Abstract
Fractures are a frequent source of morbidity in children with disabling conditions. The assessment of bone density in this population is challenging, because densitometry is influenced by dynamic forces affecting the growing skeleton and may be further confounded by positioning difficulties and surgical hardware. First-line treatment for pediatric osteoporosis involves conservative measures, including optimizing the management of underlying conditions, maintaining appropriate calcium and vitamin D intake, encouraging weight-bearing physical activity, and monitoring measurements of bone mineral density. Bisphosphonates are a class of medications that increase bone mineral density by inhibiting bone resorption. Although bisphosphonates are commonly prescribed for treatment of adult osteoporosis, their use in pediatric patients is controversial because of the lack of long-term safety and efficacy data.
INTRODUCTION
Growing awareness of bone health in pediatric patients has increasingly led practitioners to evaluate and treat children for low bone mineral density (BMD), including children with either primary bone conditions or other disabling conditions that lead to secondary osteoporosis. Bisphosphonates are a staple of osteoporosis treatment and have been used extensively in adults for conditions associated with bone fragility [1]. The literature pertaining to adults supports improvement in clinical outcomes with the use of bisphosphonates [2,3]; however, because of differences in pediatric skeletal metabolism, caution is required when attempting to extrapolate adult data to children. This review will apprise practitioners of the current literature regarding bisphosphonate treatment in children with disabilities, address controversies regarding safety and efficacy, and discuss future directions for improving the knowledge gap in treatment of children with skeleton-related conditions.
BONE MODELING AND REMODELING
Bone remodeling is a continuous, lifelong process in which mature bone is broken down by osteoclasts and new bone is formed by osteoblasts. This process underlies BMD changes in adults, as well as fracture healing and repair of skeletal microdamage. Tight coupling of bone formation and resorption is required to maintain skeletal homeostasis. In childhood, skeletal growth occurs as the result of strictly regulated uncoupling of bone formation and resorption at specific sites, termed “bone modeling” [4]. On the outer periosteal surface, the formation of bone leads to an increase in bone size, driven by genetic factors and mechanical loading forces [5,6]. Bone resorption expands the marrow cavity on the inner periosteum and sculpts the bone on the outer surface, establishing the widened, funnel-like shape of the metaphyses [7,8]. The net result of bone modeling is an overall increase in bone size and mass.
In many skeletal disorders the bone remodeling cycle is disrupted, leading to a net loss of BMD. Treatment strategies include altering the cycle to either inhibit osteoclast activity or promote osteoblast activity, with the goal of shifting the balance in favor of bone formation.
BISPHOSPHONATES
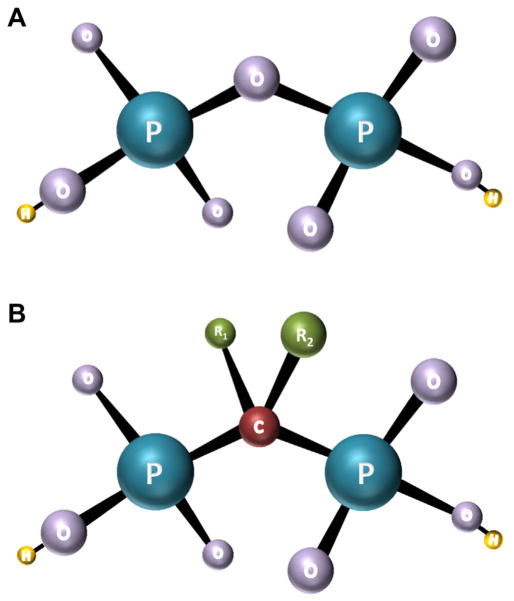
Bisphosphonates are a class of drugs that increase BMD by inhibiting osteoclast activity. They are synthetic analogs of pyrophosphate, an endogenous regulator of bone metabolism. In bisphosphonates, the central oxygen atom in pyrophosphate is replaced with a carbon atom (Figure 1). All bisphosphonates share a common phosphorus-carbon-phosphorus motif with 2 side chains (R1 and R2 in Figure 1). The R2 side chain determines the chemical properties of the drug and distinguishes individual types of bisphosphonates. This chemical structure affords a high affinity for calcium hydroxyapatite, permitting rapid and specific targeting of the skeleton.
Figure 1.
Chemical structure of pyrophosphate (A) and bisphosphonates (B). P = phosphorus, O = oxygen, H = hydrogen, C = carbon, R = side chain.
Bisphosphonates have 2 classes with distinct mechanisms of action [9]. The early compounds that do not contain nitrogen (ie, clodronate, tiludronate, and etidronate) are incorporated into the terminal pyrophosphate moiety of adenosine triphosphate, forming a nonfunctional molecule that disrupts osteoclast metabolism and apoptosis. Newer, more potent bisphosphonates that contain nitrogen (ie, pamidronate, alendronate, ibandronate, risedronate, and zoledronate) inhibit a key enzyme, farnesyl pyrophosphate synthase, in the mevalonic acid pathway. Inhibition of this enzyme blocks posttranslational modification of small guanosine triphosphatases such as Ras, Rho, and Rac, which act as signaling molecules for key components of osteoclast function. These effects disrupt osteoclast activity, reduce osteoclast recruitment, and induce apoptosis [10].
The bioavailability of oral bisphosphonates is low, with an estimated absorption rate of 0.6%–2.5% [11]. Approximately 40%–60% of each dose is incorporated into bone, and the remainder is excreted unchanged in the urine [11]. The terminal half-life of bisphosphonates in bone is estimated to exceed 10 years, reflecting release from the skeleton with bone remodeling [12]. Bisphosphonates have been detected in the urine of children up to 8 years after use of the drug is discontinued, consistent with prolonged skeletal release after the completion of treatment [13].
