Abstract
Objective
Androgen excess in women is associated with visceral adiposity. However, little is known on the mechanism through which androgen promotes visceral fat accumulation.
Design and Methods
To address this issue, we exposed female mice to chronic androgen excess using 5α-dihydrotestosterone (DHT) and studied the regulation of energy homeostasis.
Results
DHT induced a leptin failure to decrease body weight associated with visceral adiposity but without alterations in leptin anorectic action. This paralleled leptin’s failure to upregulate brown adipose tissue expression of uncoupling protein-1, associated with decreased energy expenditure. DHT decreased hypothalamic proopiomelanocortin (pomc) mRNA expression and increased POMC intensity in neuronal bodies of the arcuate nucleus while simultaneously decreasing the intensity of POMC projections to the dorsomedial hypothalamus (DMH). This was associated with a failure of the melanocortin 4 receptor agonist melanotan-II to suppress body weight.
Conclusion
Taken together, these data indicate that androgen excess promotes visceral adiposity with reduced POMC neuronal innervation in the DMH, reduced energy expenditure but without hyperphagia.
Keywords: Androgen, leptin resistance, adiposity, energy expenditure, dorsomedial hypothalamus, melanocortin
Introduction
Experimental models in mammals have shown that transient developmental exposure to androgen increases visceral adiposity in adult female offspring. Prenatal androgenization increases visceral adiposity in female non-human primates (1), heifers (2) and rats (3). Neonatal exposure to testosterone increases visceral fat deposition in adult female rats (4, 5, 6). We reported that neonatal androgenization in female mice programs visceral adiposity in adults (7). This programming is associated with diminished ability of leptin to upregulate the hypothalamic arcuate nucleus (ARC) melanocortin system (8) and dysregulation of sympathetic outflow to adipose tissue (7). In women with chronic androgen excess, plasma testosterone is positively correlated with waist circumference, an index of visceral obesity (9). Nonetheless, little is known about the mechanism through which chronic androgen excess induces adiposity in females with a preferentially visceral distribution.
In the experiments described in this report, we explored the mechanism by which chronic androgen excess in females induces visceral adiposity. We focused on the hypothalamic melanortin system using female C57BL/6 mice exposed to the non-aromatizable androgen receptor agonist 5α-dihydrotestosterone (DHT). We observed that androgen excess induces leptin failure to activate brown adipose tissue (BAT) thermogenesis and suppress body weight associated with reduced energy expenditure. We also observed decreased hypothalamic proopiomelanocortin (pomc) expression and POMC neuronal innervations into dorsomedial hypothalamus (DMH). These results suggest that androgen-induced visceral fat distribution and accumulation involves alteration of the melanocortin system between the ARC and DMH.
Methods
Animals
Nine to ten week old C57BL/6 female mice were purchased from Jackson laboratory. After one week acclimation, DHT pellets (15mg/pellet, 90-day release; Innovative Research of America #NA-161) insertions or sham operations were performed. The DHT pellet dose was chosen to elevate DHT serum concentrations to levels observed in males. Experiments were performed between 3 and 8 weeks of DHT exposure, as precised in each figure. All animal experiments were approved by Northwestern University Animal Care and Use Committee (ACUC) in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
Food intake measurement
Animals were housed individually for 1 week to acclimate to the new environment. Food intake was measured daily for 1 week following accommodation. For measurement of food intake following prolonged fasting, mice were starved for 24 hours and following refeeding, food intake was measured at the indicated time points.
Serum hormone concentrations measurement
Serum leptin and adiponectin concentrations were measured by ELISA (Linco Research, Inc.).
Measurements of energy expenditure
Energy expenditure was measured by indirect calorimetry using a computer-controlled open circuit calorimetry system (PhysioScan Oxygen Consumption/Carbon Dioxide Production System; AccuScan Instruments Inc., Columbus, OH). Heat production was normalized by total body weight (Kcal/hour/kg).
Extraction and analysis of tissue NE
NE analysis were performed as previously described (7). Briefly, BAT was weighed and homogenized in iced 0.2 N perchloric acid containing 1% Na2S2O5 (by weight) and 1 mmol/l EDTA in a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) to extract the catecholamines. After isolation and elution, aliquots of the alumina eluate were injected onto a liquid chromatographic system for catecholamine analysis.
