Abstract
Brain operation is profoundly rhythmic. Oscillations of neural excitability shape sensory, motor, and cognitive processes. Intrinsic oscillations also entrain to external rhythms, allowing the brain to optimize the processing of predictable events such as speech. Moreover, selective attention to a particular rhythm in a complex environment entails entrainment of neural oscillations to its temporal structure. Entrainment appears to form one of the core mechanisms of selective attention, likely relevant to certain psychiatric disorders. Deficient entrainment has been found in schizophrenia and dyslexia, and mounting evidence also suggests that it may be abnormal in Attention-Deficit/Hyperactivity Disorder. Accordingly, we suggest that studying entrainment in selective attention paradigms will likely reveal mechanisms underlying deficits across multiple disorders.
Keywords: attention-deficit/hyperactivity disorder, dyslexia, EEG, entrainment, schizophrenia, selective attention
Cognitive science has recently returned its focus to self-generated brain activity [1, 2]. Intrinsic brain rhythms were first detected in early electrophysiological recordings [3], but the contribution of these complex oscillations to perception and cognition is only now being elucidated. This partly reflects a long-standing emphasis on characterizing brain activity by examining signals evoked by events such as external stimuli. This approach implicitly assumes that neurons respond reflexively and background neural activity is considered noise [1]. However, ongoing oscillations of neural excitability are now known to modulate responses and shape perceptual, motoric, and cognitive processes [4-6]. For example, neural oscillations may promote or suppress detection of external stimuli [7-10].
While intrinsic rhythms modulate responses to stimuli, neural oscillations themselves also respond to the environment. Frequency and timing of ongoing oscillations can be entrained by rhythmic stimuli, aligning the temporal dynamics of neural processing to external patterns. Entrainment optimizes neural excitability to be high or low when stimuli are expected [11-13] (Figure 1). This mechanism explains why predictable rhythmic stimuli are more easily perceived than unpredictable non-rhythmic stimuli [14, 15]. Further, entrainment to particular rhythms has recently been proposed to underlie selective attention, making entrainment a relevant electrophysiological target for attention research [12, 16-18]. Here, we use the term ‘selective attention’ to refer to enhanced sensory representation and perception of certain stimuli over others. Oscillatory entrainment provides a neural basis for selective attention in the case of rhythmic stimuli such as human speech [19-23].
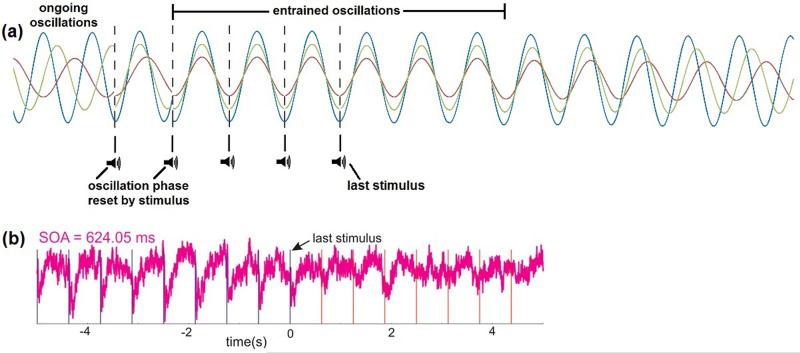
Figure 1. Entrainment of neural oscillations.
(a) Stylized example of oscillatory entrainment by rhythmic auditory stimuli. The excitability of neural ensembles oscillates at various frequencies. The phases of ongoing oscillations are reset by the first stimulus. The second stimulus establishes a rhythm, and oscillatory frequencies adjust such that phases become aligned to this rhythm. As a consequence of entrainment, neural ensembles are in a particular state of excitability when stimuli occur. Oscillations remain entrained for several cycles after the last stimulus before eventually “falling out of phase” due to frequency changes. (b) Supragranular current source density trace recorded from a monkey showing entrainment of ongoing oscillations to rhythmic auditory stimuli. Blue vertical lines represent stimuli and red lines represent when stimuli would have occurred if stimulation had continued. Oscillatory phase remained entrained to the stimulation rhythm for several cycles after the last stimulus (adapted, with permission, from [12]).
This review highlights recent advances in understanding oscillatory entrainment as a mechanism of selective attention. We then discuss new evidence of impaired entrainment in psychopathology and the implications for better characterizing related cognitive deficits. Surprisingly, entrainment has not been studied in relation to failures of selective attention. Using the example of Attention-Deficit/Hyperactivity Disorder (ADHD), we suggest that studying entrainment will elucidate substrates of selective attention deficits. This proposal is based on growing evidence that selective attention is specifically impaired in ADHD, along with findings of abnormal intrinsic oscillations in ADHD.
Entrainment of intrinsic neural oscillations
Ongoing neural oscillations can be entrained by rhythmic sensory stimuli or transcranial brain stimulation, ensuring optimal excitability across neural ensembles when stimuli occur [24] (Table 1). Entrainment is likely accomplished by repetitive phase reset of oscillations by incoming stimuli [12] (Box 1), and has been demonstrated in several frequency ranges. Entrainment entails rhythmic fluctuations of neural excitability and perception, which can outlast external stimulation [12]. For example, de Graaf et al. [25] presented visual stimuli at an alpha frequency (10.6 Hz). After an entrainment period, responses to visual targets were most accurate when targets occurred in time (in-phase) with the preceding rhythm. The periodicity of this effect correlated with individual resting alpha oscillation frequency, suggesting that rhythmic stimulation recruited intrinsic oscillations, rather than creating oscillations de novo. Similarly, Mathewson et al. [15, 26] (Figure 3) presented rhythmic visual stimuli at an alpha frequency (12 Hz). After an entrainment period, probe stimuli were only perceived if presented in-phase with the 12 Hz stimulus rhythm. Electroencephalography (EEG) revealed entrainment of alpha oscillations which continued after the entrainment period, with entrained alpha phase predicting probe stimulus perception. Entrainment and its behavioral consequences were also observed for quasi-rhythmic (jittered) entraining stimuli. This indicates entrainment is an adaptable mechanism that can operate for naturalistic, not precisely rhythmic, stimuli (Box 2). Alpha oscillations entrained by rhythmic pulses of transcranial brain stimulation altered detection of auditory [27] and visual [28] stimuli depending on entrained oscillatory phase.
Table 1.
