Abstract
Diet-induced obesity and metabolic syndrome are important contributors to cardiovascular diseases. The decreased nitric oxide (NO) bioactivity in endothelium and the impaired response of smooth muscle cell (SMC) to NO significantly contribute to vascular pathologies, including atherosclerosis and arterial restenosis after angioplasty. Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is an important mediator of NO function in both endothelial cells and SMCs, and its irreversible oxidation impair its stimulation by NO. We used C57BL/6J mice fed a high fat, high sucrose diet (HFHSD) to study the role of SMC SERCA in diet-induced obesity and metabolic syndrome. We found that HFHSD upregulated Nox2 based NADPH oxidase, induced inflammation, increased irreversible SERCA oxidation, and suppressed the response of aortic SERCA to NO. Cultured aortic SMCs from mice fed HFHSD showed increased reactive oxygen species production, Nox2 upregulation, irreversible SERCA oxidation, inflammation, and a decreased ability of NO to inhibit SMC migration. Overexpression of wild type SERCA2b or downregulation of Nox2 restored NO-mediated inhibition of migration in SMCs isolated from HFHSD-fed mice. In addition, tumor necrosis factor alpha (TNFα) increased Nox2 which induced SERCA oxidation and inflammation. Taken together, Nox2 induced by HFHSD plays significant roles in controlling SMC responses to NO and TNFα-mediated inflammation, which may contribute to the development of cardiovascular diseases in diet-induced obesity and metabolic syndrome.
Keywords: Nox2, nitric oxide, smooth muscle cell, sarco/endoplasmic reticulum Ca2+ ATPase, high fat high sucrose diet
Introduction
Diet-induced obesity and metabolic syndrome are important contributors to cardiovascular diseases, including vascular restenosis after angioplasty and atherosclerosis. Vascular smooth muscle cell (SMC) migration contributes significantly to these pathological processes. Generally, SMCs do not proliferate in arteries. However, when the endothelium is disrupted, the underlying medial SMCs migrate to the intima and form a neointima. Nitric oxide (NO), the biologically active component of endothelium-derived relaxing factor, has critical roles in the maintenance of vascular homeostasis including the regulation of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). SERCA is an important enzyme that maintains optimal intracellular calcium levels in SMCs by taking up calcium into the sarco/endoplasmic reticulum. NO stimulates SERCA activity by S-glutathiolation of the most reactive thiol on cysteine-674 (C674) resulting in inhibition of SMC migration.[1] Our previous studies in high glucose treated SMCs and in SMCs from obese Zucker rat, a model of obesity and insulin resistance, indicate that irreversible oxidation of C674 on SERCA is responsible for the failed inhibition of SMC migration by NO.[2–4]
Reactive oxygen species (ROS) are important determinants of vascular function, both as signaling intermediates for cell growth and differentiation and as modulators of pathological processes, such as restenosis and atherosclerosis.[5] Increased ROS production is the major cause of SERCA C674 irreversible oxidation.[2, 4] The NADPH oxidases are major ROS generating enzymes in the vasculature, and produce superoxide (O2−.) and either directly, or through dismutation, hydrogen peroxide (H2O2). NADPH oxidases are multi-component enzymes comprised of p22phox, gp91phox (also known as Nox2 or its homologues Nox1, 3–5) that are bound to the membrane, and p47phox, p67phox and the small G protein, Rac1, that are in the cytoplasm and contribute to NADPH oxidase activation. Normally, SMCs express mainly Nox4, responsible for a continuous, low-level of H2O2 production. Nox4 is predominantly localized at the endoplasmic reticulum and in the nucleus, and it is also speculated to be present in cytosol, plasma membrane and mitochondria.[6–8] Unlike Nox1 and Nox2, the activation of Nox4 does not require Rac1, p67phox or p47phox.[9–11] Both Nox1 and Nox2 are mainly expressed in cytosol and plasma membrane, and Nox1 is also believed to be expressed in the nucleus, endoplasmic reticulum and mitochondria.[7, 8] Though Nox1 and Nox2 have relatively low expression levels in SMCs, they are highly inducible and might account for the increased O2−. production in cardiovascular diseases.
We recently showed that mice fed with high fat high sucrose diet (HFHSD), a typical diet consumed by many Americans, become obese and glucose intolerant, recapitulating the human metabolic syndrome.[12] In addition, HFHSD-fed mice develop aortic stiffness, left ventricular hypertrophy and diastolic dysfunction; at a later stage, they become hypertensive.[12, 13] At early time points HFHSD also impairs endothelium-dependent relaxation and increases aortic SERCA oxidation.[12]. In addition, HFHSD also increased risk factors for atherosclerosis in rats, including hyperinsulinemia, hypertension, and hypertriglyceridemia.[14], accelerates atherosclerotic lesions in minipigs and in LDL receptor deficient mice,[15–17] and increases intimal hyperplasia after rat carotid endarterectomy.[18] Compared to another commonly used obesity model induced by high fat diet without sucrose,[19, 20] HFHSD fed mice gain less weight and have less insulin resistance.[21] These two obese models both develop glucose intolerance, insulin resistance, cardiac hypertrophy and myocardial dysfunction.[13, 19, 20] In the present study, we aimed to elucidate the response of aortic SMCs to NO and to explore the potential factors that may contribute to the development of cardiovascular diseases induced by HFHSD, including restenosis and atherosclerosis.
Material and Methods
Diet-induced obese mice
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee at Boston University. Male C57BL/6J mice (stock number 00664, The Jackson Laboratory, USA) were obtained at 8 weeks of age. After one week of acclimation, mice were fed control (normal diet, ND: 4.5 % fat, 0% sucrose) or HFHSD for 16 weeks (35.5% fat (lard) representing 60% calories, 16.4% sucrose)[22] ad libitum (catalog numbers D09071702 and D09071703, Research Diets, USA). The control diet was custom-formulated to match the micronutrients contained in HFHSD except for fat and sucrose. Mice were housed in rooms with 12-hour light/dark cycle and in groups of 3–4 whenever possible.
