Abstract
Heart failure is associated with pump dysfunction and remodeling but it is not yet known if the condition affects different transmural regions of the heart in the same way. We tested the hypotheses that the left ventricles of non-failing human hearts exhibit transmural heterogeneity of cellular level contractile properties, and that heart failure produces transmural region-specific changes in contractile function.
Permeabilized samples were prepared from the sub-epicardial, mid-myocardial, and sub-endocardial regions of the left ventricular free wall of non-failing (n=6) and failing (n=10) human hearts. Power, an in vitro index of systolic function, was higher in non-failing mid-myocardial samples (0.59±0.06 μW mg−1) than in samples from the sub-epicardium (p=0.021) and the sub-endocardium (p=0.015). Non-failing mid-myocardial samples also produced more isometric force (14.3±1.33 kN m−2) than samples from the sub-epicardium (p=0.008) and the sub-endocardium (p=0.026). Heart failure reduced power (p=0.009) and force (p=0.042) but affected the mid-myocardium more than the other transmural regions. Fibrosis increased with heart failure (p=0.021) and mid-myocardial tissue from failing hearts contained more collagen than matched sub-epicardial (p<0.001) and sub-endocardial (p=0.043) samples. Power output was correlated with the relative content of actin and troponin I, and was also statistically linked to the relative content and phosphorylation of desmin and myosin light chain- 1. Non-failing human hearts exhibit transmural heterogeneity of contractile properties. In failing organs, region-specific fibrosis produces the greatest contractile deficits in the mid-myocardium. Targeting fibrosis and sarcomeric proteins in the mid-myocardium may be particularly effective therapies for heart failure.
Keywords: Left ventricular function, Mechanics, Collagen, Sarcomere, Myofilament Protein
Introduction
Clinical studies have shown that transmural variation in the contractile function of human myocardium may influence clinical end-points [1, 2]. For example, a study by Wachtell et al. [2] followed 840 patients for 3,914 patient-years and showed that shortening of the middle transmural region of the left ventricular free wall is a better predictor of cardiovascular morbidity than the shortening of other regions, or ejection fraction. However, the mechanisms that underlie these results, which may include transmural variation in cellular and molecular properties, are not well understood.
Previous studies using human hearts have demonstrated that action potentials and Ca2+ transients vary systematically across the left ventricular free well, and that these effects probably reflect transmural-region specific expression of ion channels and ionic transporters [3, 4]. Less is currently known about potential transmural variation in contractile function. Therefore, the goals of this study were to test the hypotheses that human hearts exhibit transmural heterogeneity of cellular level contractile properties, and that heart failure produces transmural region-specific changes in contractile function.
The results showed that cellular level indices of systolic function (power output and isometric force) exhibit transmural heterogeneity in non-failing human hearts. Specifically, samples from the mid-myocardium developed more power and more force than samples from the sub-epicardial and sub-endocardial regions. Heart failure reduced power and force by ~20% and affected mid-myocardial samples more than tissue from the other regions. As a result, failing hearts exhibited less transmural heterogeneity. Additional data from histological and biochemical assays suggested that these contractile changes reflect a region-specific increase in fibrosis and modifications to the content and phosphorylation status of sarcomeric proteins including cardiac troponin I (cTnI), desmin and myosin light chain-1 (MLC-1).
Methods
A detailed Materials and Methods section is provided in the online supplement.
Samples were obtained from patients undergoing heart transplants at the University of Kentucky and from organ donors who did not have heart failure (Table 1). Hearts were passed to a researcher as soon as they were excised from the body. All samples used in this work were obtained from through-wall sections cut from a similar region of the distal anterior left ventricular free wall approximately 1 inch above the apex. These sections were then split transmurally into three parts of equal thickness forming the sub-epicardial, mid-myocardial, and sub-endocardial specimens. The specimens were snap frozen in liquid nitrogen and stored at −150°C. All procedures were approved by the University of Kentucky Institutional Review Board and subjects gave informed consent.
Table 1. Clinical characteristics.
| A. Patients with heart failure | |||
|---|---|---|---|
|
| |||
| Sex | Age (y) | Cardiomyopathy | Medications |
| F | 49 | Non ischemic, idiopathic | Diuretic, Inotropes, Digoxin |
| M | 65 | Ischemic | Diuretic, ACE inhibitor/ARB, β-blockers, Inotropes, Digoxin, Statin, Insulin |
| M | 63 | Ischemic | Diuretic, ACE inhibitor/ARB, β-blockers, Statin |
| M | 49 | Non ischemic, idiopathic | Diuretics, Inotropes |
| M | 39 | Non ischemic, idiopathic | Inotropes, Statin |
| M | 64 | Ischemic | Diuretics, β-blockers, Inotropes, digoxin |
| M | 61 | Ischemic | Diuretic, ACE inhibitor/ARB, Inotropes, Statin - |
| F | 23 | Non ischemic, postpartum | Diuretic, ACE inhibitor/ARB, β-blockers, Inotropes |
| M | 20 | Non ischemic, idiopathic | Diuretic, ACE inhibitor/ARB, Digoxin |
| M | 53 | Ischemic | Diuretic, β-blockers, Inotropes, Digoxin |
|
| |||
| B. Donors with non-failing hearts | |||
|
| |||
| Sex | Age (y) | Cause of death | |
|
| |||
| F | 31 | Stroke | |
| M | 59 | Stroke | |
| F | 18 | Head Trauma | |
| F | 38 | Stroke | |
| M | 28 | Head Trauma | |
| M | 33 | Head Trauma | |
2.1. Permeabilized Myocardial Samples and Functional Measurements
Chemically permeabilized myocardial preparations (Figure S1) were attached between a force transducer and a servo motor as previously described [5, 6]. A total of 141 multicellular preparations from 48 samples (3 transmural regions from each of 6 non-failing and 10 failing hearts) were analyzed in this work.