Summary of recent studies of entrainment, its role in attention, and its dysfunction in psychopathology, listed chronologically within each section.
| Reference | Species | Diagnosis | Modality | Behavioral Results | Electrophysiological Results |
|---|---|---|---|---|---|
| Entrainment of intrinsic neural oscillations | |||||
| Mathewson et al., 2010 [15] | Human | None | Visual | Backward-masked targets after rhythmic stimuli (12 Hz) were perceived more accurately when in phase with rhythm. | None. |
| Stefanics et al., 2010 [31] | Human | None | Auditory | Predictive cues and targets were presented in a semi-rhythmic stimulus stream (0.74 Hz average). Response time for targets depended on entrained delta phase and cue predictive power. | Delta oscillations were entrained to the average stimulus frequency. Entrainment was stronger for more predictive cues and weaker for less predictive cues. |
| Ng et al., 2012 [29] | Human | None | Auditory | Detection accuracy and response time for target stimuli among a noisy background depended on power and phase of theta oscillations. | Theta oscillation phase was reset by the onset of the noisy background. |
| Neuling et al., 2012 [27] | Human | None | Auditory | Stimulus detection thresholds depended on the phase of oscillations entrained by rhythmic 10 Hz transcranial brain stimulation. | Rhythmic transcranial brain stimulation (10 Hz) entrained alpha oscillations and increased alpha power. |
| Henry & Obleser, 2012 [10] | Human | None | Auditory | Detection of gaps in a continuous auditory stimulus depended on phase of 3 Hz modulation of stimulus frequency. | Delta oscillations were entrained by the frequency-modulated stimulus, and delta phase predicted behavioral performance. |
| Mathewson et al., 2012 [26] | Human | None | Visual | Backward-masked targets after rhythmic stimuli (12 Hz) were perceived more accurately when in phase with rhythm. | Rhythmic stimuli entrained alpha oscillations, and entrained alpha phase predicted target perception. |
| Cravo et al., 2013 [30] | Human | None | Visual | Contrast sensitivity was enhanced during rhythmic (2.5 Hz) compared to non-rhythmic stimuli. | Rhythmic stimuli entrained delta oscillations, and delta phase predicted contrast sensitivity performance. |
| de Graaf et al., 2013 [25] | Human | None | Visual | Responses to targets after rhythmic stimuli (10.6 Hz) were more accurate when in phase with the rhythm. | Frequency of behavioral effect correlated with individual resting alpha oscillation frequency. |
| Jaegle & Ro, 2013 [28] | Human | None | Visual | Stimulus perception depended on the phase of oscillations entrained by rhythmic 10 Hz transcranial brain stimulation. | Rhythmic transcranial brain stimulation (10 Hz) entrained alpha oscillations. |
| Entrainment as a mechanism of attention | |||||
| Lakatos et al., 2008 [11] | Macaque monkey | None | Visual & Auditory | Simultaneous, out-of-phase rhythmic visual and auditory stimulus streams (1.5 Hz average) were presented, with attention directed to one. Monkeys detected deviant stimuli in the attended stream. | Delta oscillations entrained to the rhythm of the attended stream only. Delta phase predicted reaction time, theta power, and gamma power. |
| Besle et al., 2011 [37] | Human | Epilepsy | Visual & Auditory | Simultaneous, out-of-phase rhythmic visual and auditory stimulus streams (1.5 Hz average) were presented, with attention directed to one. Participants detected deviant stimuli in the attended stream. | Delta oscillations entrained to the rhythm of the attended stream only. The degree of entrainment depended on the regularity (predictability) of stimuli. |
| Gomez-Ramirez et al., 2011 [38] | Human | Epilepsy | Visual & Auditory | Simultaneous, out-of-phase rhythmic visual and auditory stimulus streams (0.67 Hz) were presented, with attention directed to one. Participants detected deviant stimuli in the attended stream. | Delta oscillations at twice the stimulation rate (1.33 Hz) entrained to the rhythm of the attended stream only. Delta phase correlated with alpha power. |
| Lakatos et al., 2013 [12] | Macaque monkey | None | Auditory | Simultaneous, out-of-phase rhythmic auditory stimulus streams (1.6 and 1.8 Hz) were presented, with attention directed to one. Monkeys detected deviant stimuli in the attended stream. | Delta oscillations entrained to the rhythm of the attended stream only. Phase varied across auditory cortex, enhancing responses in relevant regions and suppressing responses in irrelevant regions. |
| Abnormal entrainment in psychiatric disorders | |||||
| Thomson & Goswami, 2008 [40] | Human | Dyslexia | Motor system | Rhythmic finger tapping ability correlated with written language development, suggesting a link between rhythmic processing and language. | None. |
| Lakatos et al., 2013 [32] | Human | Schizophrenia | Auditory | Participants detected deviant stimuli in rhythmic auditory stimulus streams (0.67 Hz). Patients with schizophrenia matched healthy participants on accuracy. | Compared to healthy participants, patients with schizophrenia lacked entrainment of delta oscillations to stimulus rhythms. This deficit correlated with psychotic symptoms. |
| Leong & Goswami, 2013 [42] | Human | Dyslexia | Auditory & Motor system | Compared to healthy participants, those with dyslexia showed weaker entrainment to rhythmic speech measured by finger tapping. When producing rhythmic speech, dyslexics had altered syllable rhythms (~5 Hz) and altered coupling between syllable and phoneme rhythms (12-40 Hz). | None. |
| Soltész et al., 2013 [41] | Human | Dyslexia | Auditory | Healthy participants and those with dyslexia detected deviant stimuli in rhythmic auditory stimulus streams (2 and 1.5 Hz). | Compared to healthy participants, those with dyslexia had weaker entrainment of delta oscillations and weaker contingent negative variation. |
Figure 3. Entrained oscillatory phase affects perception.
