Abstract
Objective
We determined whether whole body and subcutaneous adipose tissue (SAT) insulin resistance was proportional to regional fat mass (FM).
Design and Methods
We studied postmenopausal women (Mean±SD; age 56±4 y, n=25) who were overweight or obese (BMI 29.9±5.1 kg/m2). Whole body and regional FM were measured by dual-energy x-ray absorptiometry (DXA) and computed tomography (CT). Women were studied during basal and insulin-stimulated (3-stage euglycemic clamp) conditions. Whole-body lipolysis was assessed by [2H5]glycerol rate of appearance and abdominal and femoral SAT lipolysis by interstitial glycerol (microdialysis).
Results
Whole body insulin resistance in skeletal muscle (insulin-stimulated glucose disposal) and adipose tissue (insulin-suppressed lipolysis) were independently related to trunk FM (r=−0.336 and 0.484, respectively), but not leg FM (r=−0.142 and −0.148, respectively). Local antilipolytic insulin resistance in abdominal, but not femoral, SAT was positively related to trunk FM (r=0.552) and visceral FM (r=0.511) but not related to leg FM (r=−0.289). Whole body and abdominal, but not femoral, adipose tissue insulin sensitivity were strongly related to skeletal muscle insulin sensitivity (r=−0.727 and −0.674, respectively).
Conclusions
The association of SAT insulin sensitivity (lipolysis) with adiposity and skeletal muscle insulin sensitivity is specific to the abdominal region.
Keywords: adipose tissue, lipolysis, insulin sensitivity
Introduction
In normal weight individuals, the basal and postprandial (insulin-suppressed) release of non-esterified fatty acids (NEFA) during lipolysis of adipose tissue triglycerides is well-regulated to meet energy demands (1). In contrast, in obese individuals basal NEFA concentrations are elevated and insulin suppression of lipolysis is impaired, suggesting dysregulation with obesity (2–5). Although increased NEFA in obesity was generally thought to be proportional to fat mass, a review suggested that fasting NEFA (i.e., basal lipolysis) does not go up in proportion to total fat mass and may, in fact, be reduced per kilogram of fat with increasing obesity (at least in men) (6). Associations between reduced abdominal subcutaneous adipose tissue (SAT) basal lipolysis and increased fasted hyperinsulinemia suggests there may be a progressive downregulation of lipolysis with increasing systemic glucoregulatory insulin resistance (6). The ability of insulin to effectively suppress lipolysis is important because most individuals spend the majority of the day in a postprandial rather than fasted state. Yet, it is not known whether systemic insulin-suppressed lipolysis, like basal lipolysis, is also down-regulated with increasing adiposity and glucoregulatory insulin resistance. Moreover, it remains unclear whether local (adipose tissue-specific) insulin-suppressed lipolysis is proportional to local fat mass or indicative of impaired SAT function. In vitro data from subcutaneous (abdominal and gluteal) adipocytes of obese premenopausal women demonstrated a correlation of insulin resistance with visceral adiposity (7). Local resistance to insulin may provide insights into SAT dysfunction and redistribution away from subcutaneous and toward visceral depots with increasing obesity and may be particularly important after menopause when women begin to accumulate more visceral fat (8). Indeed, in vitro data suggested that the higher adipocyte insulin sensitivity in gluteal, compared to abdominal, that was present in obese premenopausal women (7) was no longer apparent in postmenopausal women (9). Insulin resistance at the level of the adipocyte (in vitro lipolysis) was also related to systemic hyperinsulinemia in those postmenopausal women (9), consistent with the associations observed in men at the level of abdominal tissue (artereo-venous balance) (6). Taken together, the basal lipolysis observations in men and the in vitro insulin-stimulated lipolysis observations in adipose tissue from women suggest that rate of SAT lipolysis may not be simply a function of total fat mass, but rather increase with progressive hyperinsulinemia and visceral adiposity. Our aim for the current study was to verify these observations in vivo using the reference method 3-stage hyperinsulinemic, euglycemic clamp to evaluate insulin sensitivity systemically (glucoregulatory and antilipolytic) and locally (microdialysis in abdominal and femoral SAT). We expected that any associations of SAT lipolysis with hyperinsulinemia or visceral adiposity would be particularly apparent in a cohort of overweight and obese postmenopausal women.