2.2. Biochemical Assays and Histology
Biochemical assays were performed using chemically permeabilized myocardial samples. The content and phosphorylation status of key sarcomeric proteins were evaluated using SDS-PAGE and western blots. Relative collagen content was evaluated by picrosirius red staining and bright field imaging [7].
2.3. Statistical Analysis
The experimental data were analyzed in SAS 9.1.3 (SAS Institute, Cary, NC) using linear mixed effects models incorporating 2 main effects (condition and transmural region) and their interaction. This approach matches data from different regions of the same hearts and provides more statistical power than repeated measures ANOVA tests when multiple samples are analyzed from each region. Compound symmetry was assumed for the covariance structure and post-hoc analyses were performed using Tukey-Kramer corrections. Linear regression tests were performed in MATLAB (Mathworks, Natick, MA). P values less than 0.05 were considered significant. Data are reported as mean±SEM.
Results
3.1. Cellular Level Power Output
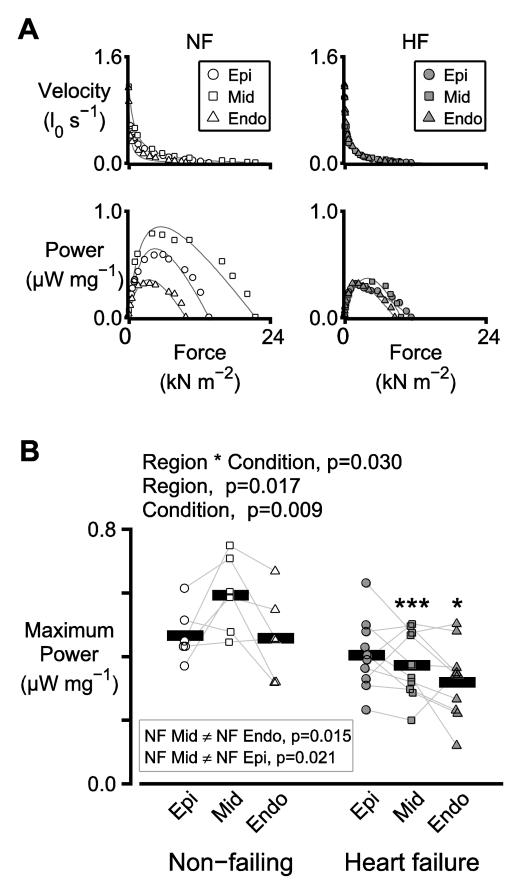
Maximum power output, an in vitro index of systolic function, was measured using multicellular permeabilized preparations. Figure 1A shows force-velocity and force-power curves measured using preparations obtained from the sub-epicardial, mid-myocardial and sub-endocardial regions of one non-failing and one failing heart.
Figure 1. Transmural heterogeneity of power output is reduced in heart failure.
A) Representative force-velocity and force-power curves measured using individual preparations from the sub-epicardial, mid-myocardial, and sub-endocardial regions of the left ventricle of one non-failing (NF, left) and one failing (HF, right) heart. B) Symbols show the mean of the power outputs measured from 2 or 3 preparations from each region for each heart. In this figure, and in all similar panels, thin lines join data points from the same heart. Thick bars show the mean data for the region. The text above the plot shows p values for the main statistical effects. Significant differences between regions, tested separately for non-failing and failing hearts, are listed in the inset box. Significant differences due to heart failure, tested separately for each region, are indicated by asterisks (* p<0.05, ** p<0.01, *** p<0.001).
Figure 1B shows maximum power output for the 6 non-failing and 10 failing hearts. There was a significant interaction between region and heart failure condition (p=0.030), which implies that heart failure status affects the sub-epicardial, mid-myocardial, and sub-endocardial regions in different ways. The post-hoc tests revealed that, in the non-failing samples, the maximum power output of the mid-myocardial preparations was greater than that of the sub-epicardial (p=0.021) and the sub-endocardial preparations (p=0.015). No significant transmural differences were observed in the heart failure samples. Heart failure thus lowered the power output of mid-myocardial samples more than it lowered the power output of sub-epicardial and sub-endocardial preparations.