In vivo leptin stimulation (8)
Mice were separated into individual cages and left to acclimate for the 1st week. After the 2nd week of basal daily food intake measurement, i.p leptin [National Hormone and Peptide Program (NHPP)] was injected daily for 4 days (25μg/20g body weight). Food intake and body weight were measured daily as previously described (8).
Gene expression analysis by real-time quantitative PCR
BAT and hypothalami were harvested in fed mice or after 6h following i.p. injections of PBS or leptin (3μg/g body weight) in 24h-fasted mice (8). Tissues were snap-frozen in liquid N2 until RNA extraction. Gene expression was quantified in tissues by real-time q-PCR (iCycler, Bio-Rad Laboratories) and normalized to β-actin expression. Briefly, total RNA was extracted from tissue in TRIzol Reagent (Invitrogen). 1 μg of RNA was reverse-transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories) with random hexamers. Primer sequences are available upon request.
Free-floating brain IHC
Mice were fasted for 24 h and refed overnight (15–18hrs). Mice were sacrificed following cardiac perfusion with 10% formalin and brains were postfixed in 10% formalin overnight at 4°C and transferred to 30% sucrose solution. Tissues were frozen on dry ice, cut into 30 μm coronal sections on a sliding microtome, collected, and stored in antifreeze solution (50% PBS, 15% ethyl glycol, and 35% glycerol) at −20°C. Brain sections were used for free-floating immunohistochemistry. Sections were washed and pretreated in methanol containing 1% H2O2, 0.3% glycine, 0.03% SDS. Sections were blocked in 3% normal donkey serum, followed by incubation with rabbit anti-ACTH [1:100, from National Hormone and Peptide Program (NHPP)] overnight at room temperature and visualized with a secondary donkey anti-rabbit-Alexa488 (Invitrogen).
Quantification of ACTH fibers
To quantify ACTH fiber density in mouse brains we took confocal images (Leica Microsystem) from anatomically closely matched sites of the PVN and ARC. In each focused image, the gain and offset was kept constant and a stack of 75 optical sections was scanned through a volume of 10.1 μm covering the florescent signal. Further image analysis was performed with image J (NIH) and three rectangles (identical in size) were chosen per stack to measure average fluorescence intensity throughout the individual optical sections. Background signals were subtracted after ensuring that no significant difference in background signal was found between groups.
Melanotan-II (MT-II) sensitivity test
Mice were kept in individual cages for 1 week for adjustment. After 24 h fasting, half of the mice were injected with 1μg/g i.p. MT-II (Bachem) and the other half with saline. Food intake was measured at the indicated time points following the MT-II injection.
Statistical analysis
Results are presented as mean ± SEM unless otherwise stated. Data were analyzed using the unpaired Student’s t test or two-way ANOVA followed by post hoc analysis using Bonferroni test as appropriate. A value of p<0.05 was considered statistically significant.
Results
Chronic DHT exposure alters body composition
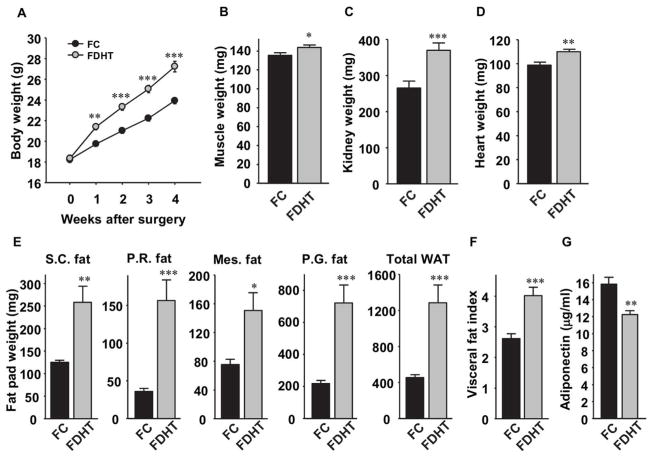
Compared to female controls (FC) chronic DHT exposure in adult female mice (FDHT) increased their body weight (Figure 1A) with corresponding increases in androgen sensitive lean mass, including calf muscle and heart muscles as well as kidney (Figure 1B–D). FDHT also showed a dramatic increase in subcutaneous and visceral fat mass (Figure 1E) with a clear and predominant visceral distribution (Figure 1F). Consistent with the development of visceral adiposity, serum levels of adiponectin were lower in FDHT (Figure 1G).