LOW BMD IN CHILDREN
Assessment of BMD in Children
As in adults, the most commonly used method to asses BMD in children is dual x-ray absorptiometry (DXA). This modality offers practical advantages, including wide availability, rapid scanning time, and low use of radiation. In children the posteroanterior spine and total body minus the head are the most accurate and reproducible sites and should be used preferentially [14]. DXA results are expressed as z scores calculated from age, gender, and ethnicity-adjusted norms, and should be derived from normative databases specific to the brand of densitometer used [15].
Children with disabilities present unique challenges with regard to evaluation by DXA [16,17]. Contractures may prevent patients from lying in the proper fully supine position. Lumbar spine evaluation may be hindered by scoliosis and the placement of surgical hardware. Proximal femoral anatomy may likewise be disrupted as a result of hip dysplasia, subluxation, or dislocation, which may require placement of surgical hardware. The lateral distal femur is frequently used as an alternate imaging site in these patients, offering the advantages of easier positioning and rare interruption by surgical hardware. This area also offers the potential for subregional analysis, where bone may be separated into primarily cortical and trabecular compartments based on its location [16].
A number of limitations inherent in DXA technology complicate its application to children. Because BMD is greatly influenced by sex steroids, children with early or delayed puberty may be compared with children at a different pubertal stage. A primary limitation is the inability of DXA to account for bone depth. DXA approximates BMD by quantifying the bone mineral content of a 2-dimensional area of interest. Areal BMD is thus expressed in grams of mineral per centimeters squared, as opposed to true volumetric BMD (defined by mineral mass in three dimensions, ie, grams per centimeters cubed). Because areal BMD is dependent on bone size, DXA z scores will be inaccurate in children with heights significantly greater or less than the mean for age. This factor is particularly relevant in children with chronic diseases, who frequently have impairments in growth and sexual maturation. A number of approaches have been proposed to address the impact of skeleton size on DXA results, including mathematical models and adjustment for height [18,19]. Longitudinal data are limited, and no consensus exists with regard to the preferred method. Practitioners thus must use a patient-specific approach when interpreting DXA results, taking into account height, bone age, and pubertal status.
Several emerging densitometry methodologies offer significant technical advantages compared with DXA. Quantitative computed tomography of the spine has the capability of assessing BMD in 3 dimensions, thus providing measurements of true volumetric bone density. This technique also assesses bone microarchitecture, distinguishing between trabecular and cortical bone [20]. Unfortunately, its applicability to routine clinical use has been hindered by high radiation exposure, expense, and availability. Quantitative computed tomography may be performed on the peripheral skeleton with use of only minimal radiation; however, interpretation of longitudinal measurements is confounded by growth effects on the size and shape of the skeleton [21]. Quantitative ultrasound is an emerging method in which BMD is determined by quantifying attenuation of ultrasound waves through bone [22]. This technique offers multiple advantages, including information about bone structure and biomechanical properties, relatively low cost, lack of radiation, and portability. However, because of a lack of standardization of technique and reference data, routine clinical use is currently limited.
Definition of Osteoporosis in Childhood
The overlying objective in identifying and treating low BMD is prevention of fractures. BMD in adults is highly predictive of fracture risk, and osteoporosis typically is diagnosed on the basis of a DXA T score less than −2.5. The implications of low BMD in childhood are less clear. Data for healthy children suggest a 1- to 2-fold increased fracture risk for every −1 decrease in z score [23,24]; however, few studies have correlated fractures and BMD in children with chronic illnesses. Given the lack of a defined relationship between DXA results and the risk of fracture, the criteria for diagnosis of pediatric osteoporosis has been an area of controversy. In 2007, an expert panel recommended that pediatric osteoporosis be diagnosed on the basis of a DXA z score less than −2 in conjunction with a clinically significant fracture history, defined as a lower extremity long-bone fracture, vertebral compression fracture, or 2 or more upper extremity long-bone fractures [14]. The current definition of osteoporosis in children is thus based on a combination of clinical and radiographic features, marking a major departure from adult practice.
Disorders Associated With Osteoporosis in Children
Conditions leading to low BMD in childhood are numerous and diverse in presentation (Table 1). In general, they may be divided into 2 broad categories: primary osteoporosis, resulting from intrinsic skeleton abnormalities, and secondary osteoporosis, where factors external to the skeleton impair mineralization. Primary osteoporosis arises from genetic disorders, whereas secondary osteoporosis results from a diversity of processes, including endocrinopathies, disuse, malnutrition, and other processes. A representative selection is included in Table 1.
Table 1.
A representative sample of disorders associated with low bone mineral density in children
| Primary bone disorders |
| Osteogenesis imperfecta |
| Fibrous dysplasia |
| Hypophosphatasia |
| Osteoporosis pseudoglioma syndrome |
| Ehlers-Danlos syndrome |
| Idiopathic juvenile osteoporosis |
| Secondary bone disorders |
| Impaired mobility |
| Cerebral palsy |
| Spina bifida |
| Spinal cord injury |
| Traumatic brain injury |
| Duchenne muscular dystrophy |
| Chronic inflammatory conditions |
| Juvenile idiopathic arthritis |
| Inflammatory bowel disease |
| Systemic lupus erythematosus |
| Nutritional deficiencies and malabsorption |
| Vitamin D deficiency |
| Calcium deficiency |
| Celiac disease |
| Anorexia nervosa |
| Liver failure |
| Cystic fibrosis |
| Infiltrative disorders |
| Leukemia |
| Inborn errors of metabolism |
| Endocrinopathies |
| Hypogonadism |
| Hyperparathyroidism |
| Hyperthyroidism |
| Growth hormone deficiency |
| Medication-induced and iatrogenic effects |
| Antiepileptics |
| Chemotherapeutics |
| Glucocorticoids |
| Radiotherapy |
Given the broad range of possible conditions, the evaluation for pediatric osteoporosis must be directed by history (including fracture history) and physical examination (with special attention to both axial and appendicular appearance and alignment). In general, a basic laboratory workup includes assessment of mineral metabolism (including calcium, phosphorus, parathyroid hormone, and vitamin D levels) and screening for chronic disease (including blood count, chemistry panel, inflammatory markers, and celiac screening).