3.2. Cellular Level Isometric Force
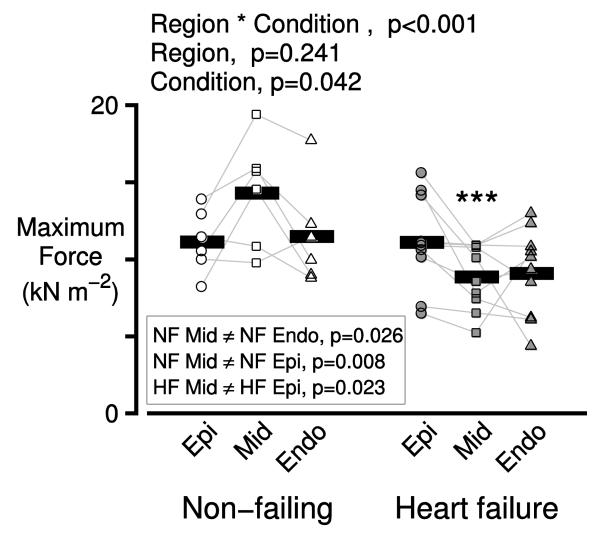
Figure 2 shows that isometric force also exhibited transmural variation in non-failing hearts and that the transmural pattern of variation was altered by heart failure (interaction term, p<0.001). Since power is the product of force and velocity, these data suggest that the transmural variation in power output primarily reflects changes in force-generating capacity rather than changes in shortening velocity (which did not exhibit significant differences, Figure S2). Post-hoc analysis showed that in non-failing hearts, the maximum force generated by samples from the mid-myocardium (14.3 ± 1.33 kN m −2) was higher than the force generated by the sub-epicardial (10.96 ± 0.74 kN m−2, p=0.008) and the sub-endocardial (11.47 ± 0.86 kN m−2, p=0.026) preparations. The samples from the failing hearts also exhibited heterogeneity, with the mid-myocardial preparations generating the least amount of force (8.80 ± 0.09 kN m−2). Heart failure thus lowered the force generating capacity of the mid-myocardial preparations more than that of the sub-epicardial and sub-endocardial samples.
Figure 2. Transmural heterogeneity of isometric force is reduced in heart failure.
Symbols show the mean of the maximum isometric forces measured from 2 or 3 preparations from each region for each heart.
3.3. Cellular Level Calcium Sensitivity
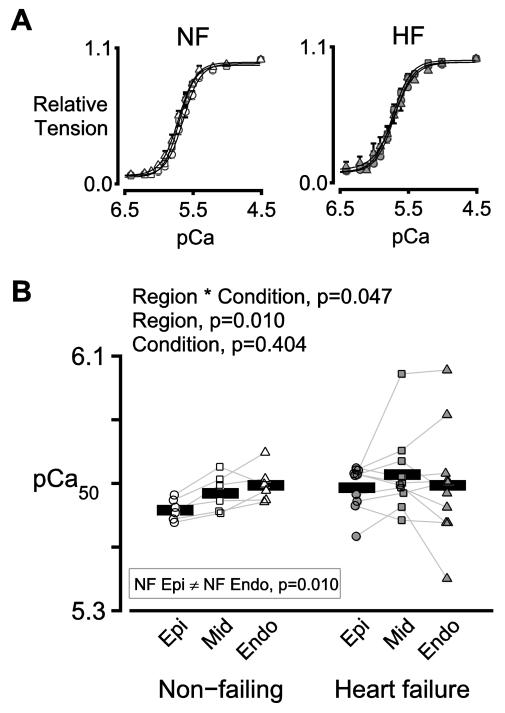
Figure 3A shows normalized isometric tension plotted as a function of the activating Ca2+ concentration (where pCa = −log [Ca2+ 10 ]) for preparations from the three transmural regions of non-failing (left panel) and failing (right panel) hearts. In the non-failing samples, Ca2+ sensitivity (assessed as the Ca2+ concentration required to produce half the maximum force) was 0.075 pCa units greater in the sub-endocardial samples than in the sub-epicardial samples (p=0.010, Figure 3). No transmural differences were observed in the failing samples. The progressive transmural change in calcium sensitivity that occurs in non-failing hearts is thus disrupted by heart failure (interaction term, p=0.047).
Figure 3. Transmural variation in Ca2+ sensitivity is disrupted in heart failure.
A) Mean values of normalized isometric tension plotted against pCa (= −log10[Ca2+]) for preparations from the three transmural regions of non-failing (left) and failing (right) hearts. Circles, squares, and triangles show data for sub-epicardial, mid-myocardial, and sub-endocardial preparations respectively. B) Symbols show the mean of the pCa50 values measured using 2 or 3 preparations from each region for each heart.
3.4. Collagen Content
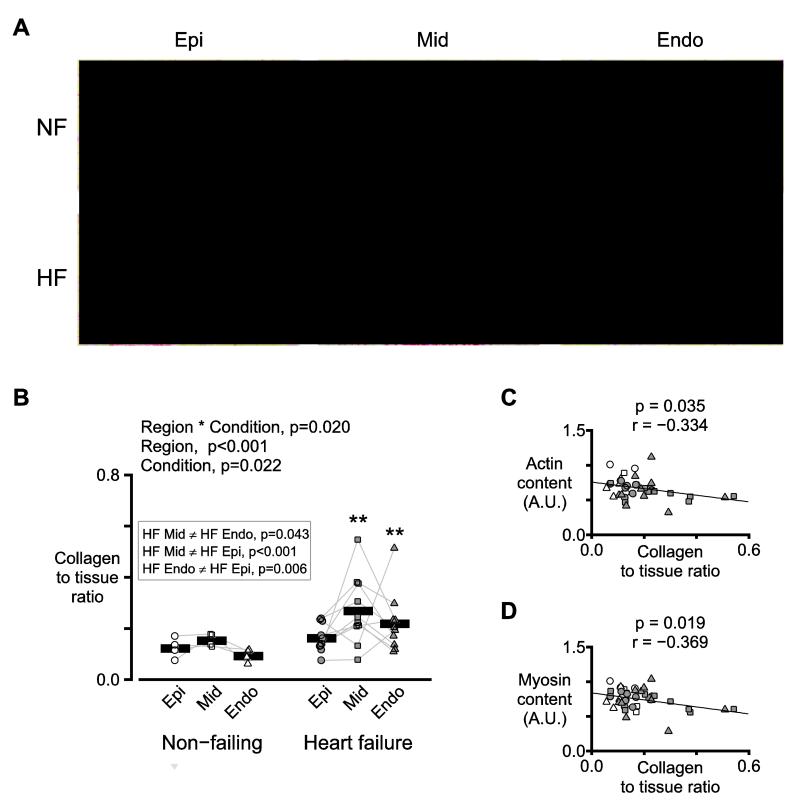
Figure 4A shows representative images of myocardial sections that had been stained with picrosirius red. Quantification showed that there was a significant interaction (p=0.020), which implied that heart failure affected the regions differentially. Furthermore, post-hoc tests revealed that, in the failing samples, the collagen to tissue ratio in the mid-myocardial sections was greater than that in the sub-epicardial (p<0.001) and sub-endocardial (p=0.043) sections. Additional experiments showed that the collagen to tissue ratio in the stained sections was negatively correlated with the relative contents of actin and myosin (Figure 4C-D) in wet tissue samples from the same biospecimens.