Figure 1. Chronic DHT exposure promotes visceral adiposity.
(A) Body weights were measured weekly. (B) Calf muscle, (C) Kidney and (D) Heart weights of mice from (A) at 5–6 weeks of DHT exposure. (E) Subcutaneous (SC), Perirenal (PR), Mesenteric (Mes), Perigonadal (PG) fat pad weights and total fat pad weight in mice from (A) at 5–6 weeks of DHT exposure. (F) Visceral fat index were calculated by dividing total Visceral fat pad weight over Subcutaneous fat pad weight. (G) Serum adiponectin concentration at 3 weeks of DHT exposure. Results represent the mean ± SE of. *p< 0.05; **p<0.01; ***p<0.001, FDHT vs. FC (n=13–14 mice).
Chronic DHT exposure provokes leptin failure to increase energy expenditure
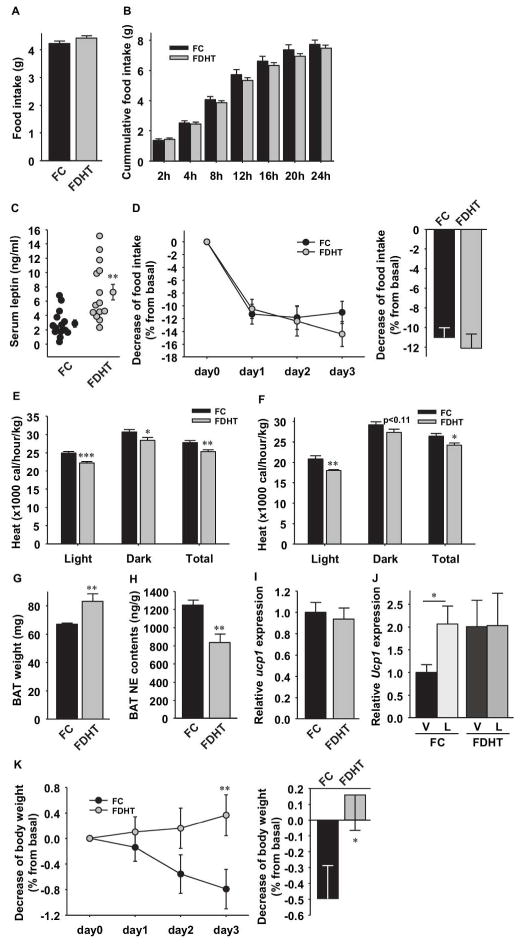
Compared to FC, FDHT did not exhibit any change in food intake in normal feeding conditions (Figure 2A) or after prolonged fasting (Figure 2B). However, consistent with their increased fat mass, FDHT exhibited hyperleptinemia suggestive of leptin resistance (Figure 2C). To investigate this issue, we performed an i.p. leptin sensitivity test. Consistent with the absence of hyperphagia, leptin decreased food intake by 11% over three days and showed similar effects in both FC and FDHT (Figure 2D). FDHT displayed decreased energy expenditure (EE) in both fed (Figure 2E) and fasted conditions (Figure 2F). BAT is the main leptin target tissue that increases EE. Surprisingly, BAT mass was increased in FDHT (Figure 2G). We thus quantified norepinephrine (NE) content in BAT. Indeed, NE is a marker of sympathetic nervous outflow in this tissue (10) and is stimulated by central leptin (11). Interestingly, BAT NE content was lower in FDHT compared to FC (Figure 2H). We then quantified the expression of the uncoupling protein 1 (Ucp1), the main regulator of thermogenesis in BAT (12). In the fed state, Ucp1 expression was similar between groups (Figure 2I). However, under fasting conditions, Ucp1 expression was higher in FDHT (Figure 2J). Expectedly, in fasted FC, leptin treatment upregulated Ucp1 expression approximately 200% (Figure 2J). In contrast, consistent with lower NE contents in BAT, in fasted FDHT, leptin failed to increase Ucp1 expression (Figure 2J). Finally and consistent these data, compared to FC, FDHT exhibited a failure of leptin to suppress body weight (Figure 2K).
