Abstract
Background
Cholinergic neurons have been identified with the acetylcholine synthetic enzyme choline acetyltransferase (ChAT). However, ChAT is difficult to localize in newly differentiated peripheral neurons making the study of cholinergic neuronal development problematic. Consequently researchers have used mouse reporter lines to indicate the presence of ChAT.
Methods
Our objective was to determine which ChAT reporter line was the most sensitive indicator of ChAT expression. We utilized two different fluorescent ChAT reporter lines (ChAT-GFP and ChAT-Cre;R26R:floxSTOP:tdTomato) together with immunolocalization of ChAT protein (ChAT-IR) to characterize the spatial and temporal expression of ChAT in myenteric neurons throughout ENS development.
Key Results
ChAT-IR cells were first seen in the intestine at E10.5, even within the migration wavefront of neural precursors. Myenteric neurons within the distal small intestine (dSI) and proximal colon were first labeled by ChAT-IR, then ChAT-GFP, and finally ChAT-Cre tdTomato. The percentage of ChAT-IR neurons is equivalent to adult levels in the dSI by E13.5 and proximal colon by P0. After these stages, the percentages remained relatively constant throughout development despite dramatic changes in neuronal density.
Conclusions and Inferences
These observations indicate that neurotransmitter expression occurs early and there is only a brief gap between neurogenesis and neurotransmitter expression. Our finding that the proportion of ChAT myenteric neurons reached adult levels during embryonic development suggests that the fate of cholinergic neurons is tightly regulated and that their differentiation might influence further neuronal development. ChAT-GFP is a more accurate indicator of early ENS cholinergic neuronal differentiation than the ChAT-Cre;R26R:floxSTOP:tdTomato reporter mouse.
Keywords: ChAT, cholinergic neurogenesis, ChAT-GFP, ChAT-Cre tdTomato, enteric nervous system
INTRODUCTION
Cholinergic neurons are the most abundant neurons in the adult enteric nervous system (ENS) (1–3). They assume a number of different physiological roles. Sensory neurons and excitatory motor neurons to both circular and longitudinal muscles represent the major populations while ascending and descending neurons make up smaller proportions. Cholinergic neurons represent about 60% of the mature ENS neurons (3), and together with non-cholinergic neurons mediate the peristaltic reflex of the bowel (4).
Neural crest cells (NCC) differentiate into neurons that subsequently express markers specific for their neurotransmitter identity. The definitive marker for cholinergic neurons is choline acetyltransferase (ChAT), the synthesizing enzyme for acetylcholine. Visualizing this protein with antisera has been problematic because ChAT is present in only small amounts and different ChAT isoforms are produced by neurons within the central versus peripheral nervous systems. The first antisera generated against ChAT stained cholinergic neurons and fibers in the central nervous system (CNS). Unfortunately, these antisera failed to immunolabel enteric neurons and other peripheral neurons demonstrated to be cholinergic from physiological and pharmacological experiments (4–6). Different ChAT isoforms were not recognized until Kimura and colleagues cloned peripheral Chat (pChat) (6). They generated antisera specific for pChAT and showed it to be distinct from cChAT, abundant in CNS cholinergic neurons (6, 7), reviewed by (8). Although pChAT immunostained a large percentage of the guinea pig ENS, cChAT immunoreactivity was also present (9). Antisera made against placental ChAT that recognizes both pChAT and cChAT are commercially available [(EMD Millipore, Temecula, CA; (10)] and have been used to localize ChAT in enteric neurons (1, 3, 11).
These issues have recently been overcome by the generation of mouse fluorescent reporter lines that detect ChAT expression. These mice provide an alternative method to visualize the presence of cholinergic neurons in the embryonic and adult nervous system. These reporter lines have been used primarily to map the location of cholinergic neurons in the CNS, and it was not until recently that the developing ENS was analyzed using these mice (12, 13). These investigators used ChAT-Cre mice in combination with different fluorescent reporters to identify cholinergic neurons in the mouse ENS (12, 13). We also initially used the ChAT-Cre mouse intercrossed R26R:floxSTOP:tdTomato animals to determine ChAT expression within the ENS, however we found a more precocious appearance of labeled neurons with a different reporter, ChAT-GFP (14). This difference led us to compare the expression of ChAT in the two different fluorescent reporters within the same preparation and to validate ChAT protein expression using immunostaining (ChAT-IR). We observed ChAT-IR in early ENS development, and its appearance was closely followed by ChAT-GFP. However, the presence of ChAT-Cre tdTomato was delayed and it was not until postnatal (P) day 13 that cholinergic neurons co-expressed all three markers. Our studies additionally indicate that the percentage of cholinergic neurons reaches adult levels early in embryonic development, an observation that suggests that cholinergic neuronal differentiation maybe an early step that establishes and/or regulates the pattern of further neuronal differentiation within the ENS.
