipopolysaccharide from E. coli K12
- PPC
potassium peroxychromate
- SEAP
secreted embryonic alkaline phosphatase
- TIRAP
Toll-interleukin 1 receptor (TIR) adaptor protein
- FBS
fetal bovine serum
- DMEM
Dulbecco’s modified Eagle’s medium
- ANOVA
analysis of variance
- MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- TBARS
thiobarbituric acid reacting substances
- MDA
malondialdehyde
- NO
nitric oxide
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TNF
tumor necrosis factor
- ELISA
enzyme-linked immuno-sorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The project described was supported by Award Number DE021888 from the National Institute of Dental & Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
REFERENCES
- 1.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46(5-6):241–281. doi: 10.3109/10408360903142326. Review. [DOI] [PubMed] [Google Scholar]
- 2.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 3.Krejsa CM, Schieven GL. Impact of oxidative stress on signal transduction control by phosphotyrosine phosphatases. Environ Health Perspect. 1998;106(Suppl 5):1179–1184. doi: 10.1289/ehp.98106s51179. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung KJ, Lee EK, Yu BP, Chung HY. Significance of protein tyrosine kinase/protein tyrosine phosphatase balance in the regulation of NF-kappaB signaling in the inflammatory process and aging. Free Radic Biol Med. 2009;47(7):983–991. doi: 10.1016/j.freeradbiomed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Brugge JS, Cotton PC, Queral AE, Barrett JN, Nonner D, Keane RW. Neurones express high levels of a structurally modified, activated form of pp60c-Src. Nature. 1985;316:554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- 6.Barnekow A, Gessler M. Activation of the pp60c-Src kinase during differentiation of monomyelocytic cells in vitro. EMBO J. 1986;5:701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287(2-3):121–149. doi: 10.1016/0304-419x(96)00003-0. Review. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. Review. [DOI] [PubMed] [Google Scholar]
- 9.Patwardhan P, Resh MD. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol Cell Biol. 2010;30(17):4094–4107. doi: 10.1128/MCB.00246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellman D, Garber EA, Cross FR, Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez Y, Maffei M, Igea A, Amata I, Gairí M, Nebreda AR, Bernadó P, Pons M. Lipid binding by the Unique and SH3 domains of c-Src suggests a new regulatory mechanism. Sci Rep. 2013;3:1295. doi: 10.1038/srep01295. doi: 10.1038/srep01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784(1):56–65. doi: 10.1016/j.bbapap.2007.08.012. Review. [DOI] [PubMed] [Google Scholar]
- 13.Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol. 2003;33:2832–2841. doi: 10.1002/eji.200324073. [DOI] [PubMed] [Google Scholar]
- 14.Paul S, Lombroso PJ. Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cell Mol Life Sci. 2003;60(11):2465–2482. doi: 10.1007/s00018-003-3123-7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 16.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19(7):2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278(3):2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- 18.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19(1):3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. Review. [DOI] [PubMed] [Google Scholar]
- 20.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. Review. [DOI] [PubMed] [Google Scholar]
- 21.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3(7):667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 22.Peden DB. The role of oxidative stress and innate immunity in O(3) and endotoxin-induced human allergic airway disease. Immunol Rev. 2011;242(1):91–105. doi: 10.1111/j.1600-065X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 23.Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K, Golenbock DT. Molecular genetic analysis of an endotoxin non-responder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194(1):79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake K. Endotoxin recognition molecules MD-2 and toll-like receptor 4 as potential targets for therapeutic intervention of endotoxin shock. Curr Drug Targets Inflamm Allergy. 2004;3(3):291–297. doi: 10.2174/1568010043343633. [DOI] [PubMed] [Google Scholar]
- 25.Ronni T, Agarwal V, Haykinson M, Haberland ME, Cheng G, Smale ST. Common interaction surfaces of the toll-like receptor 4 cytoplasmic domain stimulate multiple nuclear targets. Mol Cell Biol. 2003;23(7):2543–2555. doi: 10.1128/MCB.23.7.2543-2555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang WJ, Toledo-Pereyra LH. Toll-like receptor signaling in liver ischemia and reperfusion. J Invest Surg. 2012;25(4):271–277. doi: 10.3109/08941939.2012.687802. [DOI] [PubMed] [Google Scholar]
- 27.Essakalli M, Atouf O, Bennani N, Benseffaj N, Ouadghiri S, Brick C. [Toll-like receptors] Pathol Biol (Paris) 2009;57(5):430–438. doi: 10.1016/j.patbio.2008.04.003. Review. [DOI] [PubMed] [Google Scholar]
- 28.Liu A, Gong P, Hyun SW, Wang KZ, Cates EA, Perkins D, Bannerman DD, Puché AC, Toshchakov VY, Fang S, Auron PE, Vogel SN, Goldblum SE. TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2012;287(20):16132–16145. doi: 10.1074/jbc.M111.310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZJ. Ubiquitin signaling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. 2005. [DOI] [PubMed] [Google Scholar]
- 32.Karki R, Igwe OJ. Toll-like receptor 4-mediated nuclear factor kappa B activation is essential for sensing exogenous oxidants to propagate and maintain oxidative/nitrosative cellular stress. PLoS ONE. 2013;8(9):e73840. doi: 10.1371/journal.pone.0073840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mankan AK, Lawless MW, Gray SG, Kelleher D, McManus R. NF-κB regulation: the nuclear response. J Cell Mol Med. 2009;13:631–643. doi: 10.1111/j.1582-4934.2009.00632.x. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999;18(45):6163–6171. doi: 10.1038/sj.onc.1203174. Review. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Darnay BG, Aggarwal BB. Site-specific tyrosine phosphorylation of Ikappa B alpha negatively regulates its inducible phosphorylation and degradation. J Biol Chem. 1996;271(49):31049–31054. doi: 10.1074/jbc.271.49.31049. [DOI] [PubMed] [Google Scholar]