Figure 2. Chronic DHT exposure reduces energy expenditure without altering food intake.
(A) Daily food intake was measured in the indicated mice (n=14–15) at 5–6 weeks of DHT exposure. (B) Cumulative food intake was measured after refeeding after 24 h fasting at 3 weeks of DHT exposure. (C) Serum leptin concentration at 3 weeks of DHT exposure. (D) Suppression of food intake was assessed daily for 3 days after i.p. leptin injection (25μg/20g BW/day). Daily values (left) and 3 days averages (right) are shown (n=19–20) at 6–7 weeks of DHT exposure. (E) Daily energy expenditure (Heat) was measured under fed and (F) fasting conditions. Data are shown during the light, dark, and total cycles (n=8) at 5–6 weeks of DHT exposure. (G) BAT weight (n=13–14), (H) BAT NE content (n=4–5) and (I) BAT Ucp1 expression measured by q-PCR in fed mice (n=9) at 5–6 weeks of DHT exposure. (J) Ucp1 expression in BAT after 24h fasting followed by 6 h of 3 μg/g i.p leptin (L) or vehicle (V) treatment (n=10–17) at 7–8 weeks of DHT exposure. (K) Reduction of body weight was assessed daily for 3 days after ip leptin injection (25μg/20g BW/day). Daily values (left) and 3 days averages (right) are shown (n=19–20) at 6–7 weeks of DHT exposure. Results represent the mean ± SE. *p< 0.05; **p<0.01; ***p<0.001, FDHT vs. FC.
Chronic DHT exposure alters POMC neurons
The arcuate nucleus (ARC) is a key hypothalamic area that has a primarily role in mediating leptin’s anorectic action. It contains first-order, leptin-responsive, anorexigenic POMC/Cocaine and amphetamine-regulated transcript (CART) neurons, as well as orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons (13). In these neurons, leptin upregulates anorexigenic Pomc/Cart and downregulates orexigenic Npy/Agrp expressions, thereby decreasing both energy intake and body weight (13). Previously, we showed that neonatal testosterone exposure in mice alters the hypothalamic melanocortin system and decreases EE in adults (8). We explored the hypothesis that decreased EE in FDHT might be due to altered melanocortin system function. The hypothalamic mRNA expression of the leptin receptor was unchanged in FDHT (FC: 1.00 +/− 0.07; FDHT: 0.93 +/− 0.05, Mean +/− SE, n = 8–9). FDHT showed decreased Pomc mRNA expression (Figure 3A) without corresponding changes in mRNA expression of the other first order neuropeptides or Melanocortin 4 receptor (Mc4r) expression (Figure 3B). We therefore examined the possibility that ARC POMC neuronal function had been altered by chronic DHT exposure. We used ACTH as an accepted marker of POMC neurons (8). In the ARC of FDHT, the overall POMC fiber intensity was decreased compared to FC (Figure 3C). However, in FDHT ARC, POMC intensity was significantly increased at the level of the cell soma (Figure 3C). Accordingly, we explored whether POMC innervations of the hypothalamic nuclei were altered. In FDHT, POMC fiber intensity was reduced in the dorsomedial hypothalamus (DMH) and these mice showed a trend toward reduction in the PVN (p=0.09) compared to FC (Figure 3D). Thus, chronic DHT exposure is associated with increased POMC content in the cell body, but decreased intensity in fibers, suggesting alterations in POMC synthesis and export.
Figure 3. Chronic DHT exposure disrupts POMC neuronal function.
Relative expressions (A) Pomc, (B) Npy, Agrp, Cart and Mc4r were measured by qPCR in whole hypothalami (n=8–9) at 5–6 weeks of DHT exposure. (C) Hypothalamic POMC neuronal fibers and cell bodies intensity of the ARC were detected by IHC after ACTH staining followed by quantification as described in Materials and Methods (n=5) at 5–6 weeks of DHT exposure. (D) Hypothalamic POMC neuronal fiber intensity in DMH and PVN were detected by IHC after ACTH staining followed by quantification as described in Materials and Methods (n=5) at 5–6 weeks of DHT exposure. Results represent the mean ± SE. *p< 0.05; **p<0.01; ***p<0.001, FDHT vs. FC.