Figure 4. Collagen content is elevated in heart failure and is greatest in mid-myocardial tissue.
A) Representative images of myocardial sections from one non-failing and one failing heart stained with picrosirius red. Red staining indicates collagen. Yellow staining shows non-fibrotic tissue. B) Symbols show the mean collagen to tissue ratio measured from 1-6 sections from each region for each heart. C) Actin and D) myosin contents (determined by Lowry protein assays and gel electrophoresis) plotted against the collagen to tissue ratios from panel B. A.U. stands for arbitrary units. Symbols follow the same shape and color conventions as in panel B. The plots also show the best-fit straight lines determined by linear regression and the corresponding p and r values.
3.5. Sarcomeric Proteins
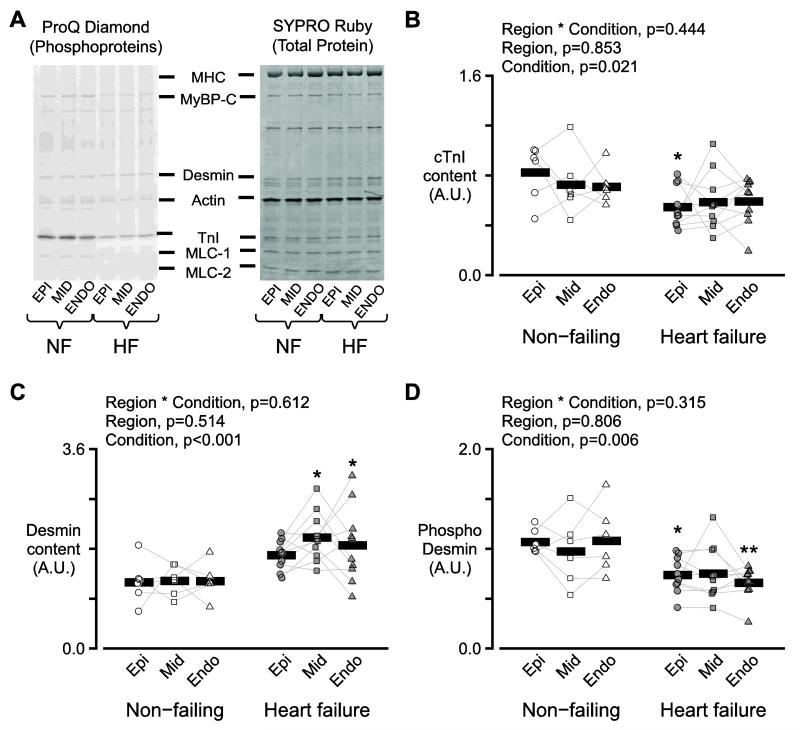
Potential changes in the isoform content and posttranslational status of sarcomeric proteins were investigated using gel electrophoresis and western blots (Figure 5, Supplementary Tables S1-S3, Figures S3-S5). The key findings were that heart failure status decreased the relative content of cardiac cTnI (p=0.021, Figure 5A, B), increased the content of desmin protein (p<0.001, Figure 5A, C), reduced desmin’s relative level of phosphorylation (p=0.006, Figure 5A, D), and increased the relative content of N2BA isoform of titin (p=0.047, Figures S3A, B).
Figure 5. Analysis of selected muscle proteins.
A) Two images of a single representative gel stained with Pro-Q Diamond (left) and SYPRO Ruby (right). The Pro-Q Diamond stain is more sensitive to phosphoproteins while SYPRO Ruby indicates total protein content. B) Symbols show the relative content of cTnI for each region for each heart. A.U. stands for arbitrary units. C) As for panel B but showing the relative content of desmin. D) As for panel B but showing the relative content of phosphorylated desmin.
3.6. Molecular Mechanisms Influencing Contractile Function
Data from human studies normally exhibit more within-group variability than results from animal-based experiments. For example, in this work, the power output of mid-myocardial preparations from non-failing hearts ranged from 0.443 to 0.747 μW mg−1, a span of 51%. Linear regression tests were used to test whether some of the variance in the functional measurements can be attributed to changes in the content and posttranslational status of sarcomeric proteins.