Additional testing should be guided by clinical suspicion. Measurement of bone turnover markers such as alkaline phosphatase, osteocalcin, and N-terminal telopeptides may suggest a state of high or low turnover. Levels must be interpreted cautiously, however, because (1) bone turnover varies depending on maturation of the skeleton; (2) normal pediatric ranges have not been well established; and (3) markers are increased during fracture healing.
General Measures for Treatment of Pediatric Osteoporosis
The initial steps in treatment of low BMD are largely conservative. Studies have shown that secondary osteoporosis in childhood is largely reversible with remission or optimization of the primary causative condition [25,26]; therefore, primary management involves the identification and treatment of underlying disorders. Maintaining appropriate vitamin D and calcium intake is essential. Target serum 25-hydroxyvitamin D levels have been an area of debate, with the Institute of Medicine recommending levels greater than 20 ng/mL for healthy children [27], and the Endocrine Society recommending 40–60 ng/mL [28]. Nutritional intake should also be addressed, because malnutrition is associated with BMD impairment. Correction of nutrition is supported by studies of patients with anorexia nervosa; these studies have shown improvement in BMD with weight gain and improved nutritional status [29].
Physical activity is essential for bone health and should be assessed in all children with low BMD. An analysis of 22 controlled trials regarding the effects of weight-bearing exercise on BMD in healthy children reported overall positive effects, particularly during early puberty [30]. Weight-bearing physical activity programs in children with impaired mobility have been shown to lead to improvements in BMD [31]. Some reports have indicated improved BMD as a result of standing on vibrating platforms [32], although data are mixed [33]. Families of children with severely compromised bone health should be counseled about the need for the child to avoid physical activities associated with high risk of fracture, including contact sports, activities involving high impact to the neck and spine (such as horseback riding and riding roller coasters), and forward flexion exercises.
Selected Disabling Conditions Associated With Osteoporosis
Osteogenesis Imperfecta
Osteogenesis imperfecta (OI) is a group of heritable disorders that constitutes the most common cause of primary osteoporosis in children and adults. Persons with OI have bone fragility as a result of defects in collagen I synthesis, which is a critical component of bone matrix. More than 90% of persons with OI can be identified by mutations in COL1A1 or COL1A2, the genes encoding type I collagen alpha chains [34]. A wide spectrum of clinical severity exists, with relatively poor genotype-phenotype correlation. Clinical features vary depending on the level of severity. In patients with moderate to severe disease, fractures develop in infancy and early childhood, leading to kyphoscoliosis, long bone deformities, short stature, and loss of mobility. Persons with mild disease present with a variable number of fractures in childhood, and their condition generally improves after puberty.
Studies relating to OI represent the largest body of literature of bisphosphonate use in children. The first study of cyclic pamidronate in children with OI was published in 1998, and since then multiple studies have reported improvements in BMD, pain, vertebral body morphology, histomorphometric analyses, and fractures [35–42]. Beneficial effects have been reported with multiple formulations, including neridronate [43], zoledronic acid [44–46], alendronate [47–49], and olpadronate [50], with studies of risedronate yielding conflicting results [51,52].
Two meta-analyses that examined the efficacy of bisphosphonate treatment for OI reported inconsistent results. In 2009 a Cochrane review of 8 randomized trials found a significant difference in fractures in only one trial, with no effect in 3 trials [53]. Two trials reported an increase in spinal BMD. In a separate analysis using different methodology, Castillo and Samson-Fang [54] also identified 8 trials for review and reported consistent effects on BMD and evidence for fracture reduction in 3 of 4 small controlled trials. The benefits of bisphosphonate treatment on the skeleton in children with OI have thus not been conclusively determined. Data suggest short-term improvements in BMD, whereas effects on fracture risk remain an area of debate.
The optimal bisphosphonate regimen in children with OI has not been standardized. Intravenous formulations are most commonly used, because oral bisphosphonates appear to have less pronounced pain effects [49]. Data are insufficient to definitively identify which children with OI will benefit from treatment with bisphosphonates. The literature disproportionately reports on patients with moderate to severe disease, and extrapolation of this data to children with mild OI is inappropriate. Currently, routine treatment of mildly affected children is not recommended [55]. Similarly, the optimal time to initiate treatment has not been determined. Most children included in controlled trials were older than 2 years; however, 2 studies in infants have shown positive effects with pamidronate treatment, including improvement in BMD [36,56], vertebral body morphology [56], bone histomorphometric measures [56], and the incidence of fracture [36].
Fibrous Dysplasia
Fibrous dysplasia (FD) is a rare disorder in which normal bone and marrow are replaced by fibro-osseous tissue [57]. FD may occur in isolation or in association with cutaneous hyperpigmentation and hyper-functioning endocrinopathies, termed McCune-Albright syndrome [58]. FD/McCune-Albright syndrome arises from somatic activating GNAS mutations, leading to bone marrow stromal cell proliferation and production of abnormal matrix prone to fracture, deformity, functional impairment, and pain.
Currently no effective medical treatments exist for persons with FD. Use of bisphosphonates has been advocated because of the prominent osteoclastogenesis present in FD tissue. Early studies of pamidronate showed positive preliminary results, with reported improvement in pain, bone turnover markers, and the radiographic appearance of FD lesions [59–61]. The authors of larger studies have been unable to replicate the radiographic effects, although they have demonstrated consistent benefits regarding pain and turnover markers [62–64]. Defining the role of bisphosphonates in the management of FD has been limited by a lack of placebo-controlled trials, and at present, their indication is limited to treatment of bone pain.
Conditions Associated With Impaired Mobility
Bone loss is a known complication of acute and chronically impaired mobility [65,66]. Many children with impaired mobility have additional risk factors for poor bone health, including anticonvulsant use, spasticity, poor nutrition, and impaired coordination leading to increased risk of falls and fractures. Antiresorptive therapy is not an intuitive choice for treatment of osteoporosis resulting from impaired bone formation. However, in part because of a lack of available anabolic medications, practitioners increasingly are using bisphosphonates for treatment of low BMD in children with these conditions.