Chronic DHT exposure alters the MC4R sensitivity
To determine whether POMC downstream targets are also affected, we tested the sensitivity of the downstream melanocortin system following i.p. injection with MT-II, the MC4R agonist. MT-II injection suppressed food intake in FC and to a lesser extent in FDHT (Figure 4A). However, while MT-II suppressed body weight in FC, the ability of MT-II to reduce body weight was lost in FDHT (Figure 4B).
Figure 4. Chronic DHT exposure alters MT-II suppression of body weight.
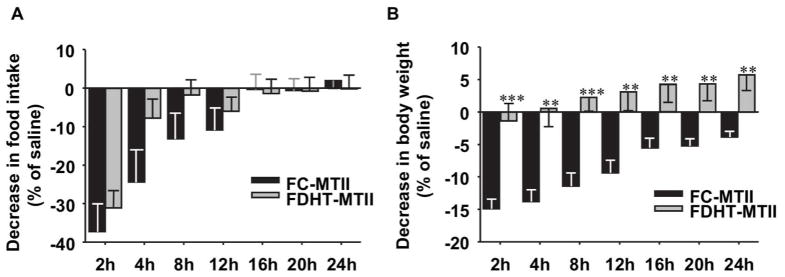
Suppression of (A) food intake and (B) body weight were measured after i.p. saline or MT-II (1 μg/g BW) injection at the indicated time points (n=6–7) at 3 weeks of DHT exposure. Results represent the mean ± SE of the % decrease following MTII injection compared to saline. *p< 0.05; **p<0.01; ***p<0.001, FDHT vs. FC.
Discussion
To examine the effect of androgen excess on the development of obesity, we used DHT, the active metabolite of testosterone that unlike testosterone cannot be aromatized into estrogen. Chronic androgen excess promotes visceral fat deposition in adult female mice. Accordingly, in male orchidectomized mice, DHT treatment increases adiposity (14). In addition, treatment of these male mice with testosterone in the presence of an aromatase inhibitor -but not testosterone alone- induced retroperitoneal fat accumulation. Together, these observations demonstrate that in both sexes, DHT is instrumental in promoting visceral fat distribution (14), probably via action on androgen receptor (AR). We observe that in FDHT, visceral obesity is associated with decreased energy expenditure. Surprisingly, in FDHT, BAT weight is increased, as is also observed in DHT-treated orchidectomized male mice (14). This could reflect an increased BAT lipid content associated with decreased BAT function in FDHT. Indeed, although BAT weight is increased in FDHT, during fasting, leptin fails to increase the expression of the thermogenic gene Ucp1 in this tissue. During fasting, Ucp1 expression is normally low in BAT due to reduced leptin levels and decreased sympathetic outflow (15, 16). Conversely, leptin increases BAT Ucp1 expression and stimulates energy expenditure, via hypothalamic sympathetic outflow (17, 18). We have used NE as a marker of sympathetic nervous outflow to BAT (7, 10). The low BAT NE content in FDHT may indirectly reflect the reduced sympathetic tone to BAT. Indeed, a disruption in sympathetic tone is observed in several rodent models of obesity, such as neonatal androgenization (7), high-fat feeding (19) and genetic leptin resistance (20). Therefore, higher Ucp1 expression during fasting along with leptin failure to further increase the expression of Ucp1 and decreased energy expenditure in FDHT suggests a disruption in communication between the central leptin signal and BAT. Thus, chronic androgen excess induces leptin’s failure to properly activate BAT Ucp1 gene expression which could decrease energy expenditure. Further studies are needed to link this leptin resistance to decreased energy expenditure.