Methods
Subjects
We retrospectively analyzed baseline data collected in healthy, sedentary postmenopausal women (n=25) previously enrolled in two studies conducted by our laboratory. Some of the data have been reported previously (10–12). Postmenopausal status was defined as cessation of menses for at least one year or hysterectomy with an FSH >30 IU/L. Women were excluded if they were currently using hormone therapy, had a history of hormone-sensitive cancer, fasted plasma glucose >5.6mmol/L, uncontrolled hypertension (resting systolic blood pressure >150 mmHg or diastolic >90 mmHg), thyroid dysfunction (TSH <0.5 or >5.0 mU/mL), hypertriglyceridemia (fasting triglycerides >4.5 mmol/L), or abnormal liver or renal function. All participants provided written informed consent to participate in the study, which was approved by the Colorado Multiple Institutional Review Board.
Body composition
Total fat mass (FM) and fat-free mass (FFM) were measured by dual-energy x-ray absorptiometry (DXA) using Lunar DPX-IQ (n=15; Software v4.38, Lunar Co., Madison, WI) or Hologic Delphi-W (n = 14; software v11.2, Hologic, Inc., Bedford, MA). The recommendations of the manufacturers were used to define the trunk and leg regions. As previously reported (13), the use of two DXA instruments could not be avoided, so orthogonal regression equations were generated from a separate cohort of subjects (n=48) measured on both instruments to adjust Lunar data to Hologic. The average between-instrument biases for Hologic vs. Lunar were: 0.17kg body mass, −0.75kg total FM, −0.93kg trunk FM, −0.34kg leg FM, and 0.92kg FFM.
Abdominal (visceral and subcutaneous) and mid-thigh (subcutaneous) fat areas were determined by computed tomography (CT) as previously described (14). Single slice images were obtained at the levels of the L2-L3 and the L4-L5 intervertebral spaces and the mid-thigh. The abdominal visceral fat areas (cm2) were manually defined by tracing the muscles of the abdominal wall. Abdominal subcutaneous fat areas (cm2) were calculated by subtracting the visceral fat areas from the total abdominal fat area. CT slice fat areas were converted to mass and used to estimate total upper body visceral and subcutaneous fat mass as previously described (15). In brief, fat areas were averaged over the two abdominal slices, multiplied by the slice thickness (10cm) to calculate fat volume (cm3), and converted to mass (0.9kg triglyceride/L tissue). Visceral and subcutaneous FM (kg) in the CT abdominal slices were then multiplied by the DXA trunk FM to estimate the proportions of total visceral and subcutaneous fat in the upper body.
Hyperinsulinemic, euglycemic clamp
Three-stage (4, 8 and 40 mU/m2/min) hyperinsulinemic, euglycemic clamps were administered as previously described (10). Briefly, clamps were performed on the Clinical and Translational Research Center (CTRC) following a, 3-day standardized diet and 12-hour fast. Plasma glucose was obtained at bedside every 5 minutes and the dextrose infusion was adjusted to sustain plasma glucose at 5 mmol/L. A primed (1.5 μmol/kg), constant (~0.1 μmol/kg/min) infusion of [2H5]glycerol (Cambridge Isotope Laboratories, Inc., Andover, MA) was delivered throughout a 90-min basal period and the three 90-min insulin stages to measure whole-body lipolysis. Blood samples were collected before (fasted) the start of infusions and at 60, 75 and 90 minutes of the basal period and each insulin stage for determination of insulin, and glycerol (concentration and isotope enrichment). Steady-state insulin and whole body glucose disposal rate (GDR; mg/kg/min) were determined from the average insulin concentration and steady-state glucose infusion rate, respectively, during the final 30 minutes of the 40 mU/m2/min dose insulin infusion.