SERCA activity
SERCA activity was measured by 45Ca2+ uptake in a postnuclear supernatant fraction, as previously described by us.[23] Briefly, the entire mouse aorta from arch to iliac bifurcation free of fat and connective tissue was incubated with 0.1 µM phenylephrine for 30 minutes followed by NO (1µM, from gas) in Kreb’s buffer for 1 minute. The aorta was homogenized and centrifuged at 4000 rpm for 2 minutes at 4°C, and the supernatant was assayed. 45Ca2+ uptake is calculated by counting the radioactivity and standardized by protein concentration. NO-stimulated activity was calculated as the difference in activities in the presence and absence of NO gas.
Aortic smooth muscle cell culture
SMCs were isolated from aortas of mice fed ND or HFHSD for 16 weeks, and cultured as previously described.[2] SMC phenotype was confirmed by α-smooth muscle actin-positive immunostaining. Cells from passages 3 to 8 were used.
Real time quantitative PCR (qPCR)
Total cellular RNA was isolated in SMCs cultured in 0.2% fetal bovine serum (FBS) Dulbecco's Modified Eagle Medium (DMEM) overnight and converted to complementary DNA. Real time qPCR was performed with synthetic TAQMAN gene specific primers (Applied Biosystems), according to the following cycling conditions: denaturation, annealing, and extension at 95°C, 55°C and 72°C for 30 seconds, 30 seconds, and 1 minute, respectively, for 40 cycles, and β-actin acts as an internal control.
Wounded monolayer migration assay
[2, 3] Briefly, SMCs were plated at a density of 106 cells/well in a 12-well plate in 0.2% FBS DMEM overnight to reach confluence. Scratch wounds were applied to the SMC monolayer with a pipette tip. Immediately after scratching, the cells were treated with 10% FBS DMEM with or without NO donor, DETA NONOate (300 µM, Cayman Chem), to stimulate cell migration. Photographs were taken at 0 hour and 6 hours at three fixed locations along the scratch with a light microscope and analyzed using NIH ImageJ software.
Small interference RNA (siRNA) transfection
[2] Using routine methods, 80% confluent SMCs were transfected with Nox2 siRNA or scrambled control siRNA (Applied Biosystems, 60 nmol/L) for 48 h according to the manufacturer’s recommendations. In some experiments, recombinant human tumor necrosis factor alpha (TNFα, 10 ng/ml, R&D Biosciences) was added overnight before SMCs were collected.
Adenovirus overexpression of LacZ or SERCA2b
For some migration assays, SMCs were infected with adenovirus to overexpress LacZ or SERCA2b at multiplicity of infection 50 in 0.2%FBS DMEM for 3 days as previously described.[2
ROS production
SMCs were plated at a density of 104 cells/well in a 96-well plate overnight in 0.2% FBS DMEM. Intracellular ROS levels were determined by incubation with 20 µM 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA; Molecular Probes) in Hanks’ balanced salt buffer for 30 minutes. SMCs were then trypsinized, washed and resuspended in Hanks’ balanced salt solution. Dichlorofluorescein fluorescence was immediately measured by a CytoFluor multiwell plate reader at excitation and emission wavelength of 530 and 580 nm, respectively. Dihydroethidium (DHE, Molecular Probes)[24] was used to detect intracellular O2−.. SMCs were treated with 5 µM DHE for 30 minutes at 37°C before measuring fluorescence at 605 nm wavelength.
Immuno-blotting
SMCs were cultured in 0.2% FBS DMEM overnight and then lysed in cell lysis buffer (Cell Signaling). Proteins were subjected to SDS-PAGE and immunoblotted with antibodies to detect Nox4 (provided by Dr. Ajah Shah), Nox2 (BD transduction), Rac1 (BD transduction), SERCA and SERCAC674-SO3H (Bethyl Laboratory Inc), TNFα (Abcam), vascular cell adhesion molecule-1 (VCAM1, Santa Cruz); p65 nuclear factor kappa B (NFκB) and phospho-p65NFκB (Cell Signaling), and GAPDH (Cell Signaling) as loading control. Total endothelial nitric oxide synthase (eNOS) and its phosphorylation at serine 1177 (Cell Signaling) were detected in aortic homogenates, and normalized to β-actin. Proteins were visualized with an ECL system (Amersham Biosciences). Densitometry analysis was performed using NIH image J software, and protein expression levels were corrected by loading control.
Immunohistochemistry (IHC) staining
[2] Aortas were paraffin-embedded, sectioned and processed as previously described.[2] Sections were immunostained with Nox2, SERCA C674-SO3H, TNFα and VCAM1 antibodies and developed with Vectastain ABC kit (Vector) followed by counterstained with hematoxylin. Images were scored by blinder analysis for quantification.[2
Bone marrow-derived macrophage adhesion assay
[25] Bone marrow-derived mononuclear cells were isolated[26] from C57BL/6J mice and cultured in high glucose DMEM supplemented with 10% FBS and 20% L929-conditioned medium. Five days later, mononuclear cells became macrophage. For macrophage adhesion assay, 106 SMCs/well in 12-well plate were plated in 0.2%FBS DMEM overnight to reach confluence, then macrophages (5×105 cells/well) were added and incubated for 1 h before the media were removed and washed 3 times with PBS to remove all unbound macrophages. Four images of macrophage adhesion to SMCs in each well were taken and the number of bound macrophage was counted per image area.
Statistical analysis
All data are presented as mean±SEM. Student t-test was used to analyze data between two groups. A probability value of <0.05 is considered statistically significant.