MATERIAL AND METHODS
Animals
The University of Wisconsin Animal Care and Use Committee approved all procedures. ChAT-Cre mice were mated with R26R:floxSTOP:tdTomato animals to produce ChAT-Cre;R26R:floxSTOP:tdTomato mice (defined as ChAT-Cre tdTomato throughout the manuscript). These animals were then mated with homozygous ChAT-GFP reporter mice, to obtain mice expressing both fluorescent reporters that detect ChAT expression. All mouse lines were obtained from Jackson Laboratories, Bar Harbor, MA. Embryos were dissected from mothers that were anesthetized with isoflurane and euthanized by cervical dislocation. Embryonic day E0.5 was defined as mid-day on the day of the vaginal plug. Postnatal (P) animals age 13 and 30 were sacrificed in the same manner while embryos, P0, and P3 animals were placed on ice and subsequently decapitated. All animals were housed in a non-sterile environment.
Tissue Preparation
To obtain embryonic (E) tissue at ages 10.5, 11.5, 13.5, and 16.5, intact embryos were fixed in 4% paraformaldehyde (PFA) for 4 hours at room temperature (RT) or overnight at 4°C. Following fixation, the embryos were washed in PBS twice and the gut was then removed for staining. P0, P3, P13, and P30 gut tissues were removed, the lumen was washed with PBS, and then placed into 4% PFA. After fixation, the tissue was rinsed twice in PBS.
Immunohistochemistry
Fixed whole-mount tissues were treated with PBS containing 0.25–1.0% Triton X-100 for 4–6 hours at RT or overnight at 4°C, washed in PBS and then incubated with primary antibodies diluted in blocking solution {PBS with 3% bovine serum albumin with 0.1% Triton X-100). They were then washed in PBS (3 × 1 hour), and finally placed into blocking solution containing the secondary antibodies as previously described [(Supp. Table 1; (15)].
Neurotransmitter and myenteric neuronal density analysis
For neurotransmitter analysis, 60x magnification images were collected randomly from the distal small intestine (SI), and proximal colon (n=3–7 images from 3 animals/age group). At E10.5–11.5 we obtained images from only proximal and distal SI since the colon is not yet innervated at these stages. Myenteric neurons were immunolabeled with Hu and then scored for the presence of ChAT-IR, GFP, and tdTomato. Neurons considered positive for ChAT-IR had significant signal in the cytoplasm and weak or no signal in the nucleus. Neurons determined positive for ChAT-GFP and ChAT-Cre tdTomato had significant signal in the cytoplasm and the nucleus. At P30 a small portion of those neurons had GFP or tdTomato only in the nucleus and were also included in these counts. tdTomato-expressing neurons in fixed tissue were visualized without immunostaining since the fluorescence remained after fixation with 4% PFA (see Figure 1, 2, and 4). For myenteric neuronal density analysis, Hu+ cells were counted (n=3 animals/age group) in 3–5 fields of view per animal from 60x magnification images randomly collected from the distal SI and proximal colon.
Figure 1.
Representative images of cholinergic neurons in the pSI at E10.5 and E11.5 and the dSI at E13.5 and E16.5. At E10.5 a small number of Hu+ neurons (blue) were found in the pSI and the majority of them were labeled with ChAT-IR (white) and ChAT-GFP (green); however, none was ChAT-Cre tdTomato+ at this stage (red). In the E11.5 pSI, the number of Hu+ neurons had increased and most were ChAT-IR and ChAT-GFP while none expressed ChAT-Cre tdTomato. At E13.5 in the dSI, ChAT-IR and ChAT-GFP signal was more intense and co-localized. A small number of these cells also expressed ChAT-Cre tdTomato. The ChAT-IR, ChAT-GFP, and small number of ChAT-CRE tdTomato neurons were found in nascent ganglia at E16.5. Scale bar for E10.5 = 50µm, all other stages = 100µm.
Figure 2.
Upper panel: Percentage of ChAT-IR neurons that were ChAT-GFP and ChAT-Cre tdTomato in the dSI and proximal colon. In the dSI, at E11.5 the majority of cholinergic neurons were only labeled with ChAT-IR, some of these were also ChAT-GFP positive, while none expressed ChAT-Cre tdTomato At E13.5, a larger percentage expressed GFP but still only a small number were ChAT-Cre tdTomato+. By E16.5 nearly all ChAT-IR neurons expressed ChAT-GFP and 14% were ChAT-Cre tdTomato+. Postnatally, virtually all ChAT-IR neurons expressed ChAT-GFP and the percentage with ChAT-Cre tdTomato increased until P13 when nearly all neurons co-expressed all three markers.
Lower panel: The proximal colon showed a gradual increase in GFP expression in ChAT-IR neurons until P0 when nearly all were ChAT-GFP+. ChAT-CRE tdTomato expression was again delayed and did not reach maximal levels until P13).
Figure 4.
Neuronal and cholinergic differentiation within the neural crest-derived migration wavefront. Hu+ neurons (blue) present within the migration wavefront of p75 positive (red) neural crest-derived cells expressed both ChAT-IR (white) and ChAT-GFP (green) in the SI at E10.5. In the colon at E13.5, the neural crest derived migration wavefront of p75+ cells contained neurons expressing only Hu and ChAT-IR since the most rostral ChAT-GFP+ cells was restricted to the SI at this stage. Scale bar = 50µm.