- 36.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86(5):787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 37.Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;(6):425–35. [PubMed] [Google Scholar]
- 38.Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Signal. 2012;10(1):33. doi: 10.1186/1478-811X-10-33. (doi: 10.1186/1478-811X-10-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G308–G318. doi: 10.1152/ajpgi.00582.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson EK, Fridovich I. The production of superoxide radical during decomposition of potassium peroxochromate (V) Biochemistry. 1974;13:3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- 41.Miesel R, Kroger H, Kurpisz M, Weser U. Induction of arthritis in mice and rats by potassium peroxochromate and assessment of disease activity by whole blood chemiluminescence and 99mpertechnetate-imaging. Free Radic. Res. 1995;23:213–227. doi: 10.3109/10715769509064035. [DOI] [PubMed] [Google Scholar]
- 42.Igwe OJ. Prooxidant-induced c-Src/nuclear factor kappa B-coupled signaling in sensory ganglia mediates cutaneous hyperalgesia. Eur J Pain. 2013;17(7):1027–1038. doi: 10.1002/j.1532-2149.2012.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Lucas K, Maes M. Role of Toll like receptor (TLR) cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48(1):190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence DS, Niu J. Protein kinase inhibitors: the tyrosine-specific protein kinases. Pharmacol Ther. 1998;77(2):81–114. doi: 10.1016/s0163-7258(97)00052-1. Review. [DOI] [PubMed] [Google Scholar]
- 46.Park SH, Won J, Lee KH. Design and characterization of non-phosphopeptide inhibitors for Src family SH2 domains. Bioorg Med Chem Lett. 2002;12(19):2711–2714. doi: 10.1016/s0960-894x(02)00523-1. [DOI] [PubMed] [Google Scholar]
- 47.Cowan-Jacob SW, Fendrich G, Manley PW, Jahnke W, Fabbro D, Liebetanz J, Meyer T. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure. 2005;13(6):861–871. doi: 10.1016/j.str.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282(22):16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 50.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to src family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 52.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 53.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 54.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 55.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK-1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278(20):18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 57.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 58.Rauert-Wunderlich H, Siegmund D, Maier E, Giner T, Bargou RC, Wajant H, Stuhmer T. The IKK inhibitor Bay 11-7082 induces cell death independent from inhibition of activation of NF-κB transcription factors. PloS One. 2013;8:e59292. doi: 10.1371/journal.pone.0059292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 60.Kang JL, Jung HJ, Lee K, Kim HR. Src tyrosine kinases mediate crystalline silica-induced NF-kappaB activation through tyrosine phosphorylation of I kappa B-alpha and p65 NF-kappaB in RAW264.7 macrophages. Toxicol Sci. 2006;90:470–477. doi: 10.1093/toxsci/kfj096. [DOI] [PubMed] [Google Scholar]
- 61.McDonald PP, Bald A, Cassatella MA. Activation of the NF-κB pathway by inflammatory stimuli in human neutrophils. Blood. 1997;89(9):3421–3433. [PubMed] [Google Scholar]
- 62.Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol. 2000;164(8):4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 63.Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;60(8):1075–1083. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 64.Béraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci U S A. 1999;96(2):429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 66.Simmonds RE, Foxwell BM. Signaling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology. 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence T. The nuclear factor NFκB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawrence T, Gilroy DW. Chronic inflammation: A failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adib-Conguy M, Moine P, Asehnoune K, Edouard A, Espevik T, Miyake K, Werts C, Cavaillon JM. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168:158–164. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- 70.Larrick JW, Wright SC. Cytotoxic mechanism of tumour necrosis factor-alpha. FASEB J. 1990;4:3215–23. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- 71.Wang P, Wu P, Siegel MI. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 72.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 73.You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF, Teitelbaum SL. Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of Ikappa Balpha. J Biol Chem. 1998;273(45):29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- 75.Khadaroo RG, Kapus A, Powers KA, Cybulsky MI, Marshall JC, Rotstein OD. Oxidative stress reprograms lipopolysaccharide signaling via Src kinase-dependent pathway in RAW264.7 macrophage cell line. J Biol Chem. 2003;278:47834–47841. doi: 10.1074/jbc.M302660200. [DOI] [PubMed] [Google Scholar]
- 76.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr and Lyn. J Exp Med. 2003;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leu TH, Charoenfuprasert S, Yen CK, Fan CW, Maa MC. Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNF alpha secretion in macrophages. Mol Immunol. 2006;43:308–316. doi: 10.1016/j.molimm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 78.Edwards JC, Quinn PJ. Decomposing potassium peroxychromate produces hydroxyl radical (.OH) that can peroxidize the unsaturated fatty acids of phospholipid dispersions. J Lipid Res. 1982;23:994–1000. [PubMed] [Google Scholar]
- 79.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18(6):2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Q, Daniel R, Chung SW, Kang AD, Eisenstein TK, Sultzer BM, Simpkins H, Wong PMC. Involvement of c-Abl tyrosine kinase in lipopolysaccharide-induced macrophage activation. J Immunol. 1998;160:3330–3336. [PubMed] [Google Scholar]