Results
HFHSD upregulates Nox2, induces inflammation, causes SERCA oxidation, decreases SERCA activity, and decreases NO bioactivity in mouse aorta
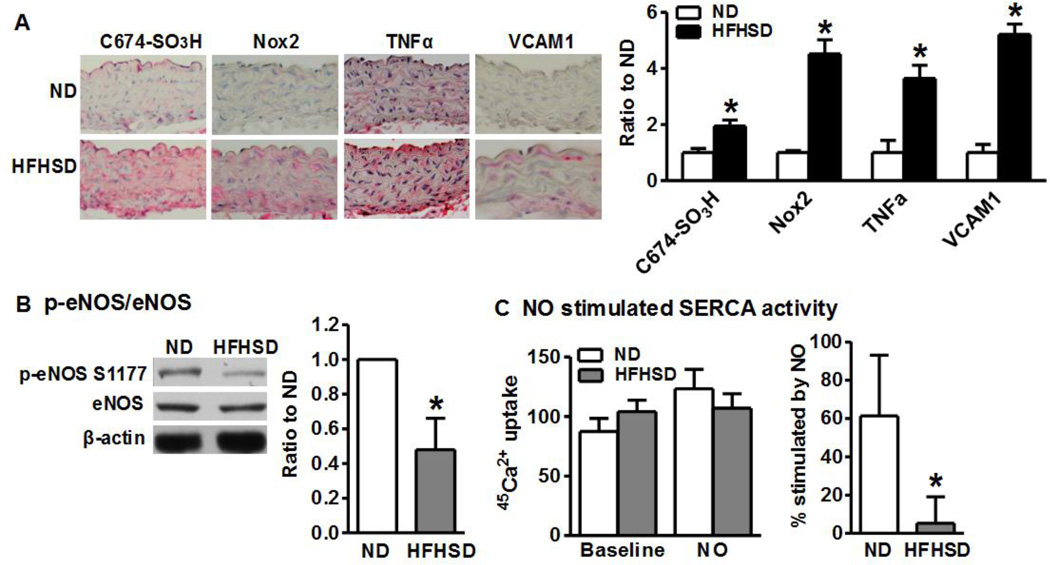
As shown in Figure 1A, similar to our previous studies at 32 weeks of diet feeding,[12] we found that irreversible oxidation of aortic SERCA C674 was significantly increased 16 weeks after HFHSD compared with ND. Although Nox2 was nearly undetectable in aorta from ND fed mice, it was increased over 4-fold by HFHSD. Consistent with our previous findings of increased TNFα mRNA level in HFHSD aorta,[12] TNFα immunostaining was significantly increased in aortas after 16 weeks HFHSD. Similarly, the inflammatory adhesion molecule, VCAM1, showed a 5-fold increase after HFHSD. We next tested NO and SERCA activity in isolated aorta. As shown in Figure 1B, HFHSD significantly down-regulated the phosphorylation of eNOS at its most critical activation site Ser-1177 compared with ND, consistent with the decreased eNOS activity. This is also consistent with impaired aortic relaxation to acetylcholine after HFHSD, as we previously reported [12]. In addition, similar to previous findings in cholesterol-fed rabbit aorta[27] there was no significant change in aortic SERCA activity caused by HFHSD (Figure 1C). However, as in the rabbits, the stimulation of aortic SERCA activity by NO that occurred in mice fed ND, did not occur in HFHSD-fed mouse aorta (Figure 1C) consistent with increased SERCA C674 oxidation in aortic SMC. Taken together, these results indicate that HFHSD increases aortic inflammation, Nox2 protein, and SERCA oxidation and decreases NO-induced SERCA activation.
Figure 1. High fat, high sucrose diet (HFHSD) increases Nox2, SERCA oxidation, inflammation and decreases NO bioactivity and NO-stimulated SERCA activity in intact aorta.
A. Representative immunostainings of aortas from mice fed HFHSD or normal diet (ND) for 16 weeks. Quantitation in graph. Pictures were taken at 40×. *p < 0.05 vs. ND, n=5–10. B. HFHSD inhibits aortic eNOS phosphorylation. *p < 0.05 vs. ND, n=3. C. HFHSD inhibits NO-stimulated aortic SERCA activity. *p < 0.05 vs. ND, n=5–8.
Nox2, SERCA oxidation, and inflammatory factors are increased in aortic SMCs from HFHSD-fed mice
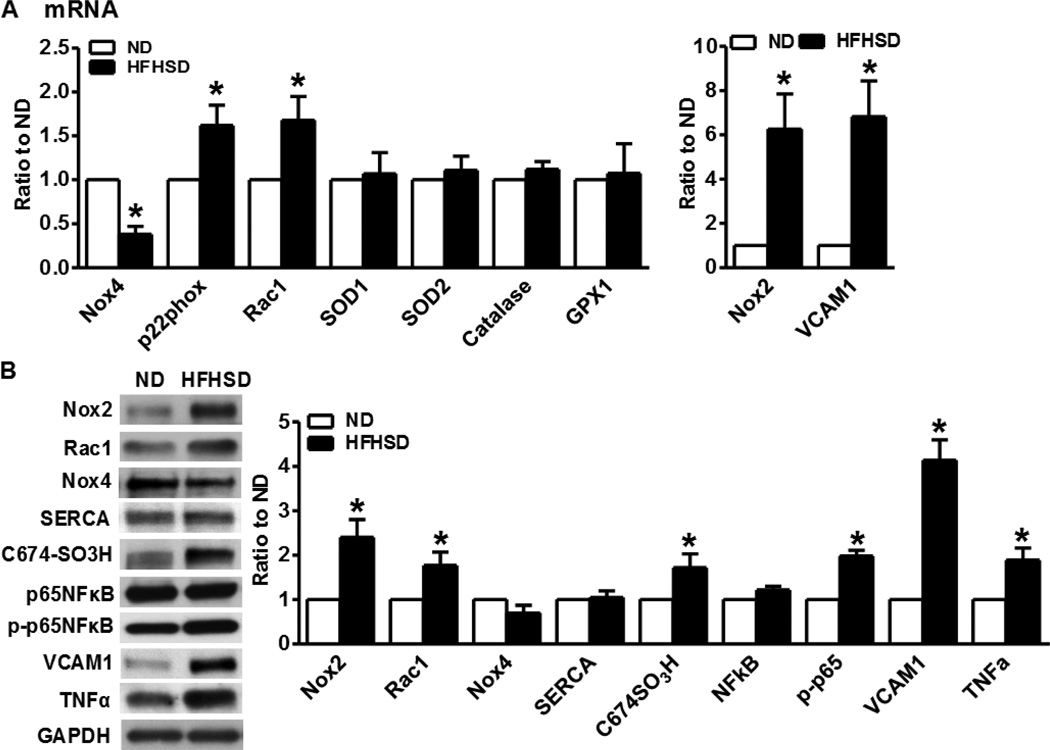