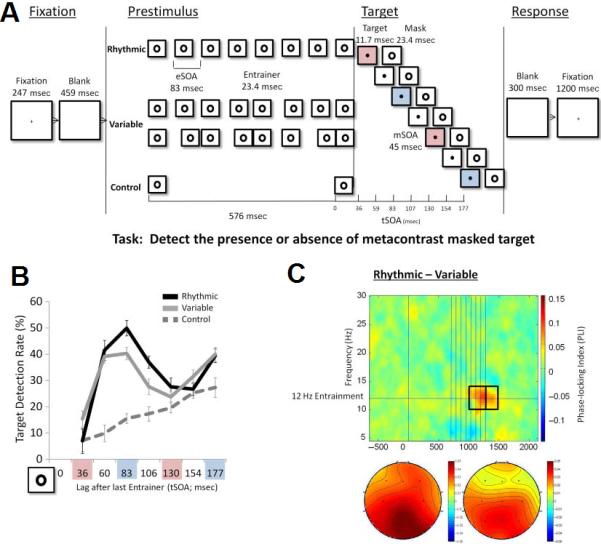
A recent study showing that the phase of entrained alpha oscillations affects stimulus detection (adapted, with permission, from [26]). (a) SOA = stimulus onset asynchrony, eSOA = entrainer SOA, tSOA = target SOA, mSOA = mask SOA. Participants were instructed to detect small circular targets that were backward-masked by an annulus. Each trial began with a fixation and blank screen, followed by an entraining period in which one of three conditions was presented. Eight annulus stimuli were presented with a fixed, 83 ms SOA (12 Hz) in the rhythmic condition and a variable SOA in the variable condition. The control condition presented only two annuli at the beginning and end of the entraining period. Targets were then presented at one of seven randomly chosen lags after the entraining period. Lags of 83 and 177 ms, indicated by blue boxes, were in-phase with the entrainment rhythm, meaning that they represented time points at which rhythmic stimuli would have occurred had entraining continued. Lags of 36 and 130 ms, indicated by pink boxes, were out-of-phase, meaning that they represented time points directly between rhythmic stimuli if entraining had continued. (b) Target detection as a function of target lag. In-phase targets (blue boxes) were detected more often than out-of-phase targets (pink boxes). For in-phase targets at 83 ms, detection rates were significantly higher for the rhythmic and variable conditions compared to the control condition. Detection rates were also significantly higher for the rhythmic than the variable condition. For in-phase targets at 177 ms, detection rates were also significantly higher for the rhythmic and variable conditions than the control condition, but the rhythmic and variable conditions did not differ. These results indicate that detection of targets was enhanced or suppressed during particular phases of the entrained oscillations. (c) Difference between phase-locking index (PLI) locked to onset of entrainment period for the rhythmic and variable conditions. PLI measures the consistency of a particular frequency’s phase at a given time across trials, reflecting phase alignment. The two marked time windows are 300 ms before and 200 ms after the end of entrainment, and the head plots show average scalp distributions of the PLI difference in these two windows. Initial fixation and entrainer stimuli are marked with vertical lines. This time-frequency plot shows increased PLI during entrainment for the rhythmic compared to the variable condition, centered on the 12 Hz entrainment frequency, coincident in time with the presentation of targets.
Ng et al. [29] used a cocktail-party paradigm in which a brief auditory stimulus occurred amid a noisy background. Theta oscillation phase was reset by the onset of background noise and modulated stimulus detection rate and response time. Cravo et al. [30] found enhanced visual contrast sensitivity predicted by entrained EEG delta phase for rhythmic but not non-rhythmic stimuli. Henry and Obleser [10] showed delta entrainment without abrupt stimulus onsets, using rhythmic frequency modulations characteristic of natural stimuli. Stefanics et al. [31] presented jittered auditory stimuli and demonstrated delta entrainment to the average stimulation frequency. Cues within the stimulus stream predicted targets with varying probability. Delta entrainment was stronger for more predictive cues and weaker for less predictive cues. The phase of entrained delta oscillations correlated with response time to target stimuli. Delta entrainment not only enhanced responses, but was sensitive to the predictive power of cues, suggesting a broad role for entrainment in temporal prediction. Similarly, the degree of entrainment depends on participant effort [32].
While the above studies demonstrate entrainment in specific frequencies, different oscillation frequencies are often coupled (Box 1) (Figure 2). For instance, the phase of entrained delta oscillations can predict gamma power [11]. Thus, entrainment has the potential to coordinate brain activity not only at the entraining rhythm, but across multiple faster time-scales, linking information in local and large-scale brain networks [33].
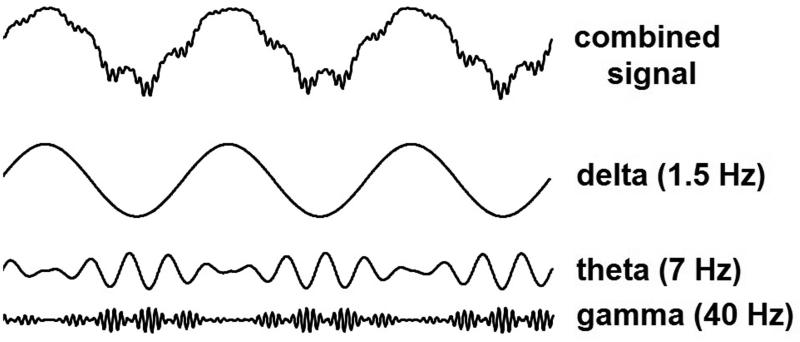
Figure 2. Hierarchical phase-amplitude coupling.
Stylized example of phase-amplitude coupling in a hierarchy of oscillations. The top trace represents a typical EEG signal comprised of several oscillatory frequencies. The other three traces are the individual oscillations that are added to create the combined signal. The phase of lower frequency oscillations determines the amplitude of higher frequencies in a hierarchical fashion. The phase of the delta oscillation is coupled to the amplitude of the theta oscillation, such that theta amplitude is larger during one phase of delta and smaller during the opposite phase. The phase of the theta oscillation is similarly coupled to the amplitude of the gamma oscillation. Gamma oscillations are “co-modulated” by both delta and theta rhythms.
Entrainment as a mechanism of attention
Recent evidence suggests that attention, even in the absence of entrainment, operates periodically. In a behavioral study, visual stimuli were presented at two locations with attention directed to one. Change detection performance at the attended location fluctuated at 4-8 Hz, i.e., at a theta rhythm [34]. Others found that detection of brief light flashes improved at attended locations depending on the phase of theta and alpha EEG oscillations [35, 36]. Thus, even seemingly stable allocation of attention appears to oscillate.
Selective attention can direct entrainment of ongoing oscillations to particular rhythmic stimuli according to task demands (Figure 4A, Table 1). Lakatos et al. [11] presented monkeys with simultaneous but out-of-phase visual and auditory stimulus streams and trained them to attend to only the cued modality stream. The phase of ongoing delta oscillations in primary visual cortex synchronized with the rhythm of the attended stream only, independent of modality. This intermodal selective entrainment effect has also been observed in human intracranial recordings [37, 38] (Figure 4B). Unimodal selective entrainment has also been observed during auditory selective attention tasks [12, 23].