Cerebral Palsy
Cerebral palsy (CP) is a heterogeneous group of nonprogressive disorders impairing movement and posture that arise from abnormalities in the motor center of the brain. CP has many causes, including perinatal infections, asphyxiation, and stroke, among others [67]. Osteoporosis is common in children with CP. Approximately 80% of patients with severe CP have low BMD, with an annual fracture incidence of 4% [68,69]. Three controlled trials of bisphosphonates for children with CP have been conducted, with pamidronate used in 1 trial [70] and risedronate used in 2 trials [71,72]. All studies demonstrated beneficial effects on BMD but were insufficiently powered to detect an effect on fracture incidence. A recent meta-analysis of 5 studies concluded that the data support probable efficacy for increasing BMD and possible efficacy in reducing fractures in children with CP [73]. Because of the relatively small numbers and lack of long-term safety and efficacy data, the authors recommended that the use of bisphosphonates be limited to children with a history of at least one fragility fracture. Oromotor dysfunction and/or gastrointestinal reflux are often associated with CP and other neuromuscular disorders, and thus important safety considerations may preclude the use of oral bisphosphonates [74].
Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is an X-linked disorder arising from mutations in the dystrophin gene, which encodes a protein critical for structural integrity of muscle fibers. Patients present with progressive muscular weakness, loss of ambulation, and death within the third to fourth decade from respiratory and/or cardiac dysfunction [75]. Treatment with corticosteroids attenuates loss of muscle function, leading to prolonged ambulation and preservation of cardiac and respiratory function [76]. The combination of chronic glucocorticoid use, severe muscle weakness, and impaired ambulation places patients at high risk for fractures, especially vertebral compression fractures which affect the majority of patients [77,78]. Few retrospective studies and no controlled studies of bisphosphonates in persons with DMD have been conducted; however, preliminary results are supportive. In a small uncontrolled study of alendronate treatment, spinal BMD was preserved despite disease progression [79]. A retrospective analysis of patients with DMD who were given pamidronate or zoledronic acid after sustaining vertebral compression fractures demonstrated improvements in pain, spinal BMD, and vertebral height ratios [80]. A recent retrospective survival analysis associated bisphosphonate use with improved life expectancy, although lack of data regarding skeleton-related outcomes and other clinical outcomes raises questions regarding the clinical applicability of these findings [81]. Regardless, a critical need exists to define bone-preserving therapies in persons with DMD, given its severe and progressive skeletal morbidity.
Spinal Cord Injury
Patients with spinal cord injuries (SCIs) experience rapid loss of bone density, particularly in the long bones of the lower limbs, resulting in a high incidence of fractures [82]. Bone loss results primarily from prolonged immobilization and muscle atrophy; however, neurologic and hormonal effects after neural injury likely play contributory roles [83]. Bisphosphonates have been used frequently in adults with SCI, but study findings are conflicting. The majority show a mild reduction in bone loss that is variably maintained, and results are insufficient to support routine use in patients without a history of fractures [84]. Data for bisphosphonate use in children with SCIs are limited to a single case report, in which a child treated with zoledronic acid for 18 months demonstrated significant gains in BMD [85]. Although use of bisphosphonates to prevent bone loss in adults with SCIs is not well supported, the potential for added benefits during bone modeling and skeletal growth justify additional investigation into the use of these medications in children.
Traumatic Brain Injury
Traumatic brain injury is another form of neurologic injury that may lead to static encephalopathy and impaired mobility in children [86]. No trials of bisphosphonate use for low BMD in this population have been conducted, and it not known whether extrapolation from studies in persons with SCI or other forms of neurologic injury, such as CP, are relevant.
Spina Bifida
Spina bifida (also known as myelomeningocele) is a congenital disorder resulting from incomplete closure of the embryonic neural tube. Clinical sequelae arise primarily from abnormal neurologic function, including paralysis, impaired sensation, and bladder and bowel incontinence. Patients with spina bifida, particularly children who are nonambulatory, have severe impairment in bone modeling because of a congenital lack of muscular forces on the skeleton [87]. Fractures are common, with a reported prevalence of around 30% [88]. Fractures frequently occur in the metaphyses and diaphyses of the affected lower extremities and are more common in patients with higher level spinal defects [88]. At present, no trials or case series dedicated to bisphosphonate treatment for persons with spina bifida have been reported, although 2 series of nonambulatory children included a patient with spina bifida [89,90]. Bisphosphonate treatment of children with spina bifida thus must be guided by literature relating to other disorders of disuse osteoporosis.
ADVERSE EFFECTS AND MONITORING WITH BISPHOSPHONATE TREATMENT
Bisphosphonate treatment has largely been well tolerated in children and adolescents. The most common adverse effect is an acute phase reaction after the initial dose, which may include flu-like symptoms such as fever, myalgia, and gastrointestinal upset. Symptoms generally begin within 24 hours and may last several days. Premedicating with nonsteroidal anti-inflammatory drugs or steroids may attenuate this reaction. Inhibition of osteoclast activity places patients at risk for hypocalcemia. For this reason, it is important to ensure that the patient has adequate vitamin D stores before treatment and to maintain appropriate dietary calcium intake during the treatment course. Practitioners may consider a short course of calcium and/or calcitriol supplementation for several days after the infusion in children at high risk for hypocalcemia.
Osteonecrosis of the jaw is a rare but potentially serious adverse effect of bisphosphonate use that is typically seen in patients with cancer who are treated with repeated high-dose intravenous infusions [91]. Although this complication has not been reported in children, it is prudent for the patient to undergo a dental examination and complete required dental work before bisphosphonates are administered. The use of bisphosphonates has been variably associated with additional adverse effects in adults, including esophagitis, atrial fibrillation, and severe arthralgias [92]. Because of the association with esophageal ulcerations, we do not recommend use of oral bisphosphonates in children with gastrointestinal disease, neuromuscular disorders carrying a high risk of gastrointestinal reflux and/or oromotor dysfunction, or an inability to report gastrointestinal pain. Children who are treated with oral formulations should be monitored for gastrointestinal symptoms at each evaluation.