In addition to the decreased NO bioactivity in endothelium, we explored the contribution of smooth muscle to cardiovascular disease phenotypes induced by HFHSD. In isolated aortic SMCs, as shown in Figure 2A, HFHSD significantly increased Nox2, Rac1 and p22phox mRNA levels but it decreased Nox4 mRNA levels. The mRNA levels of Nox1, p47phox and p67phox were very low or undetectable (not shown). HFHSD had no effects on antioxidant enzymes superoxide dismutase (SOD) 1, SOD2, catalase and glutathione peroxidase 1 (GPX1) (Figure 1A). VCAM1 mRNA was highly induced by HFHSD (Figure 2A) while TNFα mRNA was undetectable in aortic SMCs from either group (not shown). Although Nox4 mRNA was downregulated by HFHSD, its protein levels were unaffected by HFHSD (Figure 2B). Nox2, Rac1 protein levels and SERCA C674 irreversible oxidation were upregulated by HFHSD (Figure 2B). p65/NFκB phosphorylation, which activates VCAM1 gene transcription, was also upregulated by HFHSD. Similar to the IHC staining of the whole aorta, the protein levels of both TNFα and VCAM1 were upregulated in SMCs from HFHSD-fed mice (Figure 2B), indicating the induction of inflammation in SMCs by HFHSD.
Figure 2. NADPH oxidase, SERCA oxidation and VCAM1 are increased in smooth muscle cells (SMCs) from HFHSD-fed mice.
A. mRNA levels of indicated genes in SMCs isolated from aortas of HFHSD- or ND-fed mice and cultured in 0.2% FBS DMEM overnight. *p < 0.05 vs. ND, n=5. B. Representative Western blots of homogenates of aortic SMCs isolated from HFHSD- or ND-fed mice and cultured in 0.2% FBS DMEM overnight. Nox2 (58 kDa), Rac1 (21 kDa), Nox4 (66 kDa), SERCA and SERCA C674-SO3H (110 kDa), p65NFκB and p-p65NFκB (65 kDa), VCAM1 (100 kDa), TNFα (17 kDa), GAPDH (36 kDa). Quantitation of band intensities in graph. *p < 0.05 vs. ND, n=3–10.
SMCs from HFHSD have increased ROS production, macrophage adhesion, and impaired NO-mediated inhibition of migration
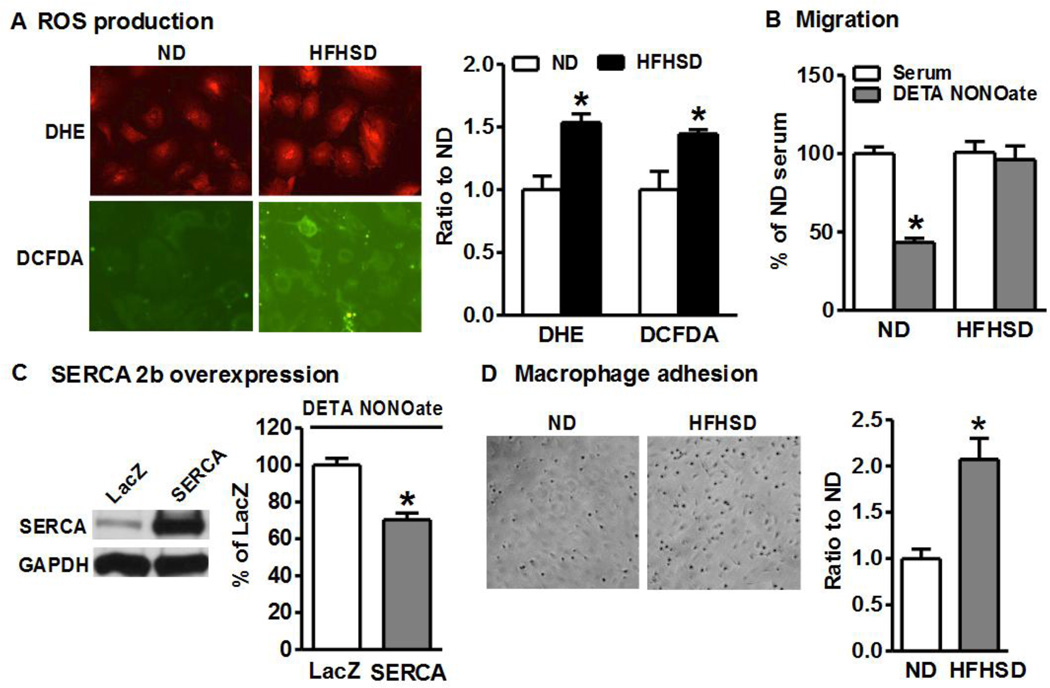
Because HFHSD upregulated Nox2 in SMCs, we measured ROS production in cultured aortic SMCs. DHE was used to detect intracellular O2−.. As shown in Figure 3A, HFHSD significantly increased intracellular O2−. levels compared with ND. DCFDA measurements also indicated that HFHSD significantly increased overall intracellular ROS production (Figure 3A).