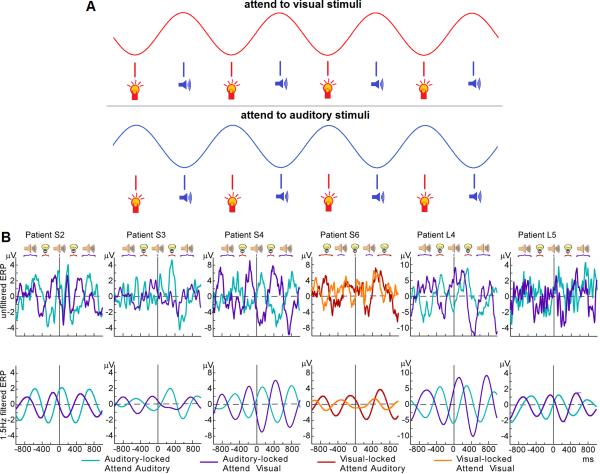
Figure 4. Oscillatory entrainment as a substrate of selective attention.
(a) Stylized example of selective oscillatory entrainment. Rhythmic visual and auditory stimulus streams are presented simultaneously in anti-phase. Attention to the visual stream entrains ongoing oscillations such that a particular phase of the oscillation in neural excitability coincides with visual stimuli. Attention to the auditory stream, however, aligns that same phase to auditory stimuli, producing an oscillation pattern that is anti-phase to the visual attention condition. Under identical stimulus conditions, attention can determine the entrainment pattern by aligning particular states of neural excitability to one stream or another. (b) Intracranial recordings from epilepsy patients who performed a selective attention task. Interleaved visual and auditory streams were presented, and the task was to detect targets in one of the two modalities. The upper plots show the unfiltered EEG recordings time-locked to auditory or visual stimuli (time point 0). The two different colors represent EEG recordings under different attention conditions (visual vs. auditory). The lower plots show the same data filtered at the stimulus frequency (1.5 Hz). The oscillatory entrainment pattern is generally anti-phase between the two attention conditions, demonstrating selective entrainment to the attended stream (adapted, with permission, from [37]). We note the caveat that this effect may also be due to a general system for predicting stimuli, which happens to appear rhythmic under rhythmic stimulus conditions. Future work may differentiate between entrainment of ongoing oscillations and more general predictability mechanisms.
When neural oscillations are selectively entrained, sensory representations of attended stimuli are both enhanced and stabilized. Non-human primates showed entrainment to attended tones in an auditory selective attention task, with frequency-dependent phase differences across tonotopically organized auditory cortex [12]. The high excitability phase of entrained oscillations was specifically aligned with the occurrence of stimuli in regions optimally tuned to process the attended frequency. Regions tuned to process frequencies approximately two octaves above or below the attended frequency showed opposite phase entrainment, with low excitability phase aligned to stimuli. This resulted in amplification of responses in regions tuned to the attended frequency and concurrent suppression of responses in differently tuned regions. Additionally, response amplitudes for attended stimuli varied less than those for ignored stimuli, indicating more stable sensory representation during entrainment.
Together, these studies suggest entrainment subserves selective attention to stimuli with a predictable temporal structure, such that fluctuations in neural excitability track one external rhythm to the exclusion of others. We propose that its mechanistic function in selective attention makes entrainment a target for further study in humans, particularly those with psychopathologies involving attentional dysfunction.
Abnormal entrainment in psychiatric disorders
Entrainment of neural oscillations has begun to be studied in psychiatric conditions (Table 1). An EEG study in schizophrenia had participants detect deviant auditory stimuli among rhythmic tone streams [32]. Healthy participants showed delta entrainment to rhythmic stimuli, while patients with schizophrenia did not entrain significantly. Greater failure to entrain correlated with diminished ability to discriminate small auditory frequency differences and with increased severity of psychotic symptoms. The authors concluded that patients with schizophrenia failed to use temporal predictability in the environment, leading to suboptimal processing of relevant events. An important caveat remains in interpreting the role of impaired entrainment in psychopathology. A failure of entrainment may underlie a loss of selective attention to rhythmic stimuli, or it may be the effect of inattention to the task. In this study [32], patients matched controls on behavioral accuracy and an increase in parieto-occipital alpha activity, indicating a similar degree of task engagement. This suggested that patients allocated similar effort to the task as controls, yet were unable to engage entrainment. Future studies will need to explicitly address the issue of cause vs. effect.
According to recent evidence, speech processing involves entraining oscillations to the rhythms of attended speech [19-23], and dysfunctional entrainment likely contributes to poor phonological processing in dyslexia [39]. Thomson et al. [40] found links between rhythmic finger tapping and written language ability in children with dyslexia, suggesting rhythmic processing contributes to both motor and language development. A recent EEG study of adults with dyslexia presented rhythmic auditory tones at delta frequencies, and found weaker delta entrainment in dyslexia compared to healthy controls [41]. Additionally, delta inter-trial phase coherence and the strength of contingent negative variation correlated with language processing and reading scores.
Delta rhythms are proposed to underlie prosody perception, while theta rhythms are linked to syllable perception [39]. Dysfunctional entrainment at these frequencies may contribute to abnormal phonological development in dyslexia [39]. To test this prediction, a behavioral study analyzed the ability of adults with dyslexia to perceive and produce rhythms in speech [42]. Entrainment to and production of syllable rhythms (~5 Hz) was found to be altered in dyslexia, as was cross-frequency coupling between syllable and phoneme rhythms (12-40 Hz). In summary, mounting evidence points to dysfunctional oscillatory entrainment in dyslexia [39-42].
The case for studying selective entrainment in ADHD
While schizophrenia and dyslexia have been linked to abnormal entrainment, there is also reason to suspect dysfunctional entrainment in ADHD. As we noted, selective entrainment to particular rhythms appears to subserve selective attention [11, 12, 17, 37, 38], but this phenomenon has yet to be examined in the putative disorder of attention, ADHD. One reason is that selective attention has long been considered intact in ADHD, a conclusion still recently supported [43, 44]. However, evidence increasingly suggests selective attention deficits in ADHD during early-stage (low-level) sensory processing. Electrophysiological studies have found robust early-stage sensory orienting deficits in ADHD [45, 46]. Behavioral studies have found deficits in effortful visual search [47] and orientation to stimuli [45]. Stevens et al. [48] found substantial deficits in adult patients with ADHD when detecting visual targets among distracters. A further experiment showed intact high-level selective attention to spatial location in ADHD, suggesting the deficits were due to impaired early-stage selective attention to targets.