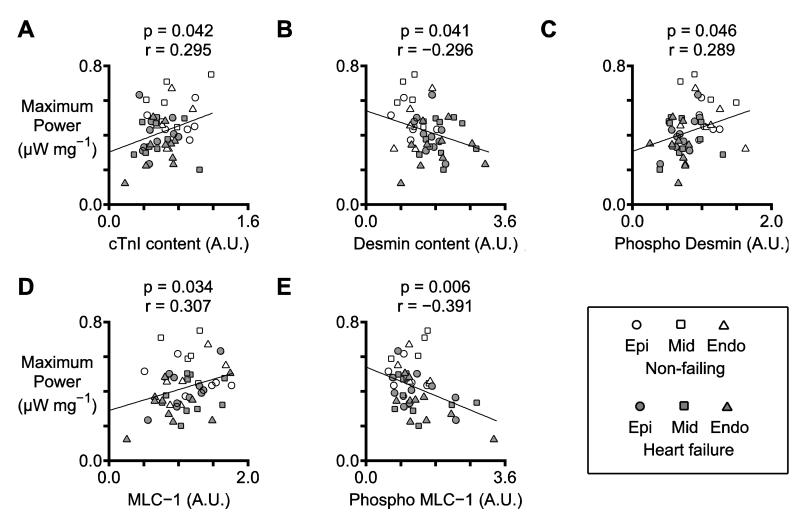
Figure 6A shows that power increased with the relative content of cTnI (p=0.042). Figure 6B-C show that power decreased with the relative content of desmin (p=0.041) but increased with its relative phosphorylation (p=0.046). Finally, Figure 6D-E show that power increased with the relative content of MLC-1 (p=0.034) but decreased with the molecule’s relative phosphorylation (p=0.006).
Figure 6. Statistically significant relationships between maximum power and biochemical data.
Each panel shows the relationship between maximum power and a selected biochemical property (for example, cTnI content). The y coordinate of each symbol indicates the mean value of maximum power measured from 2 or 3 preparations from each region for each heart. The x coordinate of each symbol shows the result of the biochemical assay performed using the matching sample. The plots also show the best-fit straight lines determined by linear regression and the corresponding p and r values. Table S4 provides p and r values for additional relationships that had p values less than 0.05.
Discussion
Clinicians already recognize the significance of transmural variation in contractile function. For example, imaging studies have shown that shortening of the middle transmural region is a better predictor of cardiovascular death than traditional measures of global ventricular function such as ejection fraction [1, 2]. The American Society of Echocardiography also notes in its guidelines that “Contraction of muscle fibers in the LV midwall may better reflect intrinsic contractility than contraction of fibers at the endocardium” [8]. The current work reveals four new cellular and molecular level results that may help to explain these clinically-relevant observations [1, 2].
First, non-failing human hearts exhibit transmural heterogeneity of contractile properties (Figures 1-3). Second, heart failure depresses power and force and has its biggest negative impact on mid-myocardial tissue. Failing hearts thus exhibit less transmural heterogeneity in cellular level contraction than non-failing hearts (Figures 1-2). Third, human heart failure produces more fibrosis in mid-myocardial tissue than it does in sub-epicardial and sub-endocardial samples (Figure 4). This may explain why heart failure produces the greatest contractile deficits in the mid-myocardium as the additional connective tissue is likely to displace functioning myocytes. Fourth, power, force and calcium sensitivity correlate with the relative content and relative phosphorylation of key sarcomeric proteins (Figure 6, Table S4). Therefore, therapies targeting these proteins may be particularly effective treatments for human heart failure.
4.1. Region-Specific Modifications in Systolic Function
During systole, the heart contracts against its afterload to eject blood from the ventricle. This can be mimicked in vitro by allowing permeabilized myocardial preparations to shorten against a controlled force [9] (Figure S13). The power output for each load is calculated by multiplying the force that the muscle generates by its shortening velocity. Figure 1 shows that the maximum power output exhibited significant transmural variation in non-failing hearts and was reduced by ~25% in failing organs (p=0.009). Further analysis suggests that these effects primarily reflect altered force generation (Figure 2) since maximum shortening velocity was not influenced by heart failure status or transmural region (Figure S2).
No previous studies have used human samples to assess transmural variation in power output. However, a few studies have measured the effect of heart failure on isometric force generation, with potentially conflicting results. For example, separate studies using epicardial biopsies [10] and samples from unspecified regions of the heart [11] have shown that isometric force is reduced by ~80% in patients with heart failure. Other works, using samples from unspecified regions of the left ventricle, suggests that there is no significant effect of heart failure on maximum isometric force [12-14]. In the present experiments, heart failure reduced the average force generated by the experimental preparations (Figure 2, p=0.042). However, post-hoc analysis of the different transmural regions showed that the effect was only significant for the mid-myocardial samples (p<0.001) and that heart failure did not significantly reduce the force generated by the sub-epicardial (p=0.935) or the sub-endocardial (p=0.120) preparations. These results imply that heart failure influences force generation in a transmural specific manner in humans. Researchers developing future studies in this area may therefore wish to consider including transmural region as an independent grouping factor when they design their experiments.
4.2. Failing Hearts have Increased Mid-Myocardial Fibrosis
Increased fibrosis was an important factor in these experiments. Figure 4A-B show that heart failure increased the relative content of collagen in the myocardial sections, and that the mid-myocardial samples of the failing hearts contained more collagen than the matching sub-epicardial (p<0.001) and sub-endocardial (p=0.043) sections. Increased collagen was also associated with lower relative contents of actin and myosin (Figure 4C-D). Additional analyses showed that force (p=0.325, Figure S6A) and maximum power p=0.012, Figure S6B) were correlated with actin content. Together these data support the hypothesis that in human heart failure, increased fibrosis depresses contractile function by replacing myocytes with extracellular matrix proteins.