Microdialysis
Regional SAT lipolysis was evaluated by placing linear microdialysis probes (BAS, Inc. custom LM-3 probes, 3 cm membrane) in both abdominal and femoral SAT as previously described (12). Briefly, the microdialysis probes were inserted under sterile technique into abdominal (2 probes lateral to the umbilicus; ~3 cm apart) and femoral (2 probes mid-thigh; ~3 cm apart) SAT. Throughout the insulin clamp procedure the probes were perfused at 2.0 μL/min with Ringer solution containing 2.5 mM glucose, 200 μM [13C]glycerol, and 5 mM ethanol. The outgoing dialysate was collected in 15-min fractions (30 μL) throughout the basal period and each insulin stage.
Whole-body lipolysis
Plasma glycerol concentrations were measured by the CTRC core laboratory. The analysis of [2H5]glycerol was performed by the Colorado Nutrition and Obesity Research Center Mass Spectrometry Core Laboratory using an adaptation of the negative ion chemical ionization gas chromatography-mass spectrometry as previously described (16). The average rate of appearance of glycerol (GLYRA) over the last 30 minutes of each stage was calculated using the steady-state equation of Steele (17): GLYRA = F/ Ep; where F is the rate of infusion for [2H5]glycerol (0.10 μmol/kg/min) and E is the plateau plasma isotope enrichment.
Regional SAT lipolysis
Dialysate samples were batched and sent to a commercial mass spectrometry laboratory (Metabolic Solutions, Inc) for the analysis of [13C]glycerol enrichment and to East Carolina University for the spectophotometric measurement of glycerol concentrations using an automated CMA600 Microdialysate Analyzer (CMA Microdialysis, Acton, MA). [13C]glycerol isotopic tracer was included in the perfusate to calibrate dialysate concentrations to the degree of equilibration of interstitial glycerol across the probe membrane as previously described (18). As an internal reference, relative recovery (RR) of glycerol across the dialysis membrane was determined from the isotopic enrichment of [13C]glycerol in the perfusate and the dialysate: RR = 1-([13C]glyceroldialysate/[13C]glycerolperfusate). Interstitial glycerol concentration was calculated from the measured dialysate glycerol concentration corrected for the RR of each probe at each individual time point and then values from both probes were averaged over the final 30 minutes (two 15-min collections) of each clamp stage and within each SAT region.
Data analysis
Exponential decay curves for glycerol (whole body GLYRA, SAT interstitial concentration) across the range of insulin concentrations were generated for each individual and suppression of lipolysis was calculated as the insulin concentration needed to half-maximally suppress glycerol (EC50) as previously described (10). The EC50 was calculated for the whole body, abdominal SAT, and femoral SAT. Pearson correlations were used to evaluate the associations among measures of whole body and regional antilipolytic insulin action (EC50), whole body glucoregulatory insulin action (GDR), and adiposity (subcutaneous and visceral fat mass). Partial correlations were used to test whether the associations of upper-body (trunk fat) and lower-body (leg fat) adiposity with measures of insulin sensitivity remained or changed after controlling for the other region. All statistical analyses were performed using SPSS software (IBM SPSS Statistics 21.0).