Pregnant women treated with bisphosphonates during childhood risk fetal exposure to bisphosphonates, because these medications easily cross the placenta. Administration of high-dose bisphosphonates to pregnant rats resulted in fetal skeleton abnormalities [93]; however, bisphosphonate toxicity has not been reported in human infants, despite numerous reports of women treated before and during pregnancy [94].
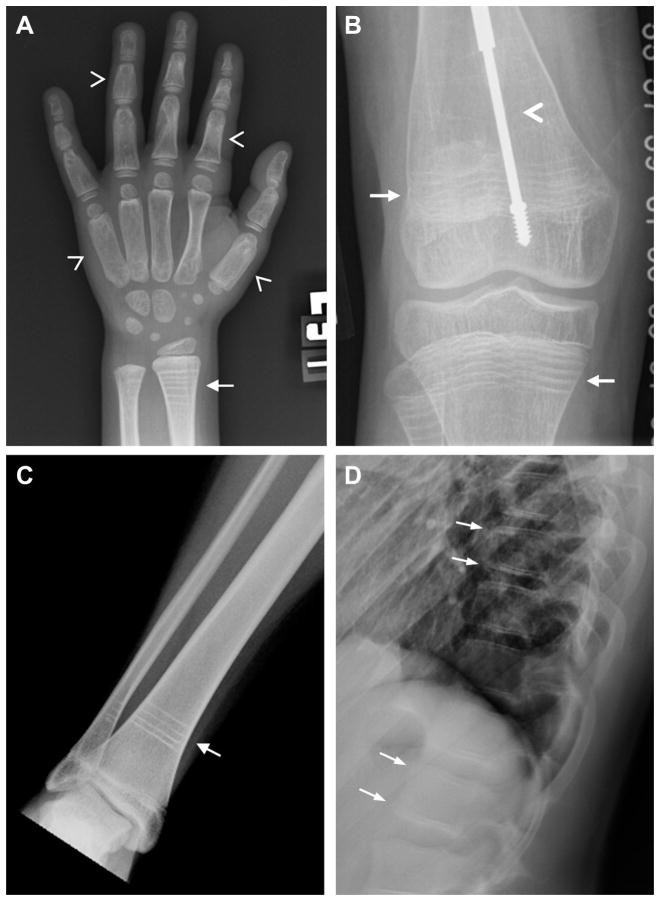
Treatment duration is an important consideration, but little evidence is available to guide practitioners. Because the uncoupling of bone formation and resorption in the growing skeleton allows selective inhibition of resorption with relative sparing of bone formation, bisphosphonates are expected to benefit growing children and adolescents more than persons who have obtained their final height [42,95]. This uncoupling is evidenced by the formation of sclerotic bands at the epiphyses, metaphyses, and vertebral bodies when bisphosphonates are given to children with open growth plates (Figure 2) [96]. These bands eventually resolve within continued bone modeling. However, long-term bone turnover suppression may have consequences for the growing skeleton. Abnormalities at the distal radius were demonstrated in children with OI even after discontinuation of bisphosphonates [95]. After the discontinuation of bisphosphonates in growing children, new bone will be formed at the growth plate and intersect with bisphosphonate-treated bone, and concern has been expressed that this bone may have an increased susceptibility to fracture [95]. Treatment with excessively high doses of bisphosphonates has led to osteopetrosis and persistent remodeling defects [97,98], which have persisted for more than 6 years after discontinuation [99].
Figure 2.
Images depicting sclerotic bands associated with bisphosphonate treatment. (A) A 10-year-old girl with fibrous dysplasia who was treated with a 3-year course of pamidronate was found to have transverse sclerotic bands at the radial epiphysis and metaphysis (arrow). Note the expansile, ground glass appearance of the fibrous dysplasia in her metacarpals and phalanges (arrowheads). (B) Radiographs from a 13-year-old boy with osteogenesis imperfecta who was treated with pamidronate for 2 years demonstrate metaphyseal bands in the distal femur and proximal tibia (arrows). An intramedullary rod has been placed for fixation of a femoral fracture (arrowhead). (C) A 13-year-old girl with fibrous dysplasia underwent a 1-year course of pamidronate at age 7 years, which caused residual sclerotic bands that appear to have migrated toward the tibial diaphysis with continued skeletal growth (arrow). (D) Spine films from the patient in (C) demonstrate sclerosis at the superior and inferior end plates of the vertebral bodies (arrows).
Long-term effects of bisphosphonates on healing of the skeleton have not been well studied in children. In one report of OI, investigators found no effect on spontaneous fracture healing but delayed healing after osteotomy procedures [100]. For this reason, practitioners frequently discontinue use of bisphosphonates several months after planned orthopedic procedures. The development of rare atypical femoral fractures in adults treated with bisphosphonates on a long-term basis raises additional concerns regarding the safety of continuous bone turnover suppression [101].
FUTURE DIRECTIONS
In recent years, insights into bone remodeling pathways have spurred the development of multiple novel bone-altering therapies. Intact parathyroid hormone and teriparatide are anabolic agents typically used in combination with bisphosphonates in adults; however, their use in pediatric patients is limited by a black box warning concerning an increased risk of osteosarcoma in treated juvenile rats [102]. Denosumab, a potent antiresorptive agent recently approved to treat osteoporosis in adults, acts through inhibition of receptor activator of nuclear kappa-B ligand [103]. Additional therapies under investigation include anabolic antisclerostin monoclonal antibodies and the antiresorptive cathepsin K inhibitor odanacatib [104,105]. These therapies offer multiple theoretical advantages compared with bisphosphonates, including high selectivity, improved promotion of bone formation, and a shorter half-life.