The increased fibrosis might have been expected to raise passive force in the samples from the failing hearts. However, linear mixed model analyses showed that neither passive force (Figure S7A) nor passive stiffness (Figure S7B) at a sarcomere length of 2.2 μm were influenced by heart failure status or transmural region (all main statistical effects > 0.05). This probably reflects a compensatory effect of titin isoform expression. Consistent with previous studies [15, 16], samples from failing hearts had a greater relative content of the large N2BA isoform of titin (p=0.047, Figure S3B). This isoform shift tends to decrease passive stiffness and seems to counteract the mechanical effect of increased fibrosis in the samples from the failing hearts.
Titin phosphorylation has also been show to change passive stiffness [17]. Although phosphorylation of N2BA isoform of titin did not change with heart failure (condition p=0.825, Figure S3C), the experimental data revealed a novel transmural heterogeneity. Sub-endocardial samples from failing hearts had greater levels of phosphorylation than samples from the other regions (p=0.037 versus mid-myocardium, p=0.006 versus sub-epicardium, Figure S3C).
4.3. Region-specific Modification in Ca2+ sensitivity
In vivo function may be particularly sensitive to changes in Ca2+ sensitivity (defined as the free Ca2+ concentration required to generate half-maximum force) since myocytes are not maximally activated during a normal heart-beat. According to a recent animal study, transmural variation in Ca2+ sensitivity may also modulate ventricular torsion [18]. In the present experiments (Figure 3) non-failing samples exhibited a statistically significant increase in Ca2+ sensitivity (p=0.010, ΔpCa50=0.075) going from the sub-epicardial to the sub-endocardial preparations. However, samples from failing hearts did not show a significant transmural trend.
Two previous studies using human myocytes isolated from unspecified ventricular locations have shown that Ca2+ sensitivity increases with heart failure [19, 20]. Another study [11] documents no change. Again, similar to the previous discussion relating to isometric force generation, it is difficult to interpret these data without more information about the transmural source of the sample [11, 19, 20].
Two additional projects have used animal tissues to assess potential transmural changes in Ca2+ sensitivity in pigs [21] and rats [22]. Both studies showed that Ca2+ sensitivity increased from the epicardium to the endocardium in healthy hearts, similar to the present work. The rodent study also showed that transmural variation in Ca2+ sensitivity was eliminated [22] 14 weeks after a myocardial infarction which is, again, broadly consistent with the current data from failing hearts (Figure 3).
Modulation of Ca2+ sensitivity is complex. The current data suggest that one molecular mechanism that may modulate the transmural variation in pCa50 in non-failing hearts (Figure 3) is phosphorylation of cMyBP-C. Post-hoc tests showed that Ser302 phosphorylation in non-failing epicardial samples was greater than in non-failing mid-myocardial tissue (p=0.047, Figure S4A-B). Regression analysis showed that pCa50 values for the non-failing preparations were negatively correlated with both total cMyBP-C phosphorylation (p=0.002, Figure S8A) and Ser302 phosphorylation (p=0.045, Figure S8B). The former finding reinforces data from a previous study using myocardial samples from unspecified regions of human hearts [14] but the new link between phosphorylation of cMyBP-C at Ser302 and pCa50 is a novel finding.
Other groups have shown that cTnI phosphorylation can also influence Ca2+ sensitivity [12, 23]. In this study, site-specific cTnI phosphorylation was reduced in heart failure samples at Ser23/24 (Figure S5, p=0.012) but there was not a significant relationship between this parameter and pCa50 (r=0.034, p=0.850, data not shown). Total cTnI phosphorylation was not correlated with pCa50 either (r=−0.011, p=0.942, data not shown). cTnI undergoes many complex posttranslational modifications – the human cardiac isoform has 14 phosphorylation sites [24]. It is therefore possible that more detailed analysis might have revealed significant relationships. For example, phosphorylation of cTnI at Ser149 has been shown to increase Ca2+ sensitivity [25] but this modification was not investigated in this study.
Another posttranslational modification that has been linked to changes in myocardial Ca2+ sensitivity is phosphorylation of MLC-2. Davis et al. [26] have reported that MLC-2 phosphorylation is greater in sub-epicardial than in sub-endocardial tissue. Data from rat trabeculae [27] suggest that this phosphorylation gradient would increase Ca2+ sensitivity in epicardial tissue. However, the situation is complex, because van der Velden et al. [28] have shown that increased phosphorylation of MLC-2 decreases pCa50 values. Unfortunately, MLC-2 phosphorylation was not studied in this work.
4.4. Proteins that Influence Power Output
The transmural heterogeneity of myocardial power output and its modification by heart failure are the key findings of this study. However, it was not possible to identify a specific sarcomeric protein that explains the functional changes.
The content of intact cTnI (Figure 5B) was reduced in biospecimens from failing hearts (p=0.021) but did not exhibit transmural variation (interaction term, p=0.853). Further analysis of these data showed that cTnI content was lower in patients with ischemic heart failure than in patients with non ischemic disease (p=0.029, Figure S9). This is consistent with a study by McDonough et al. which showed that cTnI is degraded in human hearts after ischemic injury [29]. Degradation of cTnI inhibits contractility in mice [30] and the current data show that this mechanism probably impacts human myocardial function too. Power output (Figure 6A) and force (Table S4) both correlated with the relative cTnI content, indicating that proteolyis of cTnI may be another impact factor in human disease [30].