Results
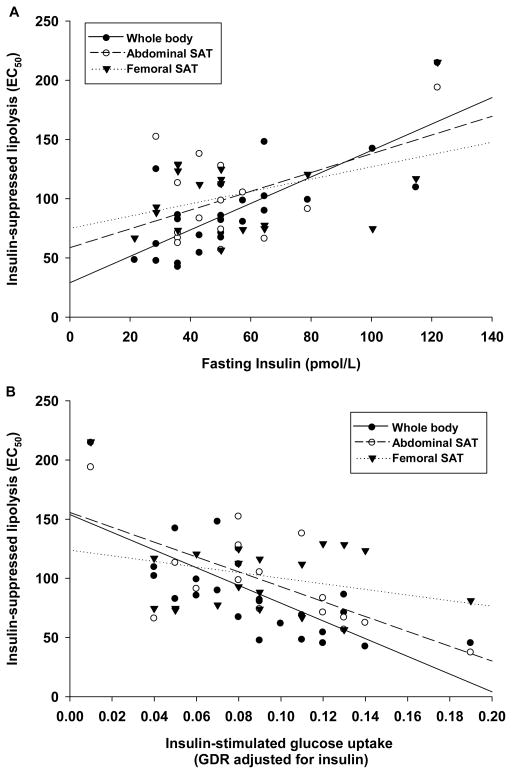
Study subjects were postmenopausal women who were sedentary and overweight or mildly obese but otherwise healthy (Table 1). None of the subjects had diabetes, smoked, or used hormone therapy or lipid- or glucose-lowering medications at the time of the study. As expected, whole body and regional SAT insulin resistance were directly correlated with fasted insulin concentration (Figure 1A). Whole body and abdominal, but not femoral, SAT insulin-suppression of lipolysis were also inversely related to whole body skeletal muscle insulin-stimulated glucose disposal (Figure1B). In addition, systemic glucoregulatory (GDR) and antilipolytic (ED50) insulin action were directly correlated with all measures of adiposity (Table 2). Local abdominal SAT insulin resistance was correlated with upper but not lower body adiposity, whereas femoral SAT insulin resistance was not correlated with any measure of adiposity (Table 2). Trunk fat mass remained significantly correlated with measures of systemic and abdominal SAT insulin sensitivity after controlling for leg fat mass. However, there was no independent relation between leg fat mass and insulin sensitivity after controlling for trunk fat mass (Table 2).
Table 1.
Subject Characteristics.
| Variable | Mean ± SD |
|---|---|
| Age (yr) | 56 ± 4 |
| Years since menopause | 10 ± 7 |
| Weight (kg) | 77.7 ± 14.6 |
| BMI (kg/m2) | 29.3 ± 5.0 |
| Total FM (kg) | 33.0 ± 9.9 |
| Trunk FM (kg) | 16.6 ± 5.7 |
| Leg FM (kg) | 11.8 ± 3.5 |
| Fat-free mass (kg) | 44.2 ± 5.8 |
| Abdominal SAT FM (kg) | 12.1 ± 4.1 |
| Visceral FM (kg) | 4.4 ± 2.1 |
| Fasted glucose (mmol/L) | 4.8 ± 0.4 |
| Whole body GDR (mg/kg/min) | 5.6 ± 2.8 |
| Whole body EC50 (pmol/L) | 88.3 ± 39.5 |
| Abdominal SAT EC50 (pmol/L) | 99.5 ± 42.0 |
| Femoral SAT EC50 (pmol/L) | 102.9 ± 35.5 |
EC50= insulin concentration needed to half-maximally suppress lipolysis; FM=fat mass; GDR= insulin-mediated glucose disposal rate; SAT= subcutaneous adipose tissue.
Figure 1.
A) Associations of whole body (r=0.874, p<0.001), abdominal (r=0.573, p<0.05), and femoral (r=0.546, p<0.01) adipose tissue insulin resistance with fasted serum insulin concentration (pmol/L); B) Inverse association of whole body (r = −0.727, p<0.001), abdominal (r = −0.674, p<0.01) and femoral (r = −0.256, p=n.s.) adipose tissue insulin resistance (EC50, pmol/L) with whole body skeletal muscle insulin sensitivity (GDR:insulin).
Table 2.
Correlations of whole body and regional fat mass with whole body skeletal muscle insulin resistance (GDR) and whole body and regional subcutaneous adipose tissue insulin resistance (EC50).
| Whole body GDR | Whole body EC50 | Abdominal SAT EC50 | Femoral SAT EC50 | |
|---|---|---|---|---|
| Pearson Correlations | ||||
| Whole body FM | −0.673* | 0.590* | 0.516* | 0.141 |
| Trunk FM | −0.651* | 0.609* | 0.557* | 0.134 |
| Leg FM | −0.603* | 0.444* | 0.302 | 0.128 |
| Abdominal subcutaneous FM | −0.565* | 0.464* | 0.538* | 0.087 |
| Abdominal visceral FM | −0.687* | 0.770* | 0.511* | 0.195 |
| Partial Correlations | ||||
| Trunk FM (adj. for Leg FM) | −0.336† | 0.484* | 0.552* | 0.048 |
| Leg FM (adj. for Trunk FM) | −0.142 | −0.148 | −0.289 | 0.028 |
p<0.05;
p=0.11;
EC50= insulin concentration needed to half-maximally suppress lipolysis; FM=fat mass (kg); GDR= insulin-mediated glucose disposal rate (mg/kg/min); SAT= subcutaneous adipose tissue.