The central regulation of energy metabolism is dependent on the activity of specialized fuel-sensing neurons within the hypothalamus; the most studied are the POMC/CART and the NPY/AgRP expressing neurons (13). Chronic androgen excess decreases Pomc mRNA expression in whole hypothalamus of FDHT mice. In addition, chronic androgen excess increases the intensity of ACTH in POMC cell bodies of the ARC while simultaneously decreasing ACTH fiber projection intensity. It has been reported that obesity can lead to the decrease of POMC post-transcriptional processing, leading to the reduction of α-melanocyte stimulating hormone, the major ARC anorexigenic peptide (21). Together, these findings suggest that POMC synthesis is decreased and/or the neuropeptide produced by its cleavage (at least ACTH) remains localized in the soma and is insufficiently exported from the ARC to other nuclei. Indeed, POMC fiber density is significantly decreased in the DMH. Interestingly, evidence suggests that leptin signaling via MC4R in the PVN controls food intake while MC4R action in other CNS sites -like the DMH- stimulate energy expenditure (22). For example, cold exposure increased Fos expression in DMH (23) suggesting that DMH is the site that regulates sympathetic nervous outflow to BAT. Importantly, Zhang et al. reported that sympathetic innervations to BAT are mediated via leptin receptor-expressing neurons in DMH -one of major POMC neuronal target site- and in medial preoptic area (mPOA) (18). Finally, Enriori et al. confirmed that leptin action in DMH increases sympathetic tone to BAT and increases thermogenesis (24). We observe that chronic DHT excess reduces POMC fiber intensity in the DMH, and that this is associated with a blunted ability of leptin to both increase BAT Ucp-1 expression. Together, these results suggest that hyperandrogenism in FDHT could alter energy expenditure via an alteration of the melanocortin pathway, in the DMH, a brain region known to be a site of leptin action. Consistent with the development of adiposity, chronic androgen excess promotes leptin resistance to control body weight. During diet-induced obesity, mice are unresponsive to the anorectic effect of leptin (25, 26). We observe that, conversely, during chronic DHT excess leptin anorectic action following i.p. injection is not affected. Instead, hyperleptinemia fails to increase energy expenditure. This interesting point is also observed in orchidectomized mice in which DHT treatment reduces energy expenditure without altering food consumption (14). POMC fiber intensity is not affected in the PVN of FDHT, a region where leptin signaling via MC4R controls food intake (22). In addition, chronic DHT excess produces a failure of MT-II, a MC4R agonist, to suppress body weight without change in MT-II ability to suppress food intake. In diet-induced obesity, the downstream melanocortin system is usually overresponsive to MT-II due to increased MC4R expression (25, 26). In contrast, MC4R expression is normal in FDHT. Thus, the inability of these anorectic compounds (leptin and MT-II) to decrease body weight could be secondary not only to a decreased POMC fibers projection but also to alterations in MC4R pathways downstream of the ARC. The primary neuronal site(s) of DHT action remains to be determined. In previous studies, only 3% of ARC POMC neurons expressed AR in rat hypothalamus (27, 28). We also observed that few POMC neurons co-expressed with AR in mice (Nohara, Mauvais-Jarvis, unpublished). Thus, we cannot eliminate an indirect effect of androgen to afferent AR-expressing neurons in a manner that indirectly alters POMC neurons. ARC and DMH receive inputs from the POA, where AR-expressing neurons have been reported (29). It is noteworthy that elevation of body temperature induced by infection depends on GABAergic neuronal activity suppression in the POA (30), highlighting the role of POA neurons on thermoregulation. Conversely, GABAergic agonist injection into DMH suppresses sympathetic outflow to BAT and reduces temperature (30). One may speculate that androgen excess could act on POA neurons, altering signaling to ARC and DMH neuronal populations.
One limitation of this study is that there is no mechanistic causality between the androgen-induced alterations in POMC neurons and the metabolic alterations in energy expenditure and adiposity detected in androgen-treated mice. Therefore, further studies are needed to determine the implications of the DMH in androgen-induced visceral fat distribution in both males and females.
In conclusion, androgen excess reduces POMC neuronal innervation in the DMH, induces leptin’s failure to decrease body weight, reduces energy expenditure and promotes visceral adiposity. These results will help better understand the central mechanisms linking androgen excess and visceral fat deposition.
What is already known about this subject?
In women with chronic androgen excess, plasma testosterone is positively correlated with visceral adipose tissue deposition.
Perinatal androgen exposure increases visceral fat deposition in adult female rodents.
The mechanism through which chronic androgen excess induces adiposity in females with a preferentially visceral distribution is unknown.
What this study adds
Chronic hyperandrogenism promotes visceral adiposity without hyperphagia.
Chronic hyperandrogenism promotes leptin failure to upregulate brown adipose tissue expression of uncoupling protein-1, associated with decreased energy expenditure.