Robust abnormalities in EEG oscillations have also been observed in ADHD. The most consistent finding has been increased theta and decreased beta power, operationalized as “theta/beta ratio.” A meta-analysis found abnormal theta/beta ratios in a substantial minority of patients with ADHD (25-40%), concluding that while this measure is not diagnostically sufficient, it may predict treatment outcome [49]. Another line of research has linked oscillatory abnormalities to symptoms through neurofeedback treatment. This procedure trains patients to adjust their EEG oscillations to typical levels (Box 3). Neurofeedback treatment has been reported to normalize EEG patterns [50-52] and reduce ADHD symptoms such as inattention, hyperactivity, and impulsivity [53-55].
Few studies of ADHD have analyzed EEG oscillations in response to sensory stimuli; most have measured oscillations during rest [56]. However, a recent study found greater gamma event-related synchronization for remembered as opposed to novel visual stimuli in typically developing children (TDC), while children with ADHD lacked this effect [57]. This was interpreted as an early-stage deficit in ADHD for selecting relevant stimuli. Occipital alpha event-related desynchronization, indicating decreased active inhibition of responses, was also found in response to visual stimuli in TDC [58]. This effect was coupled with frontal theta synchronization, interpreted as a signature of functional connectivity between occipital and frontal areas. By contrast, children with ADHD failed to show occipital desynchronization or frontal synchronization, suggesting both early-stage visual processing deficits and reduced functional connectivity [58]. Such deficits were not confirmed in a small sample of children with ADHD during a working memory task [59]. However, another working memory study of adults with ADHD replicated the occipital alpha and frontal theta effects in the short time period immediately after stimulus presentation. In later processing stages, the ADHD group showed greater alpha synchronization than controls and normal theta synchronization [60]. This pattern was interpreted as an early-stage deficit in orienting to visual information, with later stage compensatory mechanisms. The authors speculated that such compensation may have accounted for prior null findings in ADHD when methods did not distinguish early and late processing stages.
These studies indicate that not only are intrinsic oscillations abnormal in ADHD, but that they respond abnormally to external stimulation, particularly during early processing stages. Together with recent findings of abnormal early-stage selective attention, these studies suggest that entrainment of oscillations to external rhythms should be targeted for study in ADHD. This conclusion is further supported by findings of abnormal entrainment related to language and reading disability in dyslexia [41], given that ADHD often co-occurs with such difficulties [61].
Concluding remarks
Neural oscillations are fundamental to brain function, and their entrainment enhances processing for relevant time points, locations, and stimulus features. The clear advantage of enhancing neural processing by capitalizing on predictive information in the environment suggests that dysfunctional entrainment may underlie a wide range of processing difficulties across psychiatric conditions. Possible consequences of impaired entrainment include decreased access to temporal predictability, language development problems, and selective attention deficits. Future work should elucidate the role of entrainment in various psychopathologic conditions. Such knowledge can rapidly translate to treatment programs involving rhythmic training or neurofeedback.
Additional work should also delve into the specific mechanisms underlying entrainment. Systems enabling top-down control of entrainment during selective attention have yet to be elucidated. Understanding these mechanisms will require a combination of techniques such as electrophysiological recordings complemented by electrical microstimulation, optogenetics, pharmacological manipulations and computational modeling. Likewise, little is known about the development of entrainment, but longitudinal electrophysiological, neuroimaging, and behavioral studies of adolescent humans and non-human primates can characterize the origins and maturation of entrainment.
Understanding the underpinnings, ontogeny, and specific functions of entrainment will allow the design of experiments aimed at determining entrainment integrity in psychiatric disorders. On the flip side, another way to decipher function is to correlate the impairment of entrainment to specific symptoms of neuropsychiatric disorders. A more complete understanding of entrainment and its dysfunctions will be gained from examining a broad range of individuals with comorbid conditions, such as ADHD co-occurring with learning disabilities.
While this review focuses on rhythmic processing, it has been suggested that when stimulus input or its sampling is predominantly not rhythmic, oscillations are suppressed to allow more sustained sampling of input [17]. Future studies should determine whether a failure to suppress oscillatory rhythms also contributes to psychopathology. Other suggested functions of oscillations are facilitating functional connectivity [62-66] and parsing and grouping neural information [9]. It will be important to determine the role of oscillations in information modulation, information transmission, and neural syntax [67]. Knowledge of how these processes are impaired in neuropsychiatric disorders will set the stage for more targeted novel interventions.
Box 1: Mechanisms of oscillatory entrainment.
Phase reset: Phase reset refers to the modulation of the phase of ongoing oscillations by external or internal event-related input. A single attended or salient external stimulus can reset the phase of oscillations to a particular state of excitability within a sensory modality [18]. Stimuli in one sensory modality can also reset oscillatory phase in other modalities, and this phase reset can affect behavioral performance [11, 70, 71]. For example, in one study visual stimuli caused phase reset in auditory cortex, and oscillatory phase in turn correlated with response time to auditory stimuli [72]. Given that natural visual and auditory stimuli often co-occur [72], phase reset by one type of stimulus may prepare a range of sensory areas to optimally process further incoming stimuli. In the case of rhythmic stimuli, repetitive phase reset likely underlies entrainment by initially aligning oscillatory phase and frequency to an external rhythm [18] (Figure 1). After entrainment is established, it can continue for some time in the absence of phase reset, as evidenced by entrained oscillations outlasting external stimulation [12, 26]. Just like phase reset, entrainment can be cross-modal [11].
Cross frequency interactions: Neural oscillations at different frequencies form a hierarchy of coordinated activity. One mechanism of coordination is phase-amplitude coupling, in which the phase of lower frequency oscillations modulates the amplitude of higher frequency rhythms. This occurs in both hippocampus [9] and neocortex [73], allowing information processing and transfer across a hierarchy of frequency bands (time-scales) [17, 33] (Figure 2). For example, the phase of delta or theta fluctuations determines gamma amplitude, during both ongoing activity and entrainment to external rhythms [16, 74-77]. A recent study provided evidence that cross frequency coupling influences perception, showing that beta phase predicted detection of visual stimuli, but the strength of this relationship was modulated by the phase of theta and delta oscillations [78]. Attention-related entrainment of low-frequency oscillations likely coordinates brain processing across multiple oscillatory frequencies in time, and across multiple information processing units in space [33, 79]. We note the caveat that analysis of cross-frequency coupling may not be accurate if neural oscillations contain sharp changes in voltage; future analyses should take this into consideration [80].
Box 2: Entrainment to natural stimuli.