Image analysis
The stained tissue was visualized on a Nikon A1 confocal microscope. Z-series extending over the depth of the stained neurons or ganglia were captured, processed, and analyzed with Nikon Elements (Nikon, Melville, NY, USA). Cells were counted manually and the identity of a neuron was scored using a single plane where the nucleus and cytoplasm were clearly visualized from a Z-series. Although the intensity of the ChAT markers varied considerably within a ganglion, neurons were considered positive if the signal exceeded that found in the background. The brightness and contrast have been adjusted for clarity using Photoshop™ (Adobe, USA).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using an unpaired Student’s T-test and p-values < 0.05 were considered significant. Normal datasets were analyzed using one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test.
RESULTS
Myenteric cholinergic neurogenesis in the proximal small intestine (pSI)
Enteric neural crest cells (ENCC) first enter the foregut around E9.5 and are present within the primordium of the SI at E10.5 (16). We studied the proximal small intestine (pSI) at these early embryonic ages to determine when ChAT expression was first detectable. Hu immunolabeling was performed to distinguish all ENS neurons. Cholinergic neurons were identified by immunodetection (ChAT-IR), GFP, and/or tdTomato fluorescent proteins from the ChAT reporter constructs. In general, we found that Hu+ cells initially co-labelled with ChAT-IR and ChAT-GFP, while the expression of ChAT-Cre tdTomato from the ChAT-Cre construct was not detected until later in development.
At E10.5, within the pSI, a sparse population of Hu+ neurons, some of which contained ChAT-IR and ChAT-GFP, was observed (Fig. 1). Interestingly, there was no detectable expression of ChAT-Cre tdTomato in these preparations. At this age, some ChAT-GFP neurons showed caudally projecting processes (Figure 4). By E11.5, the total number of Hu+ neurons increased in the pSI and 77% of these were ChAT-IR (Figs. 1, 2 and Supp. Table. 2). Among the ChAT-IR cells, 90% also had detectable levels of ChAT-GFP expression while only 2% were ChAT-Cre tdTomato+ (Figs. 1, 2 and Supp. Table. 2). By E13.5, ChAT-GFP positive cells were more abundant along the SI (Figs. 1 and 3) and they were arranged in a proximal-distal gradient of expression with the highest numbers of cells in the pSI. In contrast, a sparse number of ChAT-Cre tdTomato positive cells were scattered evenly along the entire SI (Fig. 3).
Figure 3.
Variation of ChAT-GFP and ChAT-Cre tdTomato expression along E13.5 SI. ChAT-GFP is observed in a proximal to distal gradient along the SI with more cells in the proximal than distal SI. The most rostral ChAT-GFP+ cell is found within the cecum (see white arrow). (Some background fluorescence can be seen distal to the cecum). Individual ChAT-Cre tdTomato cells are found in small numbers scattered uniformly along the SI. The most rostral ChAT-Cre tdTomato+ cell is coincident with the most rostral ChAT-GFP+ cells (see white arrow). The lower panel shows a merge of the upper images. Scale bar = 400µm.
Myenteric cholinergic neurons at the migration wavefront of NC-derived cells
The precocious appearance of both ChAT-IR and ChAT–GFP expression prompted us to determine the spatial localization of ChAT within the migration wavefront of NC-derived cells. Neuronal differentiation, as shown by the expression of Hu, has previously been demonstrated within the migration wavefront of cells in the developing ENS of avians (17, 18). The presence of neuroblasts expressing TuJ1 has also been detected at the migration wavefront in mice (19, 20). Research from Young and colleagues (21) indicates that the most rostral Hu+ neurons are within the migration wavefront of NC-derived cells from E10.5 until E13.5. Therefore, we decided to investigate whether myenteric cholinergic neuronal differentiation occurred within the migration wavefront of p75+ NC-derived cells. We isolated E10.5–E13.5 guts from ChAT-GFP animals and immunostained them for p75, Hu, and ChAT-IR. As early as E10.5 we observed a small number of Hu+ neurons among the most terminal p75+ NC-derived cells within the SI (Fig. 4). Surprisingly, a small portion of these neurons displayed ChAT-IR and ChAT-GFP. Approximately 10% of the ChAT-GFP+ cells at the migration wavefront contained a caudally-projecting process (Fig. 4). A similar pattern of myenteric neuronal and cholinergic differentiation was observed at E11.5 and E12.5 (data not shown). At E13.5, Hu and ChAT-IR were observed at the p75+ NC-derived migration wavefront in the colon. However no ChAT-GFP+ neurons were detected at this location since the most rostral ChAT-GFP positive cells were restricted to the SI at this stage (Figs. 3 and 4). These data demonstrate early differentiation of cholinergic neurons occurs within the most distal population of NC-derived cells.