CONCLUSIONS
Pediatric osteoporosis arises from a broad range of genetic, neurologic, and metabolic disorders and is common in children with physical disabilities. Assessment of BMD in children is challenging because of the limitations of DXA in evaluating the growing skeleton. Initial management steps include optimizing treatment of underlying conditions and initiation of conservative measures such as weight-bearing physical activity and ensuring adequate nutrition. Bisphosphonates increase BMD through inhibition of bone resorption and are commonly used to treat osteoporosis in adults. Increasing evidence suggests that bisphosphonates may be beneficial for pediatric disorders of the skeleton; however, their routine use is limited by a lack of long-term safety and efficacy data. Practical considerations include significant knowledge gaps regarding optimal dosing, formulation, and the target population for bisphosphonate use in children.
The decision to initiate treatment with bisphosphonates in children must be made using clinical judgment, with the clinician weighing the potential risks and benefits for each individual patient. Based on the current literature, treatment with bisphosphonates is justified in children with significantly low BMD who have a history of fragility fractures, a high risk of morbidity from fractures, and disabling bone pain that is not responsive to conservative measures, and in the context of clinical trials.
Acknowledgments
Research support: supported by the Bone Health Program at Children’s National Medical Center and the Intramural Research Program at the National Institutes of Health.
Contributor Information
Alison M. Boyce, Division of Endocrinology and Diabetes, Children’s National Medical Center, 111 Michigan Ave NW, Washington, DC 20010; and Bone Health Program, Division of Orthopaedics and Sports Medicine, Children’s National Medical Center, Washington, DC. Disclosure: nothing to disclose.
Laura L. Tosi, Bone Health Program, Division of Orthopaedics and Sports Medicine, Children’s National Medical Center, Washington, DC Disclosure: Disclosures outside this publication: board membership, Society for Women’s Health Research (no remuneration); Medical Society for the District of Columbia (no remuneration).
Scott M. Paul, Rehabilitation Medicine Department, Mark O. Hatfield Clinical Research Center, National Institutes of Health, Bethesda, MD Disclosure: nothing to disclose.
References
- 1.Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metabol. 2012;97:2272–2282. doi: 10.1210/jc.2012-1027. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E. Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:219–233. doi: 10.1615/critreveukargeneexpr.v19.i3.40. [DOI] [PubMed] [Google Scholar]
- 5.Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27:351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16:1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 7.Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18:949–954. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 8.Rauch F, Neu C, Manz F, Schoenau E. The development of metaphyseal cortex—implications for distal radius fractures during growth. J Bone Miner Res. 2001;16:1547–1555. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 9.Ebetino FH, Hogan AM, Sun S, et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011;49:20–33. doi: 10.1016/j.bone.2011.03.774. [DOI] [PubMed] [Google Scholar]
- 10.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone. 2011;49:42–49. doi: 10.1016/j.bone.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Merck & Co. Fosamax. Whitehouse Station, NJ: Merck & Co; 2012. [Google Scholar]
- 13.Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356:1075–1076. doi: 10.1056/NEJMc062792. [DOI] [PubMed] [Google Scholar]
- 14.Bishop N, Braillon P, Burnham J, et al. Dual-energy X-ray aborptiometry assessment in children and adolescents with diseases that may affect the skeleton: The 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:29–42. doi: 10.1016/j.jocd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zemel BS, Stallings VA, Leonard MB, et al. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy x-ray absorptiometry. J Clin Densitom. 2009;12:207–218. doi: 10.1016/j.jocd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueske NM, Chan LS, Wren TA. Reliability of lateral distal femur dual-energy x-ray absorptiometry measures. J Clin Densitom. doi: 10.1016/j.jocd.2013.02.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy x-ray absorptiometry measures in children: Comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–1052. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone acquisition in healthy children and adolescents: Comparisons of dual-energy x-ray absorptiometry and computed tomography measures. J Clin Endocrinol Metab. 2005;90:1925–1928. doi: 10.1210/jc.2004-1351. [DOI] [PubMed] [Google Scholar]
- 21.Burrows M, Liu D, McKay H. High-resolution peripheral QCT imaging of bone microstructure in adolescents. Osteoporosis Int. 2010;21:515–520. doi: 10.1007/s00198-009-0913-2. [DOI] [PubMed] [Google Scholar]
- 22.Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: Technical characteristics, performance, and clinical application. Pediatr Res. 2008;63:220–228. doi: 10.1203/PDR.0b013e318163a286. [DOI] [PubMed] [Google Scholar]
- 23.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: A prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Barera G, Ricotti A, Weber G, Bianchi C, Chiumello G. Reversal of low bone density with a gluten-free diet in children and adolescents with celiac disease. Am J Clin Nutr. 1998;67:477–481. doi: 10.1093/ajcn/67.3.477. [DOI] [PubMed] [Google Scholar]
- 26.Gafni RI, McCarthy EF, Hatcher T, et al. Recovery from osteoporosis through skeletal growth: Early bone mass acquisition has little effect on adult bone density. FASEB J. 2002;16:736–738. doi: 10.1096/fj.01-0640fje. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: Institute of Medicine of the National Academies; 2010. [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 29.Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231–1237. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: A review of controlled trials. Bone. 2007;40:14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Chad KE, Bailey DA, McKay HA, Zello GA, Snyder RE. The effect of a weight-bearing physical activity program on bone mineral content and estimated volumetric density in children with spastic cerebral palsy. J Pediatr. 1999;135:115–117. doi: 10.1016/s0022-3476(99)70340-9. [DOI] [PubMed] [Google Scholar]