Figure 3. Smooth muscle cells from HFHSD-fed mice have increased ROS and macrophage adhesion, and impaired NO-inhibited migration.
A. ROS production in aortic SMCs is increased after 16 week HFHSD. Quantitation of fluorescence intensities in graph. *p < 0.05 vs. ND, n=3. B. NO donor fails to inhibit migration of cultured aortic SMCs isolated from HFHSD-fed mice. *p < 0.05 vs. serum, n=12. C. Overexpression of SERCA2b restores the inhibition of cell migration by NO donor in HFHSD SMCs. *p < 0.05 vs. LacZ (vector control), n=5. D. HFHSD enhances macrophage adhesion to cultured SMCs, quantified in graph. *p < 0.05 vs. ND, n=6.
Since SERCA cysteine-674 is irreversibly oxidized in SMCs from HFHSD (Figure 2B) and our previous studies indicate that this SERCA cysteine is crucial for NO to inhibit SMC migration,[2, 4] we measured the effect of the NO donor, DETA NONOate on SMC migration. As shown in Figure 3B, although NO donor could significantly inhibit serum-induced migration of SMCs from ND-fed mice, it failed to do so in SMCs derived from HFHSD-fed mice, indicating impaired NO function in these SMCs. As shown in Figure 3C, overexpression of SERCA2b partially restored the ability of the NO donor to inhibit migration of SMCs from HFHSD-fed mice, confirming that rescuing SERCA oxidation by overexpressing SERCA2b can restore NO function, similar to our previous studies with high glucose-treated SMCs and SMCs from obese Zucker rats.[2, 4]
Although endothelium plays a key role in regulating leukocyte adhesion, SMCs can directly regulate leukocyte recruitment by releasing chemokines where the lining endothelial cells have been activated/denuded.[28, 29] Because HFHSD significantly upregulated TNFα and activated NFκB and VCAM1, we compared macrophage adhesion to SMCs from HFHSD and ND. As shown in Figure 3D, compared with ND, HFHSD significantly increased macrophage adhesion to SMCs, which is correlated with NFκB and VCAM1 activation.
Downregulation of Nox2 restores NO function and inhibits inflammation in HFHSD SMCs
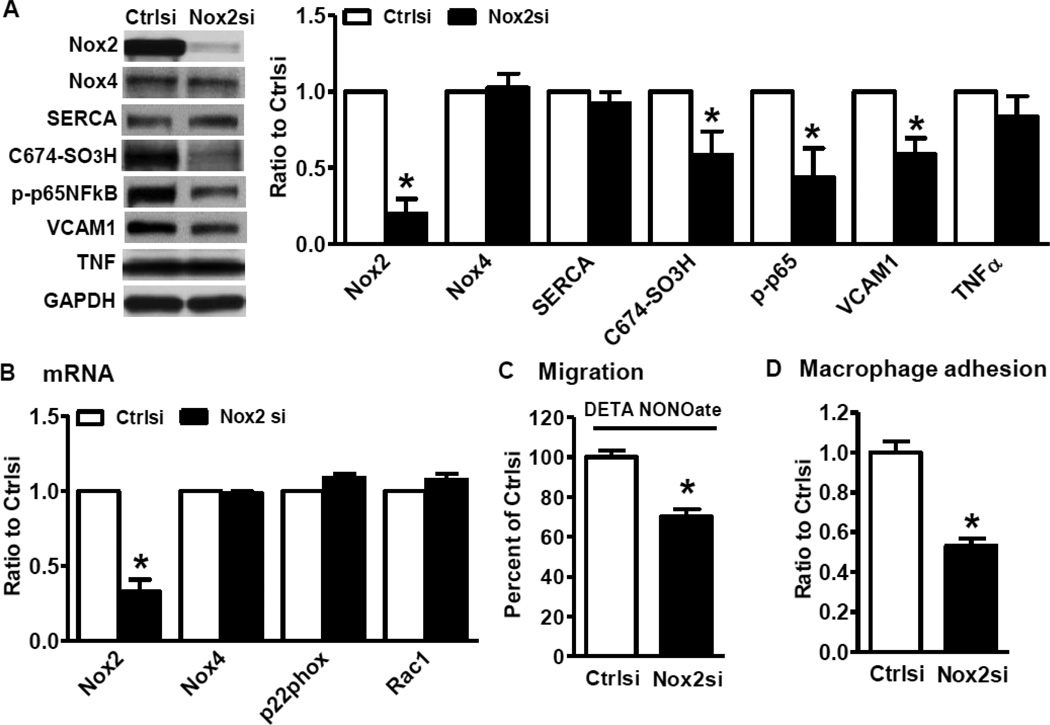
Because Nox2 was dramatically induced in HFHSD SMCs, we investigated its possible role in regulating NO function and inflammation in aortas of obese mice. As shown in Figure 4A, Nox2 siRNA specifically inhibited Nox2 protein levels, decreased irreversible SERCA oxidation, inhibited p65NFκB phosphorylation and reduced VCAM1 levels, but it had no effects on Nox4 or TNFα protein levels in SMCs from HFHSD. Downregulation of Nox2 had no effects on mRNA levels of other NADPH oxidase components (Figure 4B) and antioxidant enzymes (data not shown). Consistent with decreased irreversible SERCA oxidation following Nox2 knockdown, suppressing Nox2 also restored the inhibition of SMC migration by NO donor (Figure 4C) and reduced macrophage adhesion to SMC from HFHSD-fed mice (Figure 4D). Taken together, our data demonstrate that HFHSD-induced Nox2 upregulation contributes to impaired NO function and inflammation in aortic SMCs of diet-induced obese mice.
Figure 4. Downregulation of Nox2 restores NO function and inhibits inflammation in HFHSD SMCs.
