Abstract
Sustained activation of the JAK/STAT pathway is causal to human cancers. This pathway is less complex in Drosophila, and its dysregulation has been linked to several tumor models in this organism. Here, we discuss models of metastatic epithelial and hematopoietic tumors that are causally linked to dysregulation of JAK/STAT signaling in Drosophila. First, we focus on cancer models in imaginal discs where ectopic expression of the JAK/STAT pathway ligand Unpaired downstream of distinct tumor suppressors has emerged as an unexpected mediator of neoplastic transformation. We also discuss the collaboration between STAT and oncogenic Ras in epithelial transformation. Second, we examine hematopoietic tumors, where mutations that cause hyperactive JAK/STAT signaling are necessary and sufficient for “fly leukemia”. We highlight the important contributions that genetic screens in Drosophila have made to understanding the JAK/STAT pathway, its developmental roles, and how its function is co-opted during tumorigenesis.
Introduction
Activating mutations in JAK/STAT signaling is a causal event in human leukemia, myeloproliferative neoplasms (MPNs) and solid tumors (Lacronique et al., 1997; Jones et al., 2005; Kralovics et al., 2005; Levine et al., 2005; Moriggl et al., 2005). With respect to the former, the Jak2V617F activating mutation is present in most patients with polycytemia vera and in the majority of patients with essential thrombocytosis and myelofibrosis (reviewed in (Abdel-Wahab, 2011)). Persistent activation of Stat3 is observed all major classes of carcinoma, and cells mis-expressing dominant-active Stat3 cause tumors in immuno-compromised mice (Bromberg et al., 1999; Darnell, 2005). Hyperactivation of the JAK/STAT pathway also causes epithelial and hematopoietic tumors in flies. Interestingly, MPNs and fly hematopoietic tumors both result from over-proliferation of cells in the myeloid lineage. The powerful genetic tools available in Drosophila, coupled with the reduced genetic complexity of JAK/STAT and other signaling pathways in this organism, has led to Drosophila being adopted as a useful model for studying the role of JAK/STAT signaling in tumorigenesis.
The Drosophila genome contains a single JAK gene called hopscotch (hop) and a single STAT gene called Stat92E. Hop is most similar to Jak2 in vertebrates, while Stat92E is most homologous to Stat3 and Stat5 (reviewed in (Arbouzova and Zeidler, 2006)). Three related IL-6-like cytokines, Unpaired (Upd) (also called Outstretched), Upd2 and Upd3, bind to a gp130-like cytokine receptor called Domeless (Dome) (Fig. 1A) that subsequently activates Hop, which stimulates Stat92E. Activated Stat92E dimers induce expression of target genes including Socs36E, which encodes a negative regulator of pathway activity. Other inhibitors of the pathway include Eye transformer (ET) (also called Latran (Lat)), a second Upd receptor that antagonizes pathway signaling (Kallio et al., 2010; Makki et al., 2010), and dPIAS (or Su(var)2-10), the sole Drosophila PIAS homolog, which inhibits active Stat92E dimers.
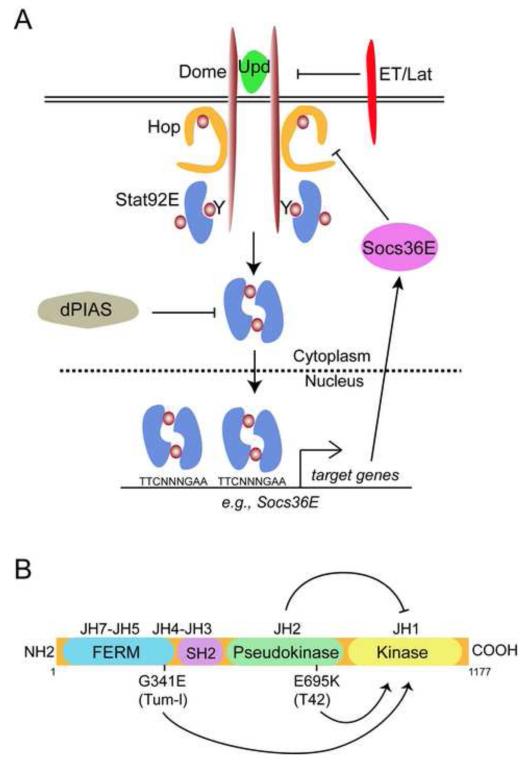
Fig. 1. The Drosophila JAK/STAT pathway.