Discussion
The primary new finding of the current study was that systemic and local SAT insulin resistance (measured in vivo) was directly correlated with abdominal (subcutaneous and visceral), but not femoral, fat mass in postmenopausal women. Whole body and abdominal, but not femoral, SAT insulin resistance were also inversely related to skeletal muscle insulin resistance. The independent association of upper body (trunk) fat with whole body and abdominal SAT insulin resistance persisted after controlling for lower body (leg) fat. In contrast, after controlling for upper body fat, the independent association of lower body fat with insulin resistance disappeared or was reversed (i.e., appeared protective).
Our data are consistent with previous studies comparing abdominal and gluteal adipocyte insulin sensitivity in vitro, but extend previous studies by measuring whole body and regional adipose tissue insulin sensitivity in vivo using microdialysis and a 3-stage hyperinsulinemic, euglycemic clamp in postmenopausal women. Previous studies in obese premenopausal women showed that abdominal adipocytes were less sensitive to the antilipolytic action of insulin than gluteal adipocytes yet insulin sensitivity in adipocytes from both regions was associated with visceral adiposity (7). Likewise, systemic antilipolytic insulin resistance was correlated with visceral adiposity in obese premenopausal women (19). Similar to previous studies we found systemic antilipolytic insulin resistance was correlated with visceral adiposity, but in contrast we found that only abdominal, not femoral, SAT insulin sensitivity was associated with visceral adiposity. Differences between studies may be attributable to the fact that our cohort was postmenopausal, our lipolysis measurements were done in vivo, or we studied femoral rather than gluteal tissue. However, our in vivo data and previous in vitro data (9) did not detect region-specific differences in SAT insulin sensitivity among postmenopausal women, so reduced insulin sensitivity in lower body fat does not appear to explain the lack of association with visceral adiposity. Our current study was also consistent with a previous study which showed a strong correlation between adipocyte insulin sensitivity in vitro and systemic fasted hyperinsulinemia among postmenopausal women (9). We extended this observation by demonstrating a strong inverse association between abdominal SAT insulin resistance and skeletal muscle insulin resistance. Our results also extend the observations made in men that abdominal SAT basal lipolysis is directly associated with increased fasted hyperinsulinemia (6). Thus, this study of postmenopausal women in vivo is consistent with and extends previous studies. Together the data suggest there may be a progressive downregulation of the ability of insulin to suppress lipolysis in the abdominal SAT depot with increasing upper body adiposity and systemic glucoregulatory insulin resistance.
Numerous studies have demonstrated elevated basal and postprandial NEFA in obese compared to lean individuals (2–5). The elevated NEFA in obesity was previously thought to be proportional to total adiposity, but a more recent review of the literature suggested basal lipolyis is not proportionally increased, and may actually be decreased, with increasing fat mass (at least in men) (6). Whether this is also true under insulin-stimulated conditions was not known, but 24-hr studies (much of which is spent in the postprandial state) suggested there may be less NEFA released per unit of fat tissue in abdominally obese, compared to lean, men (20). In support of this, our data in overweight and postmenopausal women suggest that resistance to the antilipolytic action of insulin increases directly with abdominal adiposity, resulting in disproportionately low release of NEFA. Such attenuation in adipose tissue NEFA release in abdominal obesity might be a functional adaptation that minimizes lipid mobilization in the face of increased fat availability. However, it remains unclear why such a functional adaptation would not also occur in femoral tissue.