A. Nox2 knockdown by siRNA decreases irreversible SERCA oxidation and the expression of inflammatory molecules. *p < 0.05 vs. control siRNA, n=3–7. B. Nox2 siRNA has no effects on other NADPH oxidase components mRNA. *p < 0.05 vs. control siRNA, n=3–6. C. Nox2 siRNA restored the inhibition of SMC migration by NO donor DETA NONOate. *p < 0.05 vs. control siRNA, n=6. D. Nox2 knockdown reduces macrophage adhesion to cultured SMCs isolated from HFHSD-fed mice. *p < 0.05 vs. control siRNA, n=4.
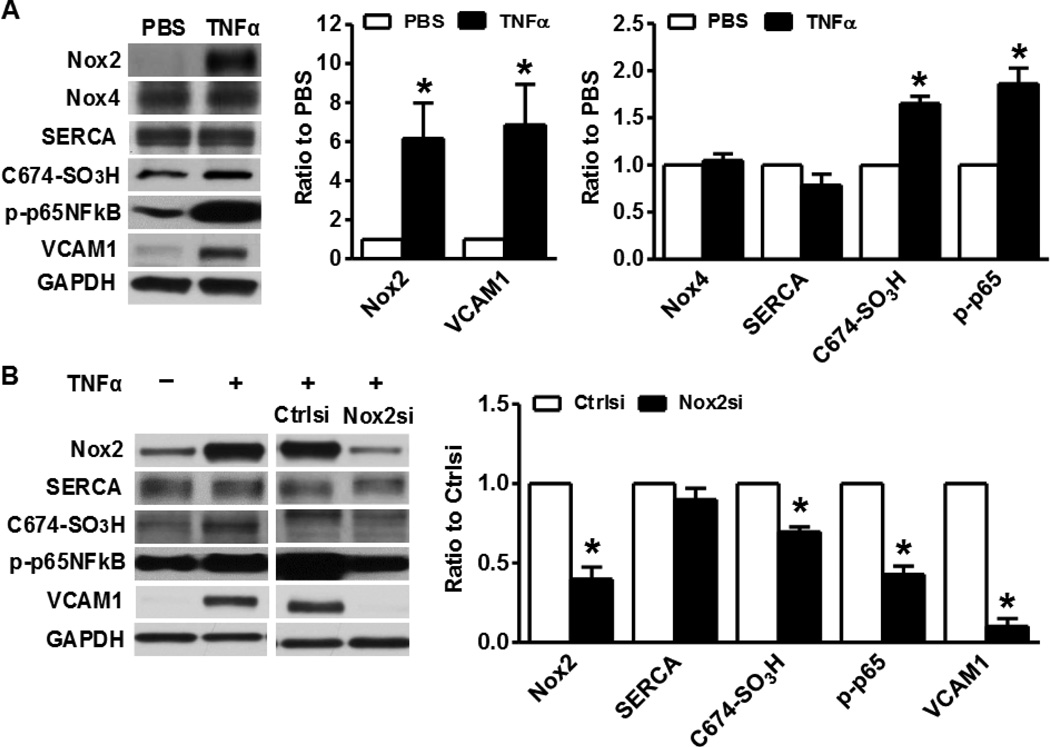
Nox2 mediates TNFα-induced SERCA oxidation and inflammation
Unlike Nox4, Nox2 is present in a very low level in SMCs, however Nox2 is dramatically induced after HFHSD. To explore the potential factors that could upregulate Nox2 in HFHSD, we treated SMCs with high palmitate, high glucose, high insulin and transforming growth factor beta 1 (TGFβ1), but none of these treatments that mimic factors associated with HFHSD was able to upregulate Nox2 (data not shown). Because HFHSD significantly upregulated TNFα levels in both aorta and aortic SMCs, and TNFα is known to activate NFκB, we reasoned that TNFα could stimulate Nox2-derived ROS production thus causing SERCA oxidation, and inducing inflammation in HFHSD SMCs. As shown in Figure 5A, overnight administration of TNFα to SMCs dramatically induced Nox2 expression, caused irreversible SERCA oxidation, activated NFκB and increased VCAM1 expression. These effects were all inhibited by silencing Nox2 (Figure 5B). These results indicate that HFHSD-stimulated TNFα in SMCs is responsible for Nox2 activation, SERCA oxidation and inflammation, and may account for the development of cardiovascular diseases induced by HFHSD.
Figure 5. Nox2 mediates TNFα-induced SERCA oxidation and inflammation in SMCs.
A. TNFα (10 ng/ml) increases Nox2 expression, SERCA oxidation, and activates NFκB and VCAM1 in SMCs. SMCs were isolated from aortas of mice fed ND. Band intensities normalized by GADPH and expressed as percentage of PBS-control. *p < 0.05 vs. PBS, n=3–6. B. Nox2 siRNA blocks the effects of TNFα on SERCA oxidation and inflammation. Quantitation of band intensities indicated in graph. *p < 0.05 vs. control siRNA, n=3–6.