Although most natural stimuli like speech are not strictly rhythmic, neural oscillations readily entrain to attended speech [19-23]. Henry and Obleser [10] demonstrated entrainment to continuous sounds rhythmically modulated without sharp onsets/offsets, showing that entrainment is not confined to streams of discreet stimuli. Lakatos et al. [12] suggested that for somewhat irregular inputs, entrainment shifts the perceived timing to an average value. Indeed, several studies show entrainment even when stimuli are not strictly rhythmic. Lakatos et al. [11] demonstrated selective entrainment to attended stimuli in monkeys with variable (jittered) stimulus timing. They varied the inter-stimulus interval (ISI) between 500 and 800 ms, with a mean of 650 ms (~1.5 Hz), and found entrainment at the delta frequency corresponding to the average ISI. Stefanics et al. [31] studied human entrainment with an ISI varying between 1200 and 1500 ms, with an average of 1350 ms (~0.74 Hz). Entrainment again occurred at the average repetition rate.
Mathewson et al. [26] compared alpha entrainment between strictly rhythmic visual streams and jittered streams (Figure 3). Rhythmic streams consisted of stimuli every 82.3 ms (~12 Hz), and resulted in both oscillatory entrainment and behavioral enhancement for subsequent in-phase probe stimulus detection. Jittered streams varied the ISI between 11.7 and 257.4 ms, with a mean of 82.3 ms and an identical duration to the rhythmic streams. The jittered condition also produced oscillatory entrainment, though with less consistency than the strictly rhythmic condition. In-phase probe detection following jittered streams also showed behavioral enhancement, which was slightly less than the enhancement following strictly rhythmic streams. Overall, these results show that the advantages of temporal predictability are also applicable to naturalistic rhythms that contain some degree of irregularity.
Cravo et al. [30] recently compared rhythmic visual stimulation to a non-rhythmic condition. While the rhythmic stimuli, presented every 400 ms (2.5 Hz) produced delta entrainment, the non-rhythmic condition produced no entrainment. The non-rhythmic stimuli were presented with randomly chosen ISI intervals of 200, 300, 400, 500, or 600 ms, and no mean frequency was reported. Taken together, these studies suggest that the amount of jitter entrainment can tolerate depends on the wavelength of the average rhythm as well as the ISI distribution (e.g., normal vs. random distribution).
Box 3: Neurofeedback treatment for ADHD.
Neurofeedback involves altering neural activity through operant conditioning. Visual or auditory stimuli, including video games and animations, are presented as a reward when particular EEG oscillations or electrophysiological potentials correspond to a target range. This procedure has been used as a non-pharmacological treatment for ADHD, due to findings of abnormal oscillatory activity (e.g., elevated theta/beta ratio) in patients with ADHD. For example, pharmacological treatment with methylphenidate improves both ADHD symptoms and normalizes neural oscillations [53], suggesting a link between abnormal oscillations and symptomatology.
Different neurofeedback procedures for ADHD treatment target different types of EEG activity, and optimal treatment may require tailoring neurofeedback to individual EEG abnormalities [81]. Some protocols aim to reward suppression of theta activity and enhancement of beta activity to normalize the theta/beta ratio, while others focus on electrophysiological potentials such as the slow cortical potential, which reflects attentiveness [81, 82]. Meta-analyses of neurofeedback treatment for ADHD have yielded moderate to large effect sizes for reduction of inattention, hyperactivity, and impulsivity symptoms [53, 55]; in one study, benefits were reported to persist for six months after treatment [54].
Neurofeedback treatment has also demonstrated concurrent changes in oscillatory activity and ADHD symptoms [50-52, 83], suggesting that oscillations and symptoms are linked. For example, neurofeedback aiming to reduce the theta/beta ratio in children with ADHD resulted in reduced theta/beta ratio, reduced parent ratings of inattention, and improved neuropsychological response times [52]. Another study applied protocols targeting theta/beta ratio and slow cortical potentials to separate groups of children with ADHD [83]. Both groups showed increased ability to voluntarily regulate electrophysiological activity as well as improvements in attention, cognition, and behavior as rated by parents and teachers. Comparison of neurofeedback to other treatments for ADHD has suggested effects comparable to psychostimulants and cognitive behavioral therapy [82]. While existing evidence suggests that neurofeedback may be an effective nonpharmacological treatment for ADHD, more randomized and well-blinded studies are needed. Neurofeedback approaches have not yet addressed modulatory mechanisms like phase reset and entrainment in perceptual processes. Investigation of oscillatory entrainment may inform neurofeedback by revealing how oscillations interact with external rhythms in ADHD. Such knowledge may provide new methods of assessing individual oscillatory abnormalities, as well as new treatment protocols involving entrainment.
Box 4: Questions for future research.
What mechanisms are involved in the top-down control of oscillatory entrainment? Given that the strong control over entrainment observed in selective attention tasks suggests modulation of stimulus related inputs at the thalamus and/or early-stage cortical targets, what pathways are involved in directing entrainment to particular environmental rhythms?
How does entrainment develop under normal conditions and in psychopathology? What links exist between the ability to entrain oscillations at various developmental stages and cognitive functions such as attention? How might the development of entrainment differ in various neurodevelopmental disorders?
What might the correlation between impaired entrainment and specific symptoms of psychiatric disorders reveal about the functions of entrainment under typical conditions?
Do links between deficient entrainment and symptoms translate across disorders, e.g. does the role of entrainment in language processing in dyslexia indicate a similar underlying mechanism for language difficulties in ADHD? Might deficient entrainment underlie the common co-occurrence of ADHD and dyslexia? Will deficient entrainment be most easily discernible in individuals with ADHD and comorbid dyslexia?
Under what conditions is it advantageous to suppress entrainment and use a more sustained sampling of sensory input? Does failure to suppress entrainment in particular frequency bands contribute to psychopathology?
Can knowledge of specific deficits of entrainment inform neurofeedback treatment of ADHD and related conditions? Can entrainment be directly utilized to modify abnormal oscillatory activity, or be used to assess individual impairments and tailor neurofeedback procedures to individual needs?
Acknowledgements
This work was supported by T32MH067763 and R01DC012947. We thank the unidentified as well as the identified (Dr. Steven Luck) reviewers for their insightful and constructive comments.
Glossary
- Amplitude
the maximum change of a periodic wave over one cycle. In extracellular electrophysiological recordings of neural ensembles, amplitude reflects the extent to which neurons in the ensemble(s) oscillate in phase at a particular frequency. In these recordings, the measure reflects the number of neurons oscillating, the degree of synchrony among them, and the magnitude of each single neuron’s oscillations.
- Contingent negative variation
an electrophysiological potential arising in the motor system reflecting anticipation of a predicted stimulus and/or motor preparation for a response. It has been suggested that the contingent negative variation at least partly reflects modulation of sensory systems by motor system predictions, and that this modulation occurs via oscillatory mechanisms [68].