Myenteric cholinergic neurogenesis in the distal SI (dSI)
We determined the percentage of myenteric Hu+ neurons expressing each ChAT marker over the course of ENS development. Compared to the pSI, the E11.5 dSI contained fewer cholinergic neurons. Only 47% of the Hu+ neurons were ChAT-IR+, 44% of these co-expressed GFP, while none were ChAT-Cre tdTomato+ (Fig. 1 and Supp. Table. 2). At E13.5, in the dSI, a large proportion of Hu+ neurons showed ChAT-IR (59%), 82% of those were also ChAT-GFP+ while only 1% co-labeled with ChAT-Cre tdTomato (Figs. 1, 2 and Supp. Table. 2). By E16.5, almost all of the ChAT-IR+ neurons were GFP+ (99%) and a small percentage (14%) also expressed ChAT-Cre tdTomato (Figs. 1, 2 and Supp. Table. 2).
At P0, the percentage of ChAT-IR neurons expressing ChAT-Cre tdTomato increased substantially to 65% (Figs 2, 3 and Supp. Table. 2). By P3, ChAT-Cre tdTomato was detected in 74% of the ChAT-IR neurons and at P13 essentially all ChAT-IR neurons showed both GFP and tdTomato expression (Figs 2, 3 and Supp. Table. 2). Interestingly, at P30 we noted that the expression pattern of GFP and tdTomato was altered in a small portion of the neurons. GFP and/or tdTomato expression was restricted to the nucleus (Fig. 5 arrowheads and Supp. Fig. 1). This altered expression of the fluorescent proteins could be the initial sign of cells marked for programmed cell death. Consistent with this is our observation that the number of Hu+ neurons is reduced from 708 ± 98 at P30 to 287 ± 13 in 3 month old animals (data not shown). Further studies would be required to confirm any apoptotic events. At all ages examined, a small percentage of the Hu+ neurons expressed either ChAT-GFP and/or ChAT-Cre tdTomato but lacked ChAT-IR (Supp. Table. 2). Within each of the ganglia, the intensity of both GFP and tdTomato expression varied considerably from neuron to neuron but remained uniform within individual neurons.
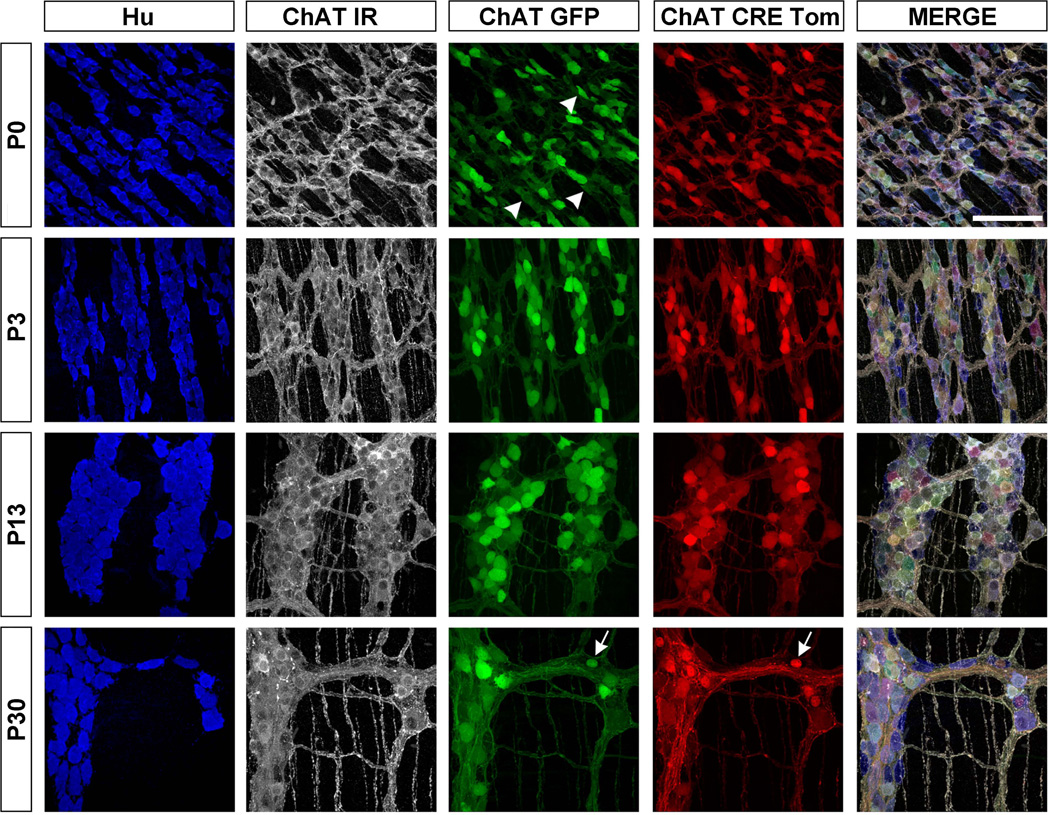
Figure 5.