(A) The Drosophila JAK-STAT pathway consists of three Upd ligands collectively referred to as Upd (green). Upd activates the receptor Dome (brown), which results in activation of Hop (orange), leading to tyrosine phosphorylation (brown circles) on Dome. Stat92E dimers (blue) bind to the phosphorylated receptor. Once bound, Stat92E is phosphorylated on tyrosine 711, generating an active Stat92E dimer that undergoes nuclear translocation, where it binds to a consensus TTCNNNGAA site and alters gene expression. Socs36E is a Stat92E target gene that encodes a negative regulator (magenta) of Dome/JAK activity. A second receptor ET/Lat (red) inhibits JAK/STAT signaling. dPIAS (gray) inhibits activated Stat92E dimers.
(B) Domain structure of Hop. Hop contains a bona fide tyrosine kinase domain (JH1, yellow), a pseudokinase domain that lacks kinase activity (JH2, green), an atypical SH2 domain (magenta), and a FERM domain (blue) that mediates attachment to cytokine receptors. In the wild type Hop protein, the JH2 domain prevents the activation of the kinase domain, JH1 (top arrow). The E695K (T42) or G341E (Tum-l) mutations lead to activation (bottom arrows) of JH1.
The central role of JAK/STAT signaling in epithelial tumors
JAK/STAT signaling and developmental growth control
The JAK/STAT pathway plays important roles during Drosophila development, particularly in imaginal discs, which are epithelial tissues set aside during embryogenesis that give rise to the adult structures (Cohen, 1993). In wing and eye imaginal discs, JAK/STAT signaling is an essential regulator of growth and patterning. Pathway activity is detected in all cells in early discs, and JAK/STAT signal transduction is required in a cell autonomous manner for growth (Luo et al., 1999; Mukherjee et al., 2005a; Ekas et al., 2006; Rodrigues et al., 2012). The level of JAK/STAT signaling regulates the size of the adult eye. Animals with reduced JAK/STAT pathway activity have small or ablated eyes, and this phenotype can be rescued by activating Stat92E (Bach et al., 2003; Ekas et al., 2006; Ekas et al., 2010). To maintain proper tissue size, the expression of Upd needs to be tightly regulated. Mis-expression of Upd in the eye or wing disc leads to dramatically overgrown tissue (Fig. 2A,B and (Bach et al., 2003; Tsai and Sun, 2004; Classen et al., 2009; Rodrigues et al., 2012)). Some studies have shown that in wild type eye discs, Notch activation at the midline induces upd in cells at the posterior midline, and Upd acts non-cell autonomously to promote growth of the eye field and allow for initiation of neurogenesis (Fig. 2C and (Chao et al., 2004; Reynolds-Kenneally and Mlodzik, 2005; Ekas et al., 2006; Tsai et al., 2007; Djiane et al., 2013)). Up-regulation of Upd can account for much of the non-autonomous growth induced by activated Notch in the eye (Chao et al., 2004; Reynolds-Kenneally and Mlodzik, 2005). However, another report finds that Upd cannot account for the overgrowth induced by sustained Notch signaling. Instead, they find that early in eye development Upd plays a central role upstream of Notch activation (Fig. 2C, green arrow, and (Gutierrez-Avino et al., 2009)). Finally, JAK/STAT signaling can also negatively regulate Notch pathway activity, as Stat92E autonomously represses expression of Serrate (Ser), which encodes a Notch ligand (Fig. 2C and (Flaherty et al., 2009)). In sum, the relationship between JAK/STAT and Notch pathways in the eye disc is complex and consists of feed-forward as well as inhibitory interactions. Although the relationship of upd as a target of Notch signaling is treated in detail below, it is worth bearing in mind that Notch may act downstream of Upd in certain developmental and pathogenic scenarios.