- 32.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res. 2011;26:1759–1766. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 33.Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: A randomized controlled pilot study. J Musculoskel Neuronal Interact. 2010;10:77–83. [PubMed] [Google Scholar]
- 34.Sykes B, Ogilvie D, Wordsworth P, et al. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Human Genet. 1990;46:293–307. [PMC free article] [PubMed] [Google Scholar]
- 35.Astrom E, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child. 2002;86:356–364. doi: 10.1136/adc.86.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkin H, Rauch F, Bishop NJ, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metabol. 2000;85:1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 37.Zacharin M, Bateman J. Pamidronate treatment of osteogenesis imperfecta—lack of correlation between clinical severity, age at onset of treatment, predicted collagen mutation and treatment response. J Pediatr Endocrinol Metab. 2002;15:163–174. doi: 10.1515/jpem.2002.15.2.163. [DOI] [PubMed] [Google Scholar]
- 38.Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: Effect of intravenous pamidronate therapy. J Bone Miner Res. 2003;18:610–614. doi: 10.1359/jbmr.2003.18.4.610. [DOI] [PubMed] [Google Scholar]
- 39.Arikoski P, Silverwood B, Tillmann V, Bishop NJ. Intravenous pamidronate treatment in children with moderate to severe osteogenesis imperfecta: Assessment of indices of dual-energy x-ray absorptiometry and bone metabolic markers during the first year of therapy. Bone. 2004;34:539–546. doi: 10.1016/j.bone.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Sumnik Z, Land C, Rieger-Wettengl G, Korber F, Stabrey A, Schoenau E. Effect of pamidronate treatment on vertebral deformity in children with primary osteoporosis. A pilot study using radiographic morphometry. Hormone Res. 2004;61:137–142. doi: 10.1159/000075589. [DOI] [PubMed] [Google Scholar]
- 41.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 42.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110:1293–1299. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adami S, Gatti D, Colapietro F, et al. Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res. 2003;18:126–130. doi: 10.1359/jbmr.2003.18.1.126. [DOI] [PubMed] [Google Scholar]
- 44.Panigrahi I, Das RR, Sharda S, Marwaha RK, Khandelwal N. Response to zolendronic acid in children with type III osteogenesis imperfecta. J Bone Miner Res. 2010;28:451–455. doi: 10.1007/s00774-009-0149-4. [DOI] [PubMed] [Google Scholar]
- 45.Vuorimies I, Toiviainen-Salo S, Hero M, Makitie O. Zoledronic acid treatment in children with osteogenesis imperfecta. Hormone Res Pediatr. 2011;75:346–353. doi: 10.1159/000323368. [DOI] [PubMed] [Google Scholar]
- 46.Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012;25:485–491. doi: 10.1515/jpem-2012-0016. [DOI] [PubMed] [Google Scholar]
- 47.DiMeglio LA, Peacock M. Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res. 2006;21:132–140. doi: 10.1359/JBMR.051006. [DOI] [PubMed] [Google Scholar]
- 48.Akcay T, Turan S, Guran T, Bereket A. Alendronate treatment in children with osteogenesis imperfecta. Indian Pediatr. 2008;45:105–109. [PubMed] [Google Scholar]
- 49.Ward LM, Rauch F, Whyte MP, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: A randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–364. doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 50.Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: A 2-year randomised placebo-controlled study. Lancet. 2004;363:1427–1431. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 51.Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: A randomized placebo-controlled study. J Bone Miner Res. 2009;24:1282–1289. doi: 10.1359/jbmr.090213. [DOI] [PubMed] [Google Scholar]
- 52.Bishop N, Harrison R, Ahmed F, et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res. 2010;25:32–40. doi: 10.1359/jbmr.090712. [DOI] [PubMed] [Google Scholar]
- 53.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochr Database Syst Rev (Online) 2008;4:CD005088. doi: 10.1002/14651858.CD005088.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Castillo H, Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: An AACPDM systematic review. Dev Med Child Neurol. 2009;51:17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 55.Glorieux FH. Treatment of osteogenesis imperfecta: Who, why, what? Hormone Res. 2007;68(suppl 5):8–11. doi: 10.1159/000110463. [DOI] [PubMed] [Google Scholar]
- 56.Munns CF, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: Clinical and histomorphometric outcome. J Bone Miner Res. 2005;20:1235–1243. doi: 10.1359/JBMR.050213. [DOI] [PubMed] [Google Scholar]
- 57.Collins MT, Riminucci R, Bianco P. Fibrous dysplasia. In: Rosen CJ, Compston JE, Lian JB, editors. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7. Washington, DC: American Society of Bone and Mineral Research; 2008. pp. 423–428. [Google Scholar]
- 58.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343:953–954. doi: 10.1016/s0140-6736(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 60.Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: Long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235–242. doi: 10.1016/j.bone.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12:1746–1752. doi: 10.1359/jbmr.1997.12.10.1746. [DOI] [PubMed] [Google Scholar]
- 62.Parisi MS, Oliveri B, Mautalen CA. Effect of intravenous pamidronate on bone markers and local bone mineral density in fibrous dysplasia. Bone. 2003;33:582–588. doi: 10.1016/s8756-3282(03)00221-7. [DOI] [PubMed] [Google Scholar]
- 63.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569–4575. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 64.Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, de Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr. 2000;89:188–193. doi: 10.1080/080352500750028816. [DOI] [PubMed] [Google Scholar]
- 65.Vandenborne K, Elliott MA, Walter GA, et al. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve. 1998;21:1006–1012. doi: 10.1002/(sici)1097-4598(199808)21:8<1006::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 66.Ceroni D, Martin X, Delhumeau C, Rizzoli R, Kaelin A, Farpour-Lambert N. Effects of cast-mediated immobilization on bone mineral mass at various sites in adolescents with lower-extremity fracture. J Bone Joint Surg. 2012;94:208–216. doi: 10.2106/JBJS.K.00420. [DOI] [PubMed] [Google Scholar]