Representative images of cholinergic neurons in the distal SI from P0 to P30. At P0, the majority of ChAT-IR neurons (white) were ChAT-GFP+ (green) and many expressed ChAT-Cre tdTomato (red). Arrowheads indicate ChAT-GFP neurons that are negative for ChAT-Cre tdTomato+. By P13 and P30 nearly all of the ChAT-IR neurons expressed ChAT-Cre tdTomato. The fluorescence of a small percentage of ChAT-GFP and ChAT-Cre tdTomato neurons at P30 was restricted to the nucleus and lacked ChAT-IR (arrows). Scale bar = 100µm.
Myenteric cholinergic neurogenesis in the proximal colon
In the proximal colon at E13.5, 73% of the Hu+ neurons showed ChAT-IR, however none of these were ChAT-GFP or ChAT-Cre tdTomato positive since these cells were restricted within the SI at this stage. (Figs. 2, 3 and Supp. Table. 2). By E16.5, the number of Hu+ neurons expressing ChAT-IR had reduced to only 40%, while the proportion of ChAT-IR cells that expressed ChAT-GFP was 84% and 7% were ChAT-Cre tdTomato+ (Fig. 2, and Supp. Table. 2). The pattern and timing of ChAT-GFP and ChAT-Cre tdTomato expression in Hu+ neurons at E13.5 and E16.5 in the colon mirrored that observed at E11.5 and E13.5 in the dSI, showing a 2–3 day delay in colonization and differentiation (Fig. 2 and Supp. Table. 2). However at P0, 57% of the Hu+ neurons were ChAT-IR and almost all of these were ChAT-GFP+, while ChAT-Cre tdTomato expression was only detected in 72% of them, similar to what we previously observed in the dSI (Fig. 2 and Supp. Table. 2). Comparable proportions of ChAT-IR, -GFP and ChAT-Cre tdTomato positive neurons were found at P3 (Fig. 2 and Supp. Table. 2). Co-expression of GFP and tdTomato proteins was apparent within most ChAT-IR neurons at P13 and the proportions of triple labeled cells then remained equivalent at P30 (Fig. 2 and Supp. Table. 2).
Gut length and myenteric neuronal density
Although both the number of ENS neurons and gut length increases substantially during embryonic and post-natal development, these changes have not been systematically studied. We wanted to determine whether neuronal cell density affects the differentiation of cholinergic neurons. Therefore, we measured the length of the gut and counted the number of Hu+ neurons within the dSI and proximal colon between E11.5 and P30 (Fig. 6). The length of the SI increased from 0.2cm at E11.5 by six-fold to reach 1.2cm at E13.5 and then more than doubled to 2.7cm by E16.5 (Fig. 6). At P0, the SI length was 8.2cm and then increased during postnatal development by more than three fold to 26.9cm at P30 (Fig. 6). In comparison, the neuronal density in the dSI was 1137 neurons/mm2 at E11.5 and then reached a peak of 7517 neurons/mm2 at E16.5 (Fig. 6 and Supp. Table. 2). At P0 the neuronal density had reduced by almost half to 3983 neurons/mm2 and continued to decline to 707 neurons/mm2 by P30 (Suppl. Table. 2). These data indicate that the increase in neuron number in the SI was greater than the rate that the length increased from E11.5 to E16.5, producing an overall neuronal density increase (Fig. 6 and Supp. Table. 2). However, at P0 the neuronal density actually decreased by almost 50%, while the gut length increased almost three fold (Fig. 6 and Supp. Table. 2).
Figure 6.
Neuronal density and gut length at different ages. A. Neuronal density of dSI increases from E11.5 to E16.5 and then declines with increasing age. Neuronal density in proximal colon is maintained at maximum values between E16.5-P3 but declines at P13 B. Neuronal density (left axis) plotted against SI length (right axis). C. Neuronal density (left axis) plotted against colon length (right axis).
Colonic length increased from 0.2cm at E11.5 by six-fold to 1.1cm by E16.5 (Fig. 6). At P0 the colonic length was 2.1cm and this tripled in length to reach 6.3cm at P30 (Fig. 6). The neuronal density at E13.5 was 2069 neurons/mm2 and similar to the SI, reached a peak of 14,692 neurons/mm2 by E16.5 (Fig. 6 and Supp. Table. 2). In contrast to the SI, there was a slower decline in the density during post-natal development as at P0 the density was equivalent to that at E16.5 (14, 136 neurons/mm2) (Fig. 6 and Supp. Table. 2). The density then reduced dramatically by P13 to 6203 neurons/mm2 and was finally at 1661 neurons/mm2 at P30 (Fig. 6 and Supp. Table. 2). Therefore, in the colon the neuronal density was maximal between E16.5-P3, decreased by 50% at P13 and then by three-fold at P30. Despite these significant changes in neuronal density and gut length (Fig. 6 and Supp. Table. 2), the percentage of cholinergic neurons remained relatively constant between E13.5-P30 in the dSI and P0–P30 in the colon, suggesting that cholinergic differentiation might be regulated independently from neuronal density (Fig. 6 and Supp. Table. 2).