The association between adipose tissue insulin resistance and fat mass in our study was unique to abdominal tissue; femoral adipose tissue insulin resistance was not related to fat mass. Although leg fat mass was associated with systemic glucoregulatory and antilipolytic insulin resistance, these correlations were no longer significant after adjusting for trunk fat mass. This is consistent with a recent study in middle-aged and older adults which showed a favorable association of thigh fat with systemic insulin sensitivity after accounting for differences in visceral adiposity (21). These findings were not surprising given that femoral fat mass generally appears benign with respect to disease risk even in the context of overweight and obesity (14, 22). Moreover, upper body subcutaneous adipose tissue is thought to be the main site of storage and release of fatty acids (23). Depot-specific differences may be due to differences in storage (re-esterification of fatty acids as triglyceride) instead of differences in NEFA release because antilipolytic insulin sensitivity (EC50) was similar between abdominal and femoral adipose tissue depots (99.5±42.0 vs. 102.9±35.5 pmol/L, respectively). Of note, as a percentage of lipolysis, basal whole body re-esterification rates are 3-fold higher in postmenopausal women compared to premenopausal women (24), but whether there are depot-specific differences in re-esterification among postmenopausal women to our knowledge remains unknown.
We acknowledge that the observed correlations do not infer causality. Studies are needed to determine whether improvement in abdominal SAT insulin sensitivity favorably reduces visceral fat accumulation and improves glucoregulatory insulin sensitivity. Evidence for this comes from studies of thiazolidinediones (TZDs) used to treat patients with type 2 diabetes. TZDs are peroxisome-proliferator activated receptorγ (PPARγ) agonists known to adipose tissue insulin sensitivity (suppression of lipolysis) (25). In addition to improving suppression of lipolysis, PPARγ is a master transcription regulator of adipogenesis, so it is not surprising that TZDs are known to increase subcutaneous fat (26). Importantly, these increases in subcutaneous fat are not accompanied by increases, but rather slight decreases, in visceral fat and improvements in skeletal muscle glucose uptake (26). Whether the reductions in visceral fat are causally related to changes in glucoregulatory insulin sensitivity remains unknown (27, 28), but the TZD data support the hypothesis that improving subcutaneous insulin sensitivity and fat storage reduces visceral fat and improves glucoregulatory insulin sensitivity.
It is important to note that the generalizability of our results may be limited; we studied a small group of sedentary and overweight to moderately obese, but otherwise healthy, postmenopausal women. Whether these results apply to men or younger adults cannot be determined. Our results are strengthened by the fact that these were well-controlled physiologic studies that used the reference methods to assess both whole body (multi-stage hyperinsulinemic-euglycemic clamp with isotope tracers) and tissue-specific (adipose tissue microdialysis) insulin-mediated suppression of lipolysis. With increasing obesity, adipose tissue remains relatively much more sensitive to the antilipolytic action of insulin when compared to insulin-stimulated glucose uptake in skeletal muscle. Nevertheless, our data suggest that the degree of adipose tissue insulin resistance varies markedly among overweight and obese postmenopausal women and is proportional to skeletal muscle insulin resistance and hyperinsulinemia.
In conclusion, abdominal, but not femoral, subcutaneous adipose tissue antilipolytic insulin resistance was related to abdominal (subcutaneous and visceral) fat mass and skeletal muscle glucoregulatory insulin resistance. We postulate that abdominal adipose tissue insulin resistance is a marker of adipose tissue dysfunction and may drive abdominal fat accumulation and skeletal muscle insulin resistance. Future studies are needed to determine whether improving subcutaneous adipose tissue antilipolytic insulin sensitivity reduces abdominal fat accumulation and skeletal muscle insulin resistance and to investigate the mechanisms by which this occurs.
What is already known about this subject
Systemic insulin resistance (both insulin-mediated glucose uptake and insulin suppression of lipolysis) is correlated with whole body and regional adiposity.
Basal release of fatty acids (fasting lipolysis) from the abdominal region per unit of adipose tissue is lower in obese vs. lean men and related to hyperinsulinemia.
Insulin-mediated lipolysis measured in vitro (isolated adipocytes) is correlated with visceral adiposity in premenopausal women and hyperinsulinemia in postmenopausal women.