Desmin, a structural protein that links myofibirils at Z-disks and maybe involved in force transmission [31], has been proposed as a potential biomarker for heart failure [32, 33]. Pawlak et al. [33] had previously shown that desmin content is increased in endocardial biopsies from patients with heart failure and this result was confirmed in the present work (Figure 5C). There was also a decrease in the total phosphorylation of desmin in samples from failing hearts (Figure 5D) [34]. Although desmin content did not vary with transmural region (p=0.514), the regression tests summarized in Figure 6B-C show that changes to the content and phosphorylation of this protein may modulate power output.
Similarly, power increased linearly with MLC-1 content (p=0.034, Figure 6D) and decreased with MLC-1’s relative phosphorylation (p=0.006, Figure 6E). Since MLC-1 binds to the lever arm of the myosin head near the convertor domain, it is ideally positioned to influence force generation and/or kinetics. For example, previous work by Morano et al. [35] and new data in Table S4 show that MLC-1 phosphorylation decreases Vmax. There are only a few studies describing MLC-1 phosphorylation but phosphorylation sites corresponding to the human isoform are known as Thr64 and Ser194/195 [36]. Intriguingly, phosphorylation of MLC-1 has recently been shown to increase the molecule’s degradation by matrix metalloproteinase-2 [37-39]. When combined with these previous findings, the new data presented in this manuscript show that MLC-1 modulates power output in human myocardium, and that loss of MLC-1 (perhaps following its phosphorylation) decreases contractility.
To the authors’ knowledge, this is the first study to identify significant correlations between the power output of human myocardium and modifications to the content and phosphorylation status of cTnI, desmin and MLC-1.
4.5. Limitations
Although the logistical difficulty of obtaining the samples meant that it was not possible to match the ages of the donors and patients (Table 1), regression analyses showed that the key contractile properties reported in this work did not vary with Age (Figure S10). It is also possible that ischemic and non ischemic heart failure produce distinct changes in contractile function (Tables S5-S10 and Figures S9 and S11). Due to the small sample size (n=5 in both heart failure groups), tests comparing ischemic and non-ischemic samples had limited statistical power.
4.6. Conclusion
This is the first study to show that (1) the contractile properties of non-failing human hearts exhibit transmural variation, and that (2) heart failure alters the transmural pattern as well as depressing overall function. These new results help to explain why in vivo shortening of the middle transmural region is a better predictor of cardiovascular death than traditional global measures of ventricular function.
Supplementary Material
Highlights.
Non-failing human hearts exhibit transmural heterogeneity of contractile properties.
Non-failing mid-myocardium develops more power than the sub-epi & subendocardium.
The transmural pattern of power is disrupted in failing human hearts.
Increased fibrosis decreases the power output of failing mid-myocardial tissue.
cTnI, desmin, and MLC-1 modulate power output in human hearts.
Acknowledgements
We thank Michael Reid, PhD for helping to establish the tissue bank, Shawn Stasko, PhD and Leonardo Ferreira, PhD for acquiring biospecimens, Parvathi Natraj BS and Eric Reid, MD for analyzing medical records, and Christopher Fry, PhD, Cheavar Blair, BS, Lin Yang, PhD and Hai Su, MS for assistance with histology and imaging.
Sources of Funding
Supported by NIH HL090749 to KSC, NIH TR000117, and the University of Kentucky Research Challenge Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current Affiliations
MIM:Markey Cancer Center, University of Kentucky, Lexington, KY
SGC:Yale School of Engineering and Applied Science, New Haven, CT
MRB:University of Toledo Medical Center, Toledo, OH
Disclosures
None.
References
- [1].de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–65. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
- [2].Wachtell K, Gerdts E, Palmieri V, Olsen MH, Nieminen MS, Papademetriou V, et al. In-treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: the Losartan Intervention For Endpoint reduction study. Journal of hypertension. 2010;28:1541–6. doi: 10.1097/HJH.0b013e328339f943. [DOI] [PubMed] [Google Scholar]
- [3].Soltysinska E, Olesen SP, Christ T, Wettwer E, Varro A, Grunnet M, et al. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Archiv : European journal of physiology. 2009;459:11–23. doi: 10.1007/s00424-009-0718-3. [DOI] [PubMed] [Google Scholar]
- [4].Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation. 2011;123:1881–90. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moss RL. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. The Journal of physiology. 1979;292:177–92. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mitov MI, Holbrook AM, Campbell KS. Myocardial short-range force responses increase with age in F344 rats. Journal of molecular and cellular cardiology. 2009;46:39–46. doi: 10.1016/j.yjmcc.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bruckner BA, Razeghi P, Stetson S, Thompson L, Lafuente J, Entman M, et al. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2004;23:36–42. doi: 10.1016/s1053-2498(03)00103-7. [DOI] [PubMed] [Google Scholar]
- [8].Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [9].McDonald KS, Wolff MR, Moss RL. Force-velocity and power-load curves in rat skinned cardiac myocytes. The Journal of physiology. 1998;511(Pt 2):519–31. doi: 10.1111/j.1469-7793.1998.519bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Velden J, Klein LJ, van der Bijl M, Huybregts MA, Stooker W, Witkop J, et al. Isometric tension development and its calcium sensitivity in skinned myocyte-sized preparations from different regions of the human heart. Cardiovascular research. 1999;42:706–19. doi: 10.1016/s0008-6363(98)00337-x. [DOI] [PubMed] [Google Scholar]