DISCUSSION
The muscular contraction that regulates peristalsis along the length of gut is mediated by cholinergic neurons via their release of the excitatory neurotransmitter, acetylcholine (22). Unfortunately, technical difficulties have made the detection of the cholinergic phenotype in ENS neuronal differentiation very problematic. The appearance of cholinergic neurons had previously been reported by the detection of 3H-acetylcholine in extracts of E11 gut (23). However, these experiments did not reveal the number or location of cholinergic neurons within the gut. The recent availability of mouse ChAT reporter lines has enabled the visualization of cholinergic neurogenesis within the ENS (12, 14, 24). ChAT-GFP expression was shown to be present in cells within E13 intestine, and the systematic study by Hao et al 2013 (12), using the ChAT-Cre;R262-YFP mouse line identified neurons as early as E11.5. Our results, using a combination of two different fluorescent ChAT reporter lines (ChAT-GFP and ChAT-Cre tdTomato) together with ChAT immunostaining (ChAT-IR), confirm the observation that the differentiation of cholinergic neurons increased in a spatial and temporal fashion with developmental age. Our findings extend their previous results to show that cholinergic neurogenesis occurs within the gut as early at E10.5 and is even present within the migration wavefront of NC-derived cells. At this age, both ChAT-IR and ChAT-GFP were present in Hu+ neurons suggesting that there is only a small time window between neurogenesis and neurotransmitter expression. Interestingly, there was no detection of ChAT-Cre activity as seen by the absence of tdTomato expression. In general, cholinergic neurons were initially marked by the expression of ChAT-IR, followed by ChAT-GFP, and finally after a delay of 2–3 days by ChAT-Cre tdTomato. We show that the proportion of cholinergic neurons reached adult levels as early at E13.5 in the dSI and P0 in the proximal colon. The difference between the ileum and colon may be accounted for in part by the progressive rostral to caudal colonization of the gut by NCC (25). Despite significant changes in neuronal density, these proportions remained relatively constant throughout ENS development. We conclude that although the ChAT-GFP reporter does not completely mark all ChAT-IR positive cells during early embryonic stages, it is a more accurate indicator of cholinergic neurogenesis within the ENS than the ChAT-Cre tdTomato mouse.
The earlier detection of ChAT expression by ChAT-IR and ChAT-GFP compared to ChAT-Cre tdTomato allowed us to show that the percentage of neurons expressing ChAT reached adult proportions during embryonic development at E13.5 in the dSI and P0 in the proximal colon. These stages are younger than those reported by Hao et al. 2013 (12) (P10 for SI and colon), and suggest that cholinergic differentiation is determined much earlier than was previously considered. Despite the delay in detection of cholinergic neurons with the ChAT-Cre tdTomato reporter mouse, maximum values were eventually observed at P13. Our data showing co-expression of all three ChAT indicators revealed that 59–61% of Hu+ neurons were cholinergic in the dSI at P30. These values are similar to the 62% reported in the adult by Hao et al., 2013 (12) and the 63% shown by Sang and Young (1996) (2). It is also similar to the total of 72% reported in the adult SI (3). Furthermore, the 55–57% ChAT positive cells in the P30 proximal colon are in close agreement with the 58% obtained by Hao et al., 2013 (12) in the adult proximal colon and 55% by Sang and Young in the adult colon (2).
It is interesting to note that the percentage of cholinergic neurons did not change significantly between E13.5-P30 in dSI and P0–P30 in the proximal colon despite dramatic alterations in neuronal density. During development, NC-derived cells proliferate and differentiate in a proximal-distal wave as they colonize the lengthening bowel (16, 26). In avians, the percentage of neurons rises from zero at E4.5 to around 55% at E6 (18). In the mouse midgut, neurogenesis occurs within a small proportion of NC-derived cells as early as E10.5 (21). It rises dramatically to reach a quarter of these cells by E12.5 and to 45% at E14.5 (21). We detected cholinergic neurogenesis within the gut as early as E10.5. At E11.5, we showed that 77% of Hu+ neurons had a cholinergic phenotype as seen by the expression of ChAT-IR. The fact that the timing of neuronal and cholinergic differentiation appears to be coincident suggests that the interval between acquiring neuronal and neurotransmitter identities is brief. In addition, the proportion of ChAT-IR positive neurons remained relatively constant throughout development despite the dramatic increases in the number of NC-derived cells. Therefore, cholinergic fate within NC-derived cells might be established very early during ENS development and there may be an intrinsic mechanism that tightly regulates the proportion of these cells amongst the pool of progenitor and neuronal cells.
Neuronal precursors undergo a terminal cell division before becoming a neuron, termed the neuron’s birthdate; the birthdates of the different enteric neuron subtypes peak at different times (27, 28). A recent study demonstrated that the birthdate of Hu+ neurons extends from E10.5 to P0 in the mouse SI (28). Subsequent to their birthdate it is thought that neurons acquire their neurotransmitter identity. Indeed, serotonergic and cholinergic neurons in the duodenum and jejunum have previously been shown to be born between E8–E16, while the peak of expression of 5HT precedes that of ChAT (27). Our data suggests that the birthdates of cholinergic neurons in the dSI and colon occur at later stages. These results suggest that 5HT could influence expression of ChAT. It is also possible that 5HT and ChAT either together or independently regulate further neuronal differentiation (29).