Discussion
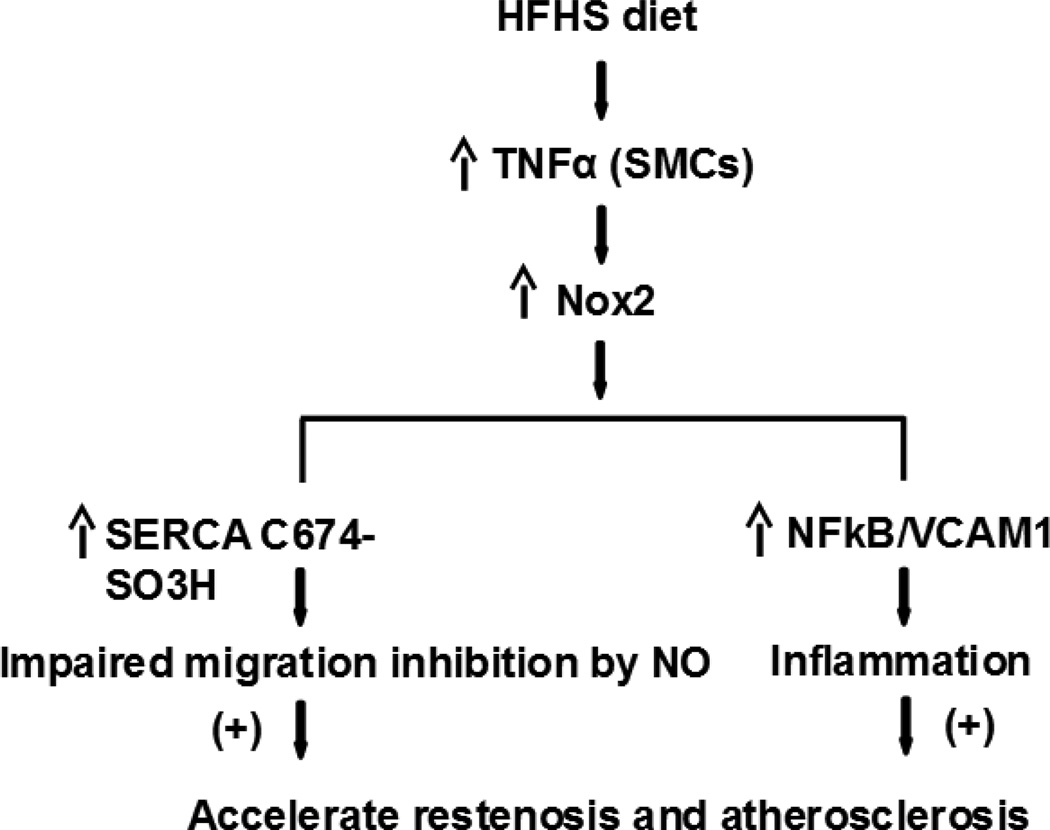
The major finding of this study is that, in a model of diet-induced obesity, the inflammatory cytokine TNFa is upregulated and stimulates Nox2 activity in intact aorta and cultured aortic SMCs, resulting in impaired NO and SERCA bioactivities. In fact, several indices of oxidative stress, including irreversible SERCA oxidation, decreased eNOS S1177 phosphorylation, and decreased NO-stimulated SERCA activity were observed in intact aorta from obese mice compared to controls. This is consistent with our previous findings of impaired endothelium-dependent aortic relaxation[12] and with the current findings of impaired migratory response of SMC to exogenous NO after HFHSD. Overexpressing SERCA2b in SMC from aortas of obese mice restored NO-inhibited migration, indicating that proper levels of redox-responsive, functional SERCA in SMCs is critical in maintaining the response to NO. As summarized in Figure 6, in aortic SMCs from obese mice, TNFα activates Nox2, which causes SERCA oxidation and impaired SMC migratory response to NO, and concomitantly, it activates the inflammatory molecules NFκB and VCAM1. These pathways can co-dependently contribute to the development of obesity-associated cardiovascular diseases, including restenosis after angioplasty and atherosclerosis.
Figure 6. Scheme of the potential mechanisms by which HFHSD contributes to cardiovascular diseases.
HFHSD-induced TNFα stimulates Nox2 and inflammation, leading to SERCA oxidation, decreased NO bioactivity, and increased SMC migration and macrophage adhesion to SMC, which can all contribute to vessel restenosis and atherosclerosis in settings of obesity.
A key early event in the development of atherosclerosis and restenosis is decreased NO bioactivity in endothelium. NO in small amounts is thought to inhibit atherosclerosis, at least in part, by inhibiting vascular SMC growth, leukocyte adhesion, and platelet adherence and aggregation. In addition, an impaired response of SMCs to NO may further contribute to development of vascular dysfunction. HFHSD-fed mice showed impaired endothelium-dependent relaxation and developed aortic stiffness, which is an early biomarker of atherosclerosis.[12, 30] We further confirmed that both NO bioactivity and NO-stimulated SERCA function were impaired in intact aorta. Our previous study in SMCs indicate that irreversible oxidation of the key reactive C674 on SERCA is responsible for the abnormal response to NO, which accounts for the increased lesion size after carotid artery endothelial denudation.[2] Upregulated ROS production is implicated in atherosclerosis and restenosis.[2, 31–33] Our studies suggest that HFHSD accelerates atherosclerotic lesions and increases intimal hyperplasia,[15–18] which might be in part via increased ROS production, irreversible SERCA oxidation, and induced inflammation.
The major source of ROS in vasculature that causes SERCA oxidation is NADPH oxidase. Our previous studies indicate that both Nox1 and Nox4 are responsible for SERCA oxidation and impaired NO bioactivity in SMCs treated with high glucose in vitro.[4] Similarly, in obese Zucker rat, a model of insulin-resistance, we showed that upregulation of TGFβ1 and Nox4 accounted for the impaired NO function and neointimal hyperplasia.[2] The present study strongly supports the conclusion that the oxidant source causing SERCA oxidation and NO reduction in aortas after HFHSD is Nox2. Compared to our previous findings, here we found that in diet-induced obese mice, which are also glucose intolerant and insulin resistant, TNFα and Nox2 were dramatically induced while Nox4 was not affected and Nox1 was undetectable in aorta and SMCs. Therefore, despite both inflammation and oxidant stress being involved in vascular pathologies associated to type 2 diabetes and insulin-resistance, the source of ROS and the inflammatory mediators may be different in different models. Here we show for the first time that high fat, high sucrose diet, but not high glucose or high insulin strongly stimulates TNFα induction and Nox2 expression and activity, and this in turn results in SERCA oxidation, impaired SERCA activity/NO response and NFκB/VCAM1 activation. In addition, increased Nox2 activity/ROS production in SMCs and aorta from HFHSD-fed mice may cause eNOS uncoupling,[34] which further contributes to the increased O2−. production. In addition to the vasculature, Nox2 is a major source of ROS in macrophages. Nox2 ablation in mice with ApoE knockout background suppresses the progression of atherogenesis possibly by reducing macrophage recruitment to the vessel.[32] Although overexpression of Nox2 in endothelial cells increases ROS production and macrophage recruitment, causing vascular dysfunction,[35, 36] the role of SMC Nox2 in regulating leukocyte adhesion has been less investigated. Here we found that Nox2 upregulation after HFHSD accounts not only for irreversible SERCA oxidation, decreased NO-stimulated SERCA activity and NO-mediated inhibition of SMC migration, but also for increased macrophage adhesion to SMCs.