Chronic hyperandrogenism reduces proopiomelanocortin neuronal innervation in the dorsomedial hypothalamus.
Acknowledgments
We acknowledge the University of Cincinnati, Mouse Metabolic Phenotyping Center (NIH grant DK059630). This work was supported by grants from National Institutes of Health (P50 HD044405, RO1 DK074970-01), the March of Dimes (6-FY07-678), the American Heart Association (11IRG5570010) and the American Diabetes Association (7-13-BS-101) to FMJ. Experiments represented in Fig.3C and D were supported by NIH R01-DK092587 (HM) and utilized the facilities of the Cell Biology and Bioimaging Core, supported in part by COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- AgRP
Agouti-related peptide
- AR
androgen receptor
- ARC
Arcuate nucleus
- BAT
Brown adipose tissue
- CART
Cocaine and amphetamine-regulated transcript
- DHT
dihydrotestosterone
- DMH
dorsomedial hypothalamus
- EE
Energy expenditure
- FC
Female controls
- FDHT
Female mice with DHT exposure
- IHC
immunohistochemistry
- MC4R
melanocortin 4 receptor
- NPY
Neuropeptide Y
- NE
norepinephrine
- POMC
proopiomelanocortin
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Author contributions: KN, HM, FMJ conceived, designed experiments. KN and AL carried out experiments, KN, CA, HM and FMJ analyzed data. KN, CA and FM-J wrote the paper. All authors were involved in writing the paper and had final approval of the submitted and published versions.
CONFLICT OF INTEREST STATEMENT
Dr. Mauvais-Jarvis received research support from Pfizer, Inc.
References
- 1.Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11:279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- 2.Reiling BA, Drackley JK, Grum LR, Berger LL. Effects of prenatal androgenization and lactation on adipose tissue metabolism in finishing single-calf heifers. J Anim Sci. 1997;75:1504–1513. doi: 10.2527/1997.7561504x. [DOI] [PubMed] [Google Scholar]
- 3.Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:E262–268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson C, Niklasson M, Eriksson E, Bjorntorp P, Holmang A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J Clin Invest. 1998;101:74–78. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 6.Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lonn M, et al. Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: comparisons with estradiol and dihydrotestosterone. Endocrinology. 2007;148:5369–5376. doi: 10.1210/en.2007-0305. [DOI] [PubMed] [Google Scholar]
- 7.Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, et al. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013;304:E1321–E1330. doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, et al. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152:1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DJ, Barth JH, Burke CW. Body fat topography in women with androgen excess. Int J Obes. 1988;12:157–162. [PubMed] [Google Scholar]
- 10.Morrison SF, Ramamurthy S, Young JB. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J Neurosci. 2000;20:9264–9271. doi: 10.1523/JNEUROSCI.20-24-09264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature. 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Moverare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, Swanson C, et al. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 2006;14:662–672. doi: 10.1038/oby.2006.75. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell NJ, Saville ME, Stock MJ. Brown fat activity in fasted and refed rats. Biosci Rep. 1984;4:351–357. doi: 10.1007/BF01140499. [DOI] [PubMed] [Google Scholar]
- 16.Champigny O, Ricquier D. Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue: evidence for diet-induced and cold-induced responses. J Nutr. 1990;120:1730–1736. doi: 10.1093/jn/120.12.1730. [DOI] [PubMed] [Google Scholar]
- 17.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226–230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor- expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C. Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol Endocrinol Metab. 2005;288:E148–154. doi: 10.1152/ajpendo.00225.2004. [DOI] [PubMed] [Google Scholar]
- 20.Blouquit MF, Geloen A, Koubi H, Edwards D, Gripois D. Decreased norepinephrine turnover rate in the brown adipose tissue of pre-obese fa/fa Zucker rats. J Dev Physiol. 1993;19:247–251. [PubMed] [Google Scholar]
- 21.Cakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC, et al. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem. 2013;288:17675–17688. doi: 10.1074/jbc.M113.475343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 24.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 27.Fodor M, Delemarre-van de Waal HA. Are POMC neurons targets for sex steroids in the arcuate nucleus of the rat? Neuroreport. 2001;12:3989–3991. doi: 10.1097/00001756-200112210-00027. [DOI] [PubMed] [Google Scholar]
- 28.Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]