The proportions of neurons expressing different neurotransmitters must be tightly regulated to ensure that the rhythmic contraction of the musculature along the length of the bowel results in movement of enteric lumenal contents. In order to determine whether this was correlated with neuronal density, we measured both ChAT-IR percentages and neuronal density at different ages throughout ENS development. Our data suggests that the proportion of cholinergic neurons is regulated independently from neuronal density in wild type animals. These data are in contrast to the alterations in neurotransmitter expression observed in mice where neuronal density has been genetically modified. Our conditional mouse knockout model of Hirschsprung’s disease (HSCR) showed an increase in the proportion of NOS expressing neurons and a decrease in the percentage of cholinergic neurons (15). These changes were correlated with reduced neuronal density in the colon just proximal to the aganglionic segment. Changes in the proportions of neurons expressing different neurotransmitters were also demonstrated in mice with a conditional deletion of Hand2 (11). Hand2 mutant mice contain reduced neuronal numbers and die around P21 with functional obstruction in the colon, a phenotype that is similar to HSCR mouse models (11). Increased neuronal density as demonstrated in mice over-expressing Noggin, has also been shown to change the percentage of myenteric serotonergic and submucosal calbindin neurons (30). Therefore, the precise role that neuronal density plays in regulating neurotransmitter expression is unclear.
GFP expression closely followed in time and number the appearance of ChAT-IR neurons. In contrast, we observed a delay of about 2–3 days between ChAT-GFP and ChAT-Cre tdTomato. The reason(s) for these differences in appearance of the markers is not clear. The detection of ChAT expression using the ChAT-Cre mouse requires the translation of Cre recombinase and a recombination event to remove the STOP sequence upstream of TdTomato within the ROSA26 locus. In contrast, ChAT-GFP is a more direct reporter of ChAT since it is a transgenic mouse in which GFP has been inserted into a BAC spanning the ChAT locus (14). The expression of GFP could be affected by the location of the transgenic ChAT-GFP construct in the mouse genome and by the activity of endogenous ChAT. Despite the fact that not all ChAT-IR positive cells are labeled with ChAT-GFP, this is clearly a more accurate indicator of cholinergic neurogenesis within the ENS than the ChAT-Cre tdTomato mouse.
In conclusion, the first marker to appear in developing cholinergic neurons was ChAT-IR, then ChAT-GFP, and lastly ChAT-Cre tdTomato. Enteric motor, sensory, and inter-neurons all produce acetylcholine, however, it is unclear how neurons assume these different phenotypes. For instance, activation of the ChAT gene may regulate downstream genes that specify a particular target and thus identity. Within the spinal cord, opposing morphogen gradients of sonic hedgehog (Shh) and bone morphogenetic protein (BMP, member of transforming growth factor β family) control cholinergic differentiation (31). Both of these factors are expressed within the gut, however their role in regulating the downstream transcriptional effectors that control enteric cholinergic neurogenesis is unknown. Eluciadation of these mechanisms will be essential in understanding how the diverse neurotransmitter phenotypes are established within the ENS.
Supplementary Material
High magnification micrograph of P30 neurons in dSI that have ChAT-GFP+ and ChAT-Cre tdTomato+ expression restricted to the nucleus (arrows). Scale bar= 50µm.
Key Messages.
-
-
In the developing enteric nervous system, our observations indicate that neurotransmitter expression occurs early and there is only a brief gap between neurogenesis and neurotransmitter expression. The proportion of ChAT myenteric neurons reaches adult levels during embryonic development, suggesting that the fate of cholinergic neurons is tightly regulated and that their differentiation might influence further neuronal development. ChAT-GFP is a more accurate indicator of early ENS cholinergic neuronal differentiation than the ChAT-Cre;R26R:floxSTOP:tdTomato reporter mouse.
-
-
In order to facilitate study of cholinergic neuronal development, our objective was to determine which ChAT reporter line was the most sensitive indicator of ChAT expression.
-
-
We utilized two different fluorescent ChAT reporter lines (ChAT-GFP and ChAT-Cre;R26R:floxSTOP:tdTomato) together with immunolocalization of ChAT protein (ChAT-IR) to characterize the spatial and temporal expression of ChAT in myenteric neurons throughout ENS development.
-
-
ChAT-IR cells were first seen in the intestine at E10.5, even within the migration wavefront of neural precursors. We found that myenteric neurons within the distal small intestine (dSI) and proximal colon were first labeled by ChAT-IR, then ChAT-GFP, and lastly by ChAT-Cre tdTomato. The percentage of ChAT-IR neurons is equivalent to adult levels in the dSI by E13.5 and proximal colon by P0.
ACKNOWLEDGEMENTS
We are grateful to Lance Rodenkirch for his excellent guidance and support in the use of the facilities of the University of Wisconsin Keck Imaging Facility. We thank Jessica Muhlenbeck and Alessandra Ruenger for assistance with cell counting.