- [11].Ambardekar AV, Walker JS, Walker LA, Cleveland JC, Jr., Lowes BD, Buttrick PM. Incomplete recovery of myocyte contractile function despite improvement of myocardial architecture with left ventricular assist device support. Circulation Heart failure. 2011;4:425–32. doi: 10.1161/CIRCHEARTFAILURE.111.961326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wijnker PJ, Boknik P, Gergs U, Muller FU, Neumann J, dos Remedios C, et al. Protein phosphatase 2A affects myofilament contractility in non-failing but not in failing human myocardium. Journal of muscle research and cell motility. 2011;32:221–33. doi: 10.1007/s10974-011-9261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Der Velden J, Klein LJ, Zaremba R, Boontje NM, Huybregts MA, Stooker W, et al. Effects of calcium, inorganic phosphate, and pH on isometric force in single skinned cardiomyocytes from donor and failing human hearts. Circulation. 2001;104:1140–6. doi: 10.1161/hc3501.095485. [DOI] [PubMed] [Google Scholar]
- [14].Hamdani N, Borbely A, Veenstra SP, Kooij V, Vrydag W, Zaremba R, et al. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. Journal of muscle research and cell motility. 2010;31:289–301. doi: 10.1007/s10974-010-9231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circulation research. 2004;95:708–16. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- [16].Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- [17].Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circulation research. 2004;94:284–95. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- [18].Campbell SG, Haynes P, Snapp WK, Nava KE, Campbell KS. Altered ventricular torsion and transmural patterns of myocyte relaxation precede heart failure in aging F344 rats. American journal of physiology Heart and circulatory physiology. 2013 doi: 10.1152/ajpheart.00797.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovascular research. 2000;46:487–95. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]
- [20].van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, et al. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovascular research. 2006;69:876–87. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- [21].van der Velden J, Merkus D, de Beer V, Hamdani N, Linke WA, Boontje NM, et al. Transmural heterogeneity of myofilament function and sarcomeric protein phosphorylation in remodeled myocardium of pigs with a recent myocardial infarction. Frontiers in physiology. 2011;2:83. doi: 10.3389/fphys.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:88–90. doi: 10.1096/fj.04-2066fje. [DOI] [PubMed] [Google Scholar]
- [23].Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovascular research. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- [24.Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, et al. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation. 2012;126:1828–37. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circulation research. 2002;91:509–16. doi: 10.1161/01.res.0000035246.27856.53. [DOI] [PubMed] [Google Scholar]
- [26].Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–41. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- [27].Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. American journal of physiology Heart and circulatory physiology. 2004;287:H2712–8. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- [28].van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovascular research. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- [29].McDonough JL, Labugger R, Pickett W, Tse MY, MacKenzie S, Pang SC, et al. Cardiac troponin I is modified in the myocardium of bypass patients. Circulation. 2001;103:58–64. doi: 10.1161/01.cir.103.1.58. [DOI] [PubMed] [Google Scholar]
- [30].Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, et al. Transgenic mouse model of stunned myocardium. Science. 2000;287:488–91. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- [31].Monreal G, Nicholson LM, Han B, Joshi MS, Phillips AB, Wold LE, et al. Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life sciences. 2008;83:786–94. doi: 10.1016/j.lfs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- [32].Chugh S, Ouzounian M, Lu Z, Mohamed S, Li W, Bousette N, et al. Pilot study identifying myosin heavy chain 7, desmin, insulin-like growth factor 7, and annexin A2 as circulating biomarkers of human heart failure. Proteomics. 2013;13:2324–34. doi: 10.1002/pmic.201200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pawlak A, Gil RJ, Kulawik T, Pronicki M, Karkucinska-Wieckowska A, Szymanska-Debinska T, et al. Type of desmin expression in cardiomyocytes - a good marker of heart failure development in idiopathic dilated cardiomyopathy. Journal of internal medicine. 2012;272:287–97. doi: 10.1111/j.1365-2796.2012.02524.x. [DOI] [PubMed] [Google Scholar]
- [34].Hamdani N, Paulus WJ, van Heerebeek L, Borbely A, Boontje NM, Zuidwijk MJ, et al. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. European heart journal. 2009;30:1863–72. doi: 10.1093/eurheartj/ehp189. [DOI] [PubMed] [Google Scholar]
- [35].Morano I, Rosch J, Arner A, Ruegg JC. Phosphorylation and thiophosphorylation by myosin light chain kinase: different effects on mechanical properties of chemically skinned ventricular fibers from the pig. Journal of molecular and cellular cardiology. 1990;22:805–13. doi: 10.1016/0022-2828(90)90091-f. [DOI] [PubMed] [Google Scholar]
- [36].Arrell DK, Neverova I, Fraser H, Marban E, Van Eyk JE. Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1. Circulation research. 2001;89:480–7. doi: 10.1161/hh1801.097240. [DOI] [PubMed] [Google Scholar]
- [37].Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, et al. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112:544–52. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- [38.Doroszko A, Polewicz D, Sawicka J, Richardson JS, Cheung PY, Sawicki G. Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic research in cardiology. 2009;104:669–79. doi: 10.1007/s00395-009-0035-1. [DOI] [PubMed] [Google Scholar]
- [39].Cadete VJ, Sawicka J, Bekar LK, Sawicki G. Combined subthreshold dose inhibition of myosin light chain phosphorylation and MMP-2 activity provides cardioprotection from ischaemic/reperfusion injury in isolated rat heart. British journal of pharmacology. 2013;170:380–90. doi: 10.1111/bph.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.