What this study adds
Systemic and abdominal, but not femoral, adipose tissue (measured via microdialysis in vivo) antilipolytic insulin resistance is correlated with skeletal muscle glucoregulatory insulin resistance in postmenopausal women.
Local adipose tissue insulin resistance in the abdominal, but not the femoral, region is correlated with adiposity.
Acknowledgments
The authors wish to thank the staffs of the University of Colorado Anschutz Medical Campus Clinical and Translational Research Center (CTRC), Department of Radiology, and Energy Balance Core of the Nutrition and Obesity Research Unit (NORC) for their assistance in conducting this study. A special thank you goes to Robert Hickner, PhD for his assistance with the microdialysis technique and glycerol assays used in this study and to Wendee Gozansky, MD for her medical oversight of the study. The authors would also like to thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts. All authors contributed extensively to the work presented in this paper. The following awards from the National Institutes of Health supported this research: R01 AG018198, R01 DK077992, K01 AG019630, T32 DK007446, P30 DK048520, UL1 TR000154
Footnotes
Competing Interests:
None of the authors have any competing interests.
References
- 1.Frayn KN, Shadid S, Hamlani R, Humphreys SM, Clark ML, Fielding BA, et al. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol. 1994;266:E308–317. doi: 10.1152/ajpendo.1994.266.3.E308. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Walfish PG. Plasma free fatty acid concentrations in obesity. New England J Med. 1963;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 3.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–1877. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42:1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 5.Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism: Clin Exper. 1992;41:264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- 6.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 2001;280:E40–49. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 8.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 9.Fried SK, Tittelbach T, Blumenthal J, Sreenivasan U, Robey L, Yi J, et al. Resistance to the antilipolytic effect of insulin in adipocytes of African-American compared to Caucasian postmenopausal women. J Lipid Research. 2010;51:1193–1200. doi: 10.1194/jlr.P000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Pelt RE, Gozansky WS, Kohrt WM. A Novel Index of Whole Body Antilipolytic Insulin Action. Obesity (Silver Spring) 2012 doi: 10.1038/oby.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villalon KL, Gozansky WS, Van Pelt RE, Wolfe P, Jankowski CM, Schwartz RS, et al. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 2011;19:2345–2350. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14:2163–2172. doi: 10.1038/oby.2006.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankowski CM, Gozansky WS, Van Pelt RE, Schenkman ML, Wolfe P, Schwartz RS, et al. Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults. Obesity (Silver Spring) 2008;16:1039–1044. doi: 10.1038/oby.2007.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrin Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 16.Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13 C and 2 H stable isotopic tracers. Annal Biochem. 1992;205:172–178. doi: 10.1016/0003-2697(92)90595-x. [DOI] [PubMed] [Google Scholar]
- 17.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 18.Hickner RC, Fisher JS, Kohrt WM. Regional differences in interstitial glycerol concentration in subcutaneous adipose tissue of women. Am J Physiol. 1997;273:E1033–E1038. doi: 10.1152/ajpendo.1997.273.5.E1033. [DOI] [PubMed] [Google Scholar]
- 19.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E551–560. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 20.McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amati F, Pennant M, Azuma K, Dube JJ, Toledo FG, Rossi AP, et al. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 2012;20:1115–1117. doi: 10.1038/oby.2011.401. [DOI] [PubMed] [Google Scholar]
- 22.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol: Endocrinol Metab. 2002;282:E1023–1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 23.Koutsari C, Jensen MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Research. 2006;47:1643–1650. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML, Zarins Z, Fattor JA, Horning MA, Messonnier L, Lehman SL, et al. Twelve weeks of endurance training increases FFA mobilization and reesterification in postmenopausal women. J Appl Physiol. 2010;109:1573–1581. doi: 10.1152/japplphysiol.00116.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism: Clin Exper. 2003;52:753–759. doi: 10.1016/s0026-0495(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrin Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 27.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 28.Miles JM, Jensen MD. Counterpoint: visceral adiposity is not causally related to insulin resistance. Diabetes care. 2005;28:2326–2328. doi: 10.2337/diacare.28.9.2326. [DOI] [PubMed] [Google Scholar]