FUNDING
This work was supported by the National Institutes of Health (NIDDK RO1DK081634) to MLE, the Central Surgical Association Foundation Turcotte Award to AG, and the American Pediatric Surgery Association Foundation Award to AG.
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
CONTRIBUTORSHIP
MLE, AJBA, AG and NRD were responsible for study concept and design. CSE, SJL, AJBA, and MLE were responsible for acquisition of data. CSE, MLE, AJBA and AG were responsible for analysis & interpretation of data. MLE, AJBA, AG, and NRD were responsible for drafting of the manuscript. MLE, AJBA, CSE and AG were responsible for critical revision of the manuscript.
References
- 1.Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. The Anatomical record. 1998;251(2):185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell and tissue research. 1996;284(1):39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- 3.Qu ZD, et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and tissue research. 2008;334(2):147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 4.Bian XC, Bornstein JC, Bertrand PP. Nicotinic transmission at functionally distinct synapses in descending reflex pathways of the rat colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2003;15(2):161–171. doi: 10.1046/j.1365-2982.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CD, Epstein ML. Monoclonal antibodies and polyvalent antiserum to chicken choline acetyltransferase. Journal of neurochemistry. 1986;46(3):968–976. doi: 10.1111/j.1471-4159.1986.tb13064.x. [DOI] [PubMed] [Google Scholar]
- 6.Tooyama I, Kimura H. A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers. Journal of chemical neuroanatomy. 2000;17(4):217–226. doi: 10.1016/s0891-0618(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. Journal of chemical neuroanatomy. 2000;18(1–2):31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 8.Bellier JP, Kimura H. Peripheral type of choline acetyltransferase: biological and evolutionary implications for novel mechanisms in cholinergic system. Journal of chemical neuroanatomy. 2011;42(4):225–235. doi: 10.1016/j.jchemneu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Chiocchetti R, et al. Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons. Cell and tissue research. 2003;311(1):11–22. doi: 10.1007/s00441-002-0652-6. [DOI] [PubMed] [Google Scholar]
- 10.Koga T, Bellier JP, Kimura H, Tooyama I. Immunoreactivity for Choline Acetyltransferase of Peripheral-Type (pChAT) in the Trigeminal Ganglion Neurons of the Non-Human Primate Macaca fascicularis. Acta histochemica et cytochemica. 2013;46(2):59–64. doi: 10.1267/ahc.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei J, Howard MJ. Targeted deletion of Hand2 in enteric neural precursor cells affects its functions in neurogenesis, neurotransmitter specification and gangliogenesis, causing functional aganglionosis. Development. 2011;138(21):4789–4800. doi: 10.1242/dev.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. The Journal of comparative neurology. 2013;521(14):3358–3370. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- 13.Gautron L, et al. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. The Journal of comparative neurology. 2013;521(16):3741–3767. doi: 10.1002/cne.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallini YN, et al. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiological genomics. 2006;27(3):391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zaitoun I, et al. Altered neuronal density and neurotransmitter expression in the ganglionated region of Ednrb null mice: implications for Hirschsprung's disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(3):e233–e244. doi: 10.1111/nmo.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Developmental dynamics : an official publication of the American Association of Anatomists. 1999;216(2):137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Conner PJ, Focke PJ, Noden DM, Epstein ML. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;226(1):91–98. doi: 10.1002/dvdy.10219. [DOI] [PubMed] [Google Scholar]
- 18.Hackett-Jones EJ, Landman KA, Newgreen DF, Zhang D. On the role of differential adhesion in gangliogenesis in the enteric nervous system. Journal of theoretical biology. 2011;287:148–159. doi: 10.1016/j.jtbi.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development. 2006;133(10):2075–2086. doi: 10.1242/dev.02375. [DOI] [PubMed] [Google Scholar]
- 20.Hao MM, Young HM. Development of enteric neuron diversity. Journal of cellular and molecular medicine. 2009;13(7):1193–1210. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn B, Bergner AJ, Turner KN, Young HM, Anderson RB. Effect of Gdnf haploinsufficiency on rate of migration and number of enteric neural crest-derived cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(1):134–141. doi: 10.1002/dvdy.21013. [DOI] [PubMed] [Google Scholar]
- 22.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Progress in histochemistry and cytochemistry. 2010;44(4):173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2(3):381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell metabolism. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. The Anatomical record. 2001;262(1):1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Developmental biology. 2007;302(2):553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. The Journal of comparative neurology. 1991;314(4):789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 28.Bergner AJ, et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. The Journal of comparative neurology. 2014;522(3):514–527. doi: 10.1002/cne.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(24):8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalazonitis A, et al. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. The Journal of comparative neurology. 2008;509(5):474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alaynick WA, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell. 2011;146(1) doi: 10.1016/j.cell.2011.06.038. 178-178 e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High magnification micrograph of P30 neurons in dSI that have ChAT-GFP+ and ChAT-Cre tdTomato+ expression restricted to the nucleus (arrows). Scale bar= 50µm.