- 67.Rosenbaum P, Paneth N, Leviton A, et al. A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 68.Mergler S, Evenhuis HM, Boot AM, et al. Epidemiology of low bone mineral density and fractures in children with severe cerebral palsy: A systematic review. Dev Med Child Neurol. 2009;51:773–778. doi: 10.1111/j.1469-8749.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 69.Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 70.Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: A randomized, placebo-controlled clinical trial. J Pediatr. 2002;141:644–651. doi: 10.1067/mpd.2002.128207. [DOI] [PubMed] [Google Scholar]
- 71.Iwasaki T, Takei K, Nakamura S, Hosoda N, Yokota Y, Ishii M. Secondary osteoporosis in long-term bedridden patients with cerebral palsy. Pediatr Int. 2008;50:269–275. doi: 10.1111/j.1442-200X.2008.02571.x. [DOI] [PubMed] [Google Scholar]
- 72.Iwasaki T, Nonoda Y, Ishii M. Long-term outcomes of children and adolescents who had cerebral palsy with secondary osteoporosis. Curr Med Res Opin. 2012;28:737–747. doi: 10.1185/03007995.2011.645562. [DOI] [PubMed] [Google Scholar]
- 73.Fehlings D, Switzer L, Agarwal P, et al. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: A systematic review. Dev Med Child Neurol. 2012;54:106–116. doi: 10.1111/j.1469-8749.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 74.Sullivan PB. Gastrointestinal disorders in children with neurodevelopmental disabilities. Dev Disabil Res Rev. 2008;14:128–136. doi: 10.1002/ddrr.18. [DOI] [PubMed] [Google Scholar]
- 75.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 76.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochr Database Syst Rev (Online) 2008;1:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 77.Mayo AL, Craven BC, McAdam LC, Biggar WD. Bone health in boys with Duchenne muscular dystrophy on long-term daily deflazacort therapy. Neuromuscu Disord. 2012;22:1040–1045. doi: 10.1016/j.nmd.2012.06.354. [DOI] [PubMed] [Google Scholar]
- 78.King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 79.Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil. 2005;86:284–288. doi: 10.1016/j.apmr.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 80.Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis Int. 2012;23:2703–2711. doi: 10.1007/s00198-012-1911-3. [DOI] [PubMed] [Google Scholar]
- 81.Gordon KE, Dooley JM, Sheppard KM, MacSween J, Esser MJ. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics. 2011;127:e.353–e.358. doi: 10.1542/peds.2010-1666. [DOI] [PubMed] [Google Scholar]
- 82.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporosis Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 83.Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol. 2006;65:555–565. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 84.Bryson JE, Gourlay ML. Bisphosphonate use in acute and chronic spinal cord injury: A systematic review. J Spinal Cord Med. 2009;32:215–225. doi: 10.1080/10790268.2009.11760776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ooi HL, Briody J, McQuade M, Munns CF. Zoledronic acid improves bone mineral density in pediatric spinal cord injury. J Bone Miner Res. 2012;27:1536–1540. doi: 10.1002/jbmr.1598. [DOI] [PubMed] [Google Scholar]
- 86.Pinto PS, Meoded A, Poretti A, Tekes A, Huisman TA. The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings—part 2. J Neuroimaging. 2012;22:e18–e41. doi: 10.1111/j.1552-6569.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 87.Ralis ZA, Ralis HM, Randall M, Watkins G, Blake PD. Changes in shape, ossification and quality of bones in children with spina bifida. Dev Med Child Neurol Suppl. 1976:29–41. doi: 10.1111/j.1469-8749.1976.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 88.Dosa NP, Eckrich M, Katz DA, Turk M, Liptak GS. Incidence, prevalence, and characteristics of fractures in children, adolescents, and adults with spina bifida. J Spinal Cord Med. 2007;30(suppl 1):S5–S9. doi: 10.1080/10790268.2007.11753961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sholas MG, Tann B, Gaebler-Spira D. Oral bisphosphonates to treat disuse osteopenia in children with disabilities: A case series. J Pediatr Orthop. 2005;25:326–331. doi: 10.1097/01.bpo.0000150810.35794.e8. [DOI] [PubMed] [Google Scholar]
- 90.Steelman J, Zeitler P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J Pediatr. 2003;142:417–423. doi: 10.1067/mpd.2003.137. [DOI] [PubMed] [Google Scholar]
- 91.Migliorati CA, Epstein JB, Abt E, Berenson JR. Osteonecrosis of the jaw and bisphosphonates in cancer: A narrative review. Nat Rev Endocrinol. 2011;7:34–42. doi: 10.1038/nrendo.2010.195. [DOI] [PubMed] [Google Scholar]
- 92.Abrahamsen B. Bisphosphonate adverse effects, lessons from large databases. Curr Opin Rheumatol. 2010;22:404–409. doi: 10.1097/BOR.0b013e32833ad677. [DOI] [PubMed] [Google Scholar]
- 93.Patlas N, Golomb G, Yaffe P, Pinto T, Breuer E, Ornoy A. Transplacental effects of bisphosphonates on fetal skeletal ossification and mineralization in rats. Teratology. 1999;60:68–73. doi: 10.1002/(SICI)1096-9926(199908)60:2<68::AID-TERA10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 94.Djokanovic N, Klieger-Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy? J Obstet Gynaecol Can. 2008;30:1146–1148. doi: 10.1016/S1701-2163(16)34026-9. [DOI] [PubMed] [Google Scholar]
- 95.Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone. 2007;40:821–827. doi: 10.1016/j.bone.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 96.van Persijn van Meerten EL, Kroon HM, Papapoulos SE. Epi- and metaphyseal changes in children caused by administration of bisphosphonates. Radiology. 1992;184:249–254. doi: 10.1148/radiology.184.1.1609087. [DOI] [PubMed] [Google Scholar]
- 97.Otero JE, Gottesman GS, McAlister WH, et al. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res. 2013;28:419–430. doi: 10.1002/jbmr.1752. [DOI] [PubMed] [Google Scholar]
- 98.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 99.Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: Novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. 2008;23:1698–1707. doi: 10.1359/jbmr.080511. [DOI] [PubMed] [Google Scholar]
- 100.Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004;19:1779–1786. doi: 10.1359/JBMR.040814. [DOI] [PubMed] [Google Scholar]
- 101.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: A systematic review and meta-analysis. J Bone Miner Res. 2013;28:1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 103.Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: A review of preclinical and clinical data. Clin Ther. 2012;34:521–536. doi: 10.1016/j.clinthera.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 105.Gauthier JY, Chauret N, Cromlish W, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]