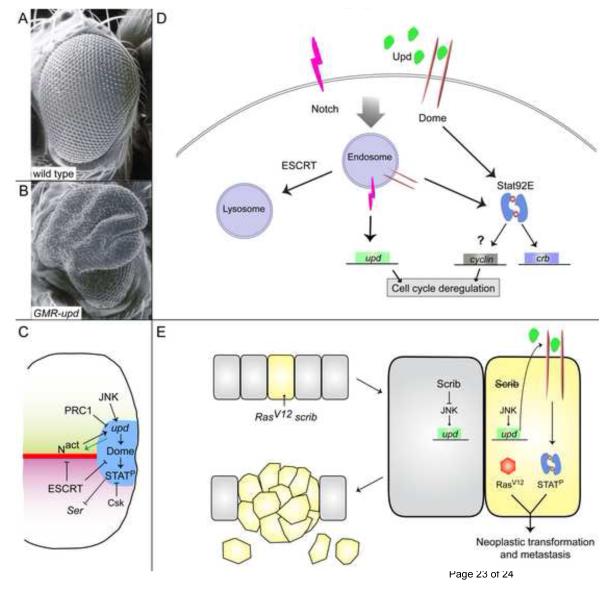
Fig. 2. JAK/STAT signaling in eye development and imaginal disc-derived tumors.
(A,B) Scanning electron micrograph of a wild type (A) or GMR-upd (B) adult eye. Note the dramatic overgrowth in B.
(C) Model of regulation of JAK/STAT pathway signaling in the developing eye imaginal disc. Notch signaling (Nact) induces upd in cells at the posterior midline (blue area). Upd activates Dome and Stat92E (labelled STATP) in adjacent cells. PRC1 normally repress upd expression, while JNK signaling can induce it. ESCRT components normally restrict Notch and Dome activity, while Csk represses Stat92E activity. In addition, early in eye development, Upd can act upstream of Notch and induce Notch activity at the midline (green arrow). Activated Stat92E (STATP) also restricts Notch activity by repressing expression of Ser, which encodes a Notch ligand.
(D) Model of regulation of JAK/STAT pathway signaling by ESCRT components. Active Notch (magenta) and Dome (brown) receptors are trafficked into endosomes, where they can induce target genes like upd (green) and crb (blue), respectively. ESCRT factors promote the trafficking of Notch and Dome into the lysosome for degradation. In ESCRT mutants, Notch and Dome are trapped in an activated state in endosomes, where their unbridled activity causes cell cycle deregulation and transformation.
(E) Model of metastatic tumors caused by gain of RasV12 and loss of scrib in the eye disc. In wild type epithelial cells (gray), Scrib repressed JNK activity. In a cell that has RasV12 and lacks scrib (yellow), JNK signaling is now activated and upd genes are ectopically expressed (green). The ectopic Upd protein (green) leads to autocrine and paracrine (not depicted) activation of Dome (brown) and Stat92E (blue). The autonomous collaboration of RasV12 and activated Stat92E causes neoplastic transformation and metastasis.
While sustained activation of JAK/STAT signaling accelerates cell cycle progression (Bach et al., 2003; Mukherjee et al., 2005a; Rodrigues et al., 2012), few direct molecular links have been made between this pathway and cell cycle progression or cellular growth. Cyclin D transcripts are upregulated in Upd-overexpressing discs (Tsai and Sun, 2004), and the relationship may be conserved as Cyclin D is a Stat5 target in vertebrate hematopoiesis (Matsumura et al., 1999). Several screens have been have been carried out in search of JAK/STAT targets in eye disc development (Bach et al., 2003; Mukherjee et al., 2005b; Flaherty et al., 2009). One group has linked Bone Morphogenetic Protein (BMP) signaling with over-growth downstream of JAK/STAT pathway activity in the eye disc (Bach et al., 2003). However, in the wing disc, ectopic JAK/STAT signaling causes increased growth without autonomously increasing BMP signal transduction, and several other developmental regulators involved in tissue growth such as Wingless, Hippo and dMyc are similarly unaffected by increased JAK/STAT activity (Rodrigues et al., 2012). In sum, although it is apparent that the JAK/STAT pathway is a major regulator of developmental growth, it is not yet clear how pathway activity leads to increased cell division and mass accumulation.