In summary, our study indicates that the upregulation Nox2 by HFHSD in aortic SMC contributes to irreversible SERCA oxidation and impaired NO response of SMCs. Downregulation of Nox2 can restore NO-mediated inhibition of SMC migration and reduce TNFα-mediated inflammation, indicating that targeting Nox2 may be beneficial in controlling the development of vascular diseases characterized by SMC migration and inflammation such as atherosclerosis and restenosis, particularly in settings of diet-induced obesity and metabolic syndrome.
Acknowledgments
This work was supported by American Diabetes Association award 7-09-JF-69, NIH R01 HL031607, and R37 HL104017. The authors thank Dr. Lan Huang and support from the National Natural Science Foundation of China (31301167) and China Scholarship Council (2011761016).
Abbreviations
- DCFDA
2’,7’-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- DMEM
Dulbecco's Modified Eagle Medium
- FBS
fetal bovine serum
- GPX1
glutathione peroxidase 1
- H2O2
hydrogen peroxide
- HFHSD
high fat high sucrose diet
- IHC
immunohistochemistry
- ND
normal diet
- NO
nitric oxide
- O2−.
superoxide
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- SERCA
sarco/endoplasmic reticulum Ca2+ ATPase
- siRNA
small interference RNA
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- TGFβ1
transforming growth factor beta 1
- TNFα
tumor necrosis factor alpha
- VCAM1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None declared.
References
- 1.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, et al. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:783–790. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 2.Tong X, Hou X, Jourd’heuil D, Weisbrod RM, Cohen RA. Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circulation Research. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong X, Schröder K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free Radical Biology & Medicine. 2009;47:1578–1583. doi: 10.1016/j.freeradbiomed.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. Journal of Molecular and Cellular Cardiology. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circulation Research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 6.Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. -Review and perspective- Circulation Journal : Official Journal of the Japanese Circulation Society. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 7.Von Löhneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Molecular and Cellular Biology. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH Oxidases in Vascular Pathology. Antioxidants & Redox Signaling. 2013:00. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambasta RK, Kumar P, Griendling KK, Schmidt HHHW, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. The Journal of Biological Chemistry. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 10.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Meng D, Lv D-D, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovascular Research. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 12.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King Ca, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, et al. The Polyphenols Resveratrol and S17834 Prevent the Structural and Functional Sequelae of Diet-Induced Metabolic Heart Disease in Mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard RJ, Faria DJ, Menges JE, Martin DA. Effects of a high-fat, sucrose diet on serum insulin and related atherosclerotic risk factors in rats. Atherosclerosis. 1993;100:229–236. doi: 10.1016/0021-9150(93)90209-d. [DOI] [PubMed] [Google Scholar]
- 15.Xi S, Yin W, Wang Z, Kusunoki M, Lian X, Koike T, et al. A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. International Journal of Experimental Pathology. 2004;85:223–231. doi: 10.1111/j.0959-9673.2004.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabolism. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyalala JO, Luo S, Campbell DN, Brown AT, Moursi MM. The effects of acarbose treatment on intimal hyperplasia in a rat carotid endarterectomy model of diet-induced insulin resistance. Vascular and Endovascular Surgery. 2010;44:560–567. doi: 10.1177/1538574410377019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yuan M, Bradley KM, Dong F, Anversa P, Ren J. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension. 2012;59:680–693. doi: 10.1161/HYPERTENSIONAHA.111.181867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Editor D. Letter to the Editor Akt 2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. 2013:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar B, Pacini G, Ahrén B. Differential development of glucose intolerance and pancreatic islet adaptation in multiple diet induced obesity models. Nutrients. 2012;4:1367–1381. doi: 10.3390/nu4101367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 23.Adachi T, Matsui R, Weisbrod RM, Najibi S, Cohen RA. Reduced sarco/endoplasmic reticulum Ca(2+) uptake activity can account for the reduced response to NO, but not sodium nitroprusside, in hypercholesterolemic rabbit aorta. Circulation. 2001;104:1040–1045. doi: 10.1161/hc3501.093798. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radical Biology & Medicine. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 25.Bayat H, Xu S, Pimentel D, Cohen RA, Jiang B. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:127–134. doi: 10.1161/ATVBAHA.107.150250. [DOI] [PubMed] [Google Scholar]
- 26.Wang H-W, Liu P-Y, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Medicine. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 28.Simon DI. Inflammation and Vascular Injury. Circulation Journal. 2012;76:1811–1818. doi: 10.1253/circj.cj-12-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins PN. Molecular biology of atherosclerosis. Physiological Reviews. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 30.Stamatelopoulos K, Karatzi K, Sidossis LS. Noninvasive methods for assessing early markers of atherosclerosis: the role of body composition and nutrition. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12:467–473. doi: 10.1097/MCO.0b013e32832f0d99. [DOI] [PubMed] [Google Scholar]
- 31.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, et al. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. The Journal of Clinical Investigation. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. American Journal of Physiology Heart and Circulatory Physiology. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 33.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. Nox1 Plays a Key Role in Diabetes Accelerated Atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 34.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circulation Research. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 35.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circulation Research. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 36.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, et al. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE−/− mice. Cardiovascular Research. 2012;94:20–29. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]