- Event-related synchronization/event-related desynchronization
increases and decreases, respectively, in the spectral power or amplitude of an oscillatory frequency in response to a stimulus event. Event related synchronization and desynchronization quantify the extent to which the oscillations of distinct neurons synchronize or desynchronize at a particular frequency in response to external stimulation.
- Inter-trial phase coherence
coherence refers to a stable relationship between the phases of oscillatory waves. Inter-trial phase coherence in EEG measures the stability of oscillatory phase across trials time-locked to stimuli and indicates the stability of entrainment by rhythmic stimuli.
- Neural oscillations
rhythmic fluctuations of neural excitability which, when synchronized across large numbers of neurons, appear as periodic waves in electrophysiological recordings. Neural oscillations occur in a hierarchy of distinguishable frequency bands, including delta (0.5 – 4 Hz), theta (4 – 10 Hz), alpha (8 – 12 Hz), beta (10-30 Hz), and gamma (30 – 100 Hz) [69]. Oscillations are further quantified in terms of phase and amplitude or spectral power.
- Phase
indicates the particular point in the cycle of an oscillation. Oscillatory phase can be measured in degrees, with one cycle comprising 360° or 2π radians. Phase indicates the particular point in the cycle of a given oscillation. The oscillations of distinct neurons or neural ensembles are in phase (synchronized) when their peaks and troughs coincide. Anti-phase fluctuations occur when the peak of one coincides with the trough of the other and vice-versa.
- Phoneme
a distinct unit of speech within a word that differentiates word meaning, e.g. the “j” and “c” sounds that differentiate “jog” from “cog”. Gamma oscillations are proposed to subserve perception of prosody [39].
- Prosody
rhythmic changes in volume, pitch, and tempo of speech that carry semantic meaning. Delta oscillations are proposed to underlie perception of prosody [39].
- Spectral power
the square of the amplitude. Spectral power, also simply called power, is a commonly reported measure of neural synchrony at a particular frequency.
- Syllable
a unit of speech containing a vowel sound and a consonant sound, e.g. “da.” Most syllables across language follow a consonant-vowel pattern. Theta oscillations are proposed to subserve perception of syllabic rhythms [39].
References
- 1.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Sporns O. Networks of the brain. MIT Press; 2011. [Google Scholar]
- 3.Stone JL, Hughes JR. Early history of electroencephalography and establishment of the American Clinical Neurophysiology Society. J Clin Neurophysiol. 2013;30:28–44. doi: 10.1097/WNP.0b013e31827edb2d. [DOI] [PubMed] [Google Scholar]
- 4.Thut G, et al. The functional importance of rhythmic activity in the brain. Curr Biol. 2012;22:R658–R663. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Buzsáki G. Rhythms of the brain. Oxford University Press; 2006. [Google Scholar]
- 6.Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder CE, et al. Dynamics of active sensing and perceptual selection. Curr Opin Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanrullen R, et al. Ongoing EEG phase as a trial-by-trial predictor of perceptual and attentional variability. Front Psychol. 2011;2:60. doi: 10.3389/fpsyg.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzsaki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14:345–367. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry MJ, Obleser J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proc Natl Acad Sci U S A. 2012;109:20095–20100. doi: 10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakatos P, et al. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 12.Lakatos P, et al. The spectrotemporal filter mechanism of auditory selective attention. Neuron. 2013;77:750–761. doi: 10.1016/j.neuron.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnal LH, Giraud AL. Cortical oscillations and sensory predictions. Trends Cogn Sci. 2012;16:390–398. doi: 10.1016/j.tics.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Rohenkohl G, et al. Temporal expectation improves the quality of sensory information. J Neurosci. 2012;32:8424–8428. doi: 10.1523/JNEUROSCI.0804-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathewson KE, et al. Rescuing stimuli from invisibility: Inducing a momentary release from visual masking with pre-target entrainment. Cognition. 2010;115:186–191. doi: 10.1016/j.cognition.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder CE, Lakatos P. The gamma oscillation: master or slave? Brain Topogr. 2009;22:24–26. doi: 10.1007/s10548-009-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakatos P, et al. The leading sense: supramodal control of neurophysiological context by attention. Neuron. 2009;64:419–430. doi: 10.1016/j.neuron.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zion Golumbic EM, et al. Temporal context in speech processing and attentional stream selection: a behavioral and neural perspective. Brain Lang. 2012;122:151–161. doi: 10.1016/j.bandl.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding N, Simon JZ. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc Natl Acad Sci U S A. 2012;109:11854–11859. doi: 10.1073/pnas.1205381109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder CE, et al. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zion Golumbic EM, et al. Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party”. Neuron. 2013;77:980–991. doi: 10.1016/j.neuron.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thut G, et al. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol. 2011;2:170. doi: 10.3389/fpsyg.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Graaf TA, et al. Alpha-band rhythms in visual task performance: phase-locking by rhythmic sensory stimulation. PLoS One. 2013;8:e60035. doi: 10.1371/journal.pone.0060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathewson KE, et al. Making waves in the stream of consciousness: entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation. J Cogn Neurosci. 2012;24:2321–2333. doi: 10.1162/jocn_a_00288. [DOI] [PubMed] [Google Scholar]
- 27.Neuling T, et al. Good vibrations: oscillatory phase shapes perception. Neuroimage. 2012;63:771–778. doi: 10.1016/j.neuroimage.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Jaegle A, Ro T. Direct control of visual perception with phase-specific modulation of posterior parietal cortex. J Cogn Neurosci. 2013 doi: 10.1162/jocn_a_00494. [DOI] [PubMed] [Google Scholar]
- 29.Ng BSW, et al. A precluding but not ensuring role of entrained low-frequency oscillations for auditory perception. J Neurosci. 2012;32:12268–12276. doi: 10.1523/JNEUROSCI.1877-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cravo AM, et al. Temporal expectation enhances contrast sensitivity by phase entrainment of low-frequency oscillations in visual cortex. J Neurosci. 2013;33:4002–4010. doi: 10.1523/JNEUROSCI.4675-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanics G, et al. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci. 2010;30:13578–13585. doi: 10.1523/JNEUROSCI.0703-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakatos P, et al. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33:11692–11702. doi: 10.1523/JNEUROSCI.0010-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landau AN, Fries P. Attention samples stimuli rhythmically. Curr Biol. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busch NA, et al. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besle J, et al. Tuning of the human neocortex to the temporal dynamics of attended events. J Neurosci. 