Tumors due to epigenetic misregulation of upd
In the last decade, there has been substantial interest in the contribution of epigenetic changes to tumorigenesis. Polycomb Repressor Complexes (PRCs) are essential to this process. PRCs bind specific DNA sequences known as Polycomb Response Elements (PREs), and this interaction leads to gene silencing (reviewed in (Schwartz and Pirrotta, 2007). One complex in particular, PRC1, has been implicated in tumor formation both in flies and mammals (Beuchle et al., 2001; Oktaba et al., 2008; Mills, 2010). Further work has established that loss-of-function mutations in several PRC1 components within Drosophila imaginal discs lead to the formation of tumors (Classen et al., 2009; Gonzalez et al., 2009; Feng et al., 2011). These tumors hyper-proliferate and lose normal epithelial organization. Intriguingly, although many PRC1 mutations cause cells to die, they also cause the over-proliferation of neighboring wild type cells (Classen et al., 2009; Feng et al., 2011), suggesting that a secreted signal mediates proliferation downstream of PRC1 loss. Indeed, the regulatory regions of the upd, upd2 and upd3 loci contain PREs that are bound by PRC1 proteins (Fig. 2C and (Classen et al., 2009; Gonzalez et al., 2009). These PREs lead to the silencing of the upd loci in wild type animals, but in PRC1 mutants, upd genes are de-repressed. Furthermore, preventing Upd expression or JAK/STAT activation in PRC1 mutants suppresses tumor growth (Classen et al., 2009; Gonzalez et al., 2009; Feng et al., 2011).
ESCRT mutants ectopically activate JAK/STAT signaling
Unexpectedly, mutations in endocytic genes revealed a link between endocytosis and tumor formation in epithelia (reviewed in (Vaccari and Bilder, 2009)). During endocytosis, cargo sorting and multi-vesicular body formation require three large protein complexes, the Endosomal Sorting Complexes Required for Transport (ESCRT-I, -II and -III) (reviewed in (Rusten et al., 2012)). Unbiased genetic screens searching for new tumor suppressors in Drosophila imaginal discs have revealed that many components of the endocytic machinery are essential to suppress overgrowth and to maintain epithelial organization (Lu and Bilder, 2005; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Herz et al., 2006; Menut et al., 2007; Morrison et al., 2008). Mutations in most endosomal and ESCRT components lead to tumor formation in Drosophila imaginal discs. In many cases, the mutant cells are eliminated from the tissue and the overgrowths are comprised of neighboring wild type cells. For example, cells mutant for vps25, which encodes an ESCRT-II component, are not able to contribute to adult tissue, yet eyes with vps25 mutant clones are dramatically overgrown (Thompson et al., 2005; Vaccari and Bilder, 2005; Herz et al., 2006). Similar results have been reported for erupted (ept), the Drosophila homolog of TSG101 that encodes an ESCRT-I and -II component (Moberg et al., 2005). Subsequent work characterized many other ESCRT components and found that mutations in most caused similar overgrown eye phenotypes despite the fact that the mutant clones died (Herz and Bergmann, 2009; Vaccari et al., 2009; Woodfield et al., 2013). In these cases, ESCRT mutants trap the Notch receptor in endosomes, where it signals aberrantly and continues to induce transcription of upd (Fig. 2C,D and (Lu and Bilder, 2005; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Herz et al., 2006; Vaccari et al., 2008; Rodahl et al., 2009; Vaccari et al., 2009)). Overgrowth correlates with ectopic Upd expression, and reducing the genetic dose of Stat92E suppresses the non-autonomous overgrowth caused by vps25 and ept clones (Moberg et al., 2005; Vaccari and Bilder, 2005; Herz et al., 2006). Interesting recent work has identified endocytic mutants that have increased JAK/STAT activity without a corresponding increase in Notch signaling (Thomas and Strutt, 2014). The outcome of aberrant JAK/STAT activation, however, remains the same: loss of epithelial structure and ectopic growth, which are hallmarks of neoplastic transformation.