2011;31:3176–3185. doi: 10.1523/JNEUROSCI.4518-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Ramirez M, et al. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. J Neurosci. 2011;31:18556–18567. doi: 10.1523/JNEUROSCI.2164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn Sci. 2011;15:3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Thomson JM, Goswami U. Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. J Physiol Paris. 2008;102:120–129. doi: 10.1016/j.jphysparis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Soltész F, et al. Differential entrainment of neuroelectric delta oscillations in developmental dyslexia. PLoS One. 2013;8:e76608. doi: 10.1371/journal.pone.0076608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leong V, Goswami U. Assessment of rhythmic entrainment at multiple timescales in dyslexia: Evidence for disruption to syllable timing. Hear Res. 2013 doi: 10.1016/j.heares.2013.07.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAvinue LP, et al. A componential analysis of visual attention in children with ADHD. J Atten Disord. 2012 doi: 10.1177/1087054712461935. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 44.Laasonen M, et al. Project DyAdd: visual attention in adult dyslexia and ADHD. Brain Cogn. 2012;80:311–327. doi: 10.1016/j.bandc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Ortega R, et al. Exogenous orienting of visual-spatial attention in ADHD children. Brain Res. 2012;1493:68–79. doi: 10.1016/j.brainres.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone SJ, et al. Ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2013;124:644–657. doi: 10.1016/j.clinph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Mullane JC, Klein RM. Literature review: visual search by children with and without ADHD. J Atten Disord. 2008;12:44–53. doi: 10.1177/1087054707305116. [DOI] [PubMed] [Google Scholar]
- 48.Stevens AA, et al. Increased sensitivity to perceptual interference in adults with attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2012;18:511–520. doi: 10.1017/S1355617712000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arns M, et al. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17:374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- 50.Gevensleben H, et al. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int J Psychophysiol. 2009;74:149–157. doi: 10.1016/j.ijpsycho.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Gevensleben H, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psychiatry. 2009;50:780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 52.Bakhshayesh AR, et al. Neurofeedback in ADHD: a single-blind randomized controlled trial. Eur Child Adolesc Psychiatry. 2011;20:481–491. doi: 10.1007/s00787-011-0208-y. [DOI] [PubMed] [Google Scholar]
- 53.Lofthouse N, et al. A review of neurofeedback treatment for pediatric ADHD. J Atten Disord. 2012;16:351–372. doi: 10.1177/1087054711427530. [DOI] [PubMed] [Google Scholar]
- 54.Gevensleben H, et al. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur Child Adolesc Psychiatry. 2010;19:715–724. doi: 10.1007/s00787-010-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arns M, et al. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- 56.Basar E, Guntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- 57.Lenz D, et al. Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia. 2010;48:1985–1993. doi: 10.1016/j.neuropsychologia.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Mazaheri A, et al. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 59.Gomarus HK, et al. Do children with ADHD and/or PDD-NOS differ in reactivity of alpha/theta ERD/ERS to manipulations of cognitive load and stimulus relevance? Clin Neurophysiol. 2009;120:73–79. doi: 10.1016/j.clinph.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 60.Missonnier P, et al. EEG anomalies in adult ADHD subjects performing a working memory task. Neuroscience. 2013;241:135–146. doi: 10.1016/j.neuroscience.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Cheung CH, et al. Aetiology for the covariation between combined type ADHD and reading difficulties in a family study: the role of IQ. J Child Psychol Psychiatry. 2012;53:864–873. doi: 10.1111/j.1469-7610.2012.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Cashdollar N, et al. Hippocampus-dependent and -independent theta-networks of active maintenance. Proc Natl Acad Sci U S A. 2009;106:20493–20498. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson KL, et al. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20:1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- 65.Luo H, et al. Auditory cortex tracks both auditory and visual stimulus dynamics using low-frequency neuronal phase modulation. PLoS Biol. 2010;8:e1000445. doi: 10.1371/journal.pbio.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romei V, et al. Sounds reset rhythms of visual cortex and corresponding human visual perception. Curr Biol. 2012;22:807–813. doi: 10.1016/j.cub.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schyns PG, et al. Cracking the code of oscillatory activity. PLoS Biol. 2011;9:e1001064. doi: 10.1371/journal.pbio.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnal LH. Predicting “When” Using the Motor System's Beta-Band Oscillations. Front Hum Neurosci. 2012;6:225. doi: 10.3389/fnhum.2012.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sohal VS. Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry. 2012;71:1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romei V, et al. Sounds reset rhythms of visual cortex and corresponding human visual perception. Curr Biol. 2012;22:807–813. doi: 10.1016/j.cub.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fiebelkorn IC, et al. Ready, set, reset: Stimulus-locked periodicity in behavioral performance demonstrates the consequences of cross-sensory phase reset. J Neurosci. 2011;31:9971–9981. doi: 10.1523/JNEUROSCI.1338-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorne JD, et al. Cross-modal phase reset predicts auditory task performance in humans. J Neurosci. 2011;31:3853–3861. doi: 10.1523/JNEUROSCI.6176-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 74.Tort ABL, et al. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoch T, et al. Modulation of the amplitude of gamma-band activity by stimulus phase enhances signal encoding. Eur J Neurosci. 2011;33:1223–1239. doi: 10.1111/j.1460-9568.2011.07593.x. [DOI] [PubMed] [Google Scholar]
- 76.Onslow ACE, et al. Quantifying phase-amplitude coupling in neuronal network oscillations. Prog Biophys Mol Bio. 2011;105:49–57. doi: 10.1016/j.pbiomolbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Ozkurt TE, Schnitzler A. A critical note on the definition of phase-amplitude cross-frequency coupling. J Neurosci Methods. 2011;201:438–443. doi: 10.1016/j.jneumeth.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Fiebelkorn IC, et al. Cortical cross-frequency coupling predicts perceptual outcomes. Neuroimage. 2013;69:126–137. doi: 10.1016/j.neuroimage.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kramer MA, et al. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods. 2008;170:352–357. doi: 10.1016/j.jneumeth.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Kropotov JD. Quantitative EEG, event-related potentials and neurotherapy. Elsevier Inc.; 2009. Attention deficit hyperactivity disorder. [Google Scholar]
- 82.Moriyama TS, et al. Evidence-based information on the clinical use of neurofeedback for ADHD. Neurotherapeutics. 2012;9:588–598. doi: 10.1007/s13311-012-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leins U, et al. [Neurofeedback for children with ADHD: a comparison of SCP- and theta/beta-protocols]. Prax Kinderpsychol Kinderpsychiatr. 2006;55:384–407. [PubMed] [Google Scholar]