One important question is whether JAK/STAT signaling plays an autonomous role within tumor cells themselves. To examine this, two independent groups generated imaginal discs composed almost entirely of ESCRT mutant cells and found autonomous activation of JAK/STAT signaling (Gilbert et al., 2009; Woodfield et al., 2013). Within ept tumors, removing one genetic copy of Stat92E is sufficient to alter cell size and cell cycle dynamics, and FACS analysis reveals fewer cells entering S-phase following Stat92E reduction (Gilbert et al., 2009). In addition, Stat92E phosphorylation and stabilization of Dome within endocytic vesicles is detected within ept tumors. While the site of active Dome signaling is debated (Devergne et al., 2007; Vidal et al., 2010), one possibility is that Dome is stabilized in an active state in ESCRT mutants, bypassing the requirement for a secreted ligand (Fig. 2C,D). Moreover, within endocytic tumors, reducing Stat92E activity significantly rescues the loss of epithelial polarity (Gilbert et al., 2009; Woodfield et al., 2013; Thomas and Strutt, 2014). These results suggest that the role of JAK/STAT signaling extends beyond simple regulation of proliferation and affects other cell behaviors such as adhesion. One potential effector of this latter function is the apical determinant Crumbs (Crb) (Gilbert et al., 2009). Crb deregulation is itself sufficient to induce neoplastic overgrowth (Lu and Bilder, 2005). While crb is a direct target of Stat92E in posterior spiracles (Lovegrove et al., 2006), it is yet to be resolved whether this relationship also exists in endocytic tumors or if crb deregulation is a secondary effect of impaired vesicle recycling (Fig. 2D and (Gilbert et al., 2009; Thomas and Strutt, 2014)).
Oncogenic cooperation: the role of JAK/STAT signaling in polarity-deficient tumors
Many neoplastic tumor suppressor genes identified in genetic screens in Drosophila encode regulators of epithelial polarity (Bilder, 2004). In particular, scribbled (scrib) regulates septate junctions and maintains the separation between apical and basal membranes (Bilder and Perrimon, 2000). Loss of scrib in whole tissues leads to epithelial disorganization and tumor formation (Bilder et al., 2000), but clones mutant for scrib in proximity to wild type cells are eliminated by a process called cell competition (Brumby and Richardson, 2003; Igaki et al., 2006). However, in cooperation with another oncogene, such as an activated form of Ras called RasV12, the tumorigenic potential of scrib mutant cells is unleashed, and the mutant cells (referred to as RasV12 scrib) metastasize (Brumby and Richardson, 2003; Pagliarini and Xu, 2003).
RasV12 scrib mutant cells display high Jun N-terminal kinase (JNK) signaling, which can induce expression of all three upd genes, leading to systemic JAK/STAT pathway activation (Brumby and Richardson, 2003; Igaki et al., 2006; Pastor-Pareja et al., 2008; Wu et al., 2010). Activated Stat92E and RasV12 then autonomously cooperate to cause massive overgrowth and metastasis (Fig. 2E and (Wu et al., 2010)). In a separate tumor model, RasV12 combined with loss of a JAK/STAT inhibitor leads to metastatic tumor formation, confirming the carcinogenic cooperativity of the two pathways (Herranz et al., 2012). Indeed, preventing JNK activation in scrib mutant cells prevents both Stat92E activation and neoplastic transformation, and preventing Stat92E activation in RasV12 scrib tumors suppresses both over-proliferation and metastasis (Wu et al., 2010). One model to explain these observations is that expression of Upd ligands in tissues that are damaged, either by direct injury or by cell death, is a mechanism for compensatory proliferation to restore tissue size (Wu et al., 2010). Another interesting aspect of the upregulation of Upd ligands in tumors is that it leads to the proliferation in circulating blood cells called hemocytes (Pastor-Pareja et al., 2008). These cells in turn adhere to tumors and reduce their growth, suggesting that JAK/STAT signaling also plays roles indirectly in altering the tumor microenvironment by affecting hemocyte numbers.
The JAK/STAT pathway as a major driver of hematopoietic tumors
The role of JAK/STAT signaling in melanotic tumors, a “fly leukemia” model
Decades before the discovery of oncogenic mutations in JAK/STAT signaling in myeloproliferative neoplasms, studies in Drosophila linked JAK/STAT signaling to lethal blood cell tumors referred to as fly leukemia (Corwin and Hanratty, 1976; Hanratty and Ryerse, 1981). Hemocytes are derived from an embryonic pool as well as from the larval lymph gland (reviewed in (Evans et al., 2003)). The anterior lobe of the lymph gland is subdivided into a niche called the posterior signaling center; a medullary zone where multipotent progenitors called pro-hemocytes reside; and a cortical zone where the pro-hemocytes differentiate into plasmatocytes, crystal cells and lamellocytes (Fig. 3A and (Jung et al., 2005)). Plasmatocytes make up 95% of circulating hemocytes and function as professional phagocytes to remove bacteria and apoptotic cells. Crystal cells account for 5% of total hemocytes and are required for melanization of foreign tissue. Lamellocytes are absent or at very low levels in wild type animals but are rapidly induced by parasitic wasp infection, and they function to encapsulate objects too large to be phagocytosed (Sorrentino et al., 2002). Although the cell autonomous function of JAK/STAT signaling in maintenance of prohemocytes is debated (Krzemien et al., 2007; Minakhina and Steward, 2010; Mondal et al., 2011), there is agreement that Stat92E is required for plasmatocyte differentiation. This occurs at least in part through JAK/STAT pathway regulation of pannier, which encodes a GATA factor, and of u-shaped, which encodes a Friend of Gata factor (Fig. 3A,B and (Sorrentino et al., 2007; Gao et al., 2009; Minakhina et al., 2011)).
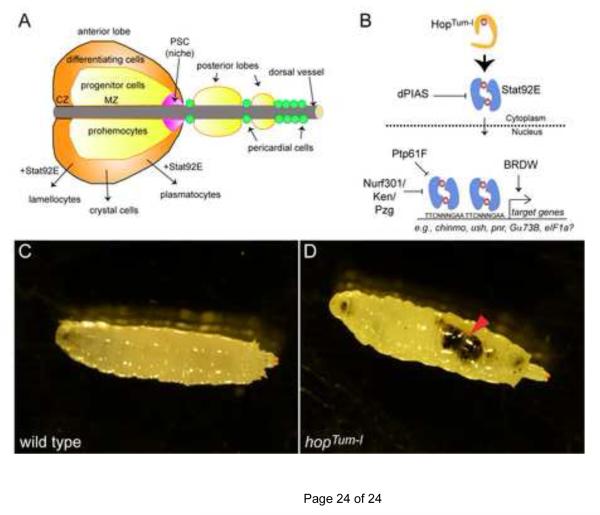
Fig. 3. JAK/STAT signaling in hematopoiesis and melanotic tumors.
(A) The lymph gland is the larval hematopoietic organ. In the anterior lobe, there are three zones. Cells in the posterior signaling center (PSC, magenta) form the niche for multipotent progenitors called prohemocytes that reside in the medullary zone (MZ, yellow). Prohemocytes give rise to all Drosophila blood lineages, plasmatocytes, crystal cells and lamellocytes. Differentiation of hemocytes occurs in the cortical zone (CZ, orange).
(B) Model of Stat92E dimer activity (blue) and gene regulation downstream of HopTum-l. See text for details.
(C,D) Micrograph of a wild type larva (C) or a hopTum-l larva (D) reared at the restrictive temperature of 29°C. There is a large melanotic tumor in the abdomen of the hopTum-l larva (D, arrowhead) but none in the wild type control (C).
Tumorous-lethal (Tum-l) is a dominant, temperature-sensitive mutation in the hop locus that leads to overproliferation of hemocytes and formation of melanotic tumors (Fig. 3C,D). Melanotic tumors are black masses of hemocytes that - in the case of dysregulated JAK/STAT signaling - are invasive and correlated with lethality (Hanratty and Ryerse, 1981; Hanratty and Dearolf, 1993; Lanot et al., 2001; Minakhina and Steward, 2006). hopTum-l is caused by a G341E substitution in the JAK homology 4 (JH4) domain (Fig. 1B). A second dominant mutation in hop, called T42, is functionally identical to Tum-l and is caused by a E695K substitution in the JH2 domain (Fig. 1B) (Luo et al., 1997). Both hop mutations result in a hyperactive kinase, which hyperphosphorylates Stat92E, leading to increased association of Stat92E with DNA (Fig. 3B and (Harrison et al., 1995; Luo et al., 1995; Luo et al., 1997)). Interestingly, the V617F mutation in Jak2 also results in a hyperactive kinase and this mutation resides in the Jak2 JH2 domain, which normally represses the function of the JH1 kinase domain (Ungureanu et al., 2011; Bandaranayake et al., 2012).
Several groups have shown that the hopTum-l melanotic phenotype is due to hyperactivation of JAK/STAT signaling as heterozygosity of Stat92E suppresses the lethality and the tumorigenic phenotype associated with both hopTum-l and hopT42 (Hou et al., 1996; Yan et al., 1996; Luo et al., 1997). In hopTum-l animals, the number of plasmatocytes and lamellocytes in circulation is dramatically increased (Silvers and Hanratty, 1984; Luo et al., 1995; Lanot et al., 2001). In particular, the number of lamellocytes increases 30 fold compared to controls and comprises 40 to 70% of the total hemocyte pool. The tumors caused by the hopTum-l lesion represent bona fide transplantable neoplasms. When hopTum-l larval lymph glands are injected into wild-type adult flies, melanotic masses and invasive hemocytes are observed in various tissues (Hanratty and Ryerse, 1981; Luo et al., 1995). Interestingly, over-expression of wild-type Hop or HopTum-l in the lymph gland is sufficient to generate melanotic tumors (Harrison et al., 1995; Luo et al., 1995; Zettervall et al., 2004). Sustained Stat92E signaling is sufficient to induce melanotic tumors, as these lesions are observed in animals mutant for dPIAS as well as those mis-expressing a dominant-active Stat92E (Hari et al., 2001; Ekas et al., 2010). Furthermore, removing one copy of dPIAS enhances the hopTum-l tumor incidence, while over-expression of dPIAS significantly suppresses it (Betz et al., 2001). Taken together, these studies strongly suggest that sustained activation of the JAK/STAT pathway in lymph gland derived-hemocytes is necessary and sufficient to generate melanotic tumors.
The role of pathway modulators and target genes in melanotic tumor formation
Through various screens, several genes have been identified as modulators or effectors of JAK/STAT signaling in tumorigenesis (Fig. 3B). RNAi screens revealed two factors that regulate Stat92E activity (Baeg et al., 2005; Muller et al., 2005). The hopTum-l tumor index is suppressed by heterozygosity of BRWD3, which encodes a bromodomain and WD40 domain protein, or by mis-expression of Ptp61F, a protein tyrosine phosphatase. Ptp61F has been proposed to de-phosphorylate activated Stat92E dimers and BRWD3 may regulate access of Stat92E dimers to chromatin (Fig. 3B). Loss-of-function mutations in Nurf301, a large nucleosome remodeling factor (NURF) subunit, induce melanotic tumors, increase lamellocyte differentiation and enhance the hopTum-l phenotype (Badenhorst et al., 2002; Kwon et al., 2008). Microarray analysis of Nurf301and hopTum-l mutant larvae showed a large overlap of upregulated genes, suggesting that NURF normally represses JAK/STAT targets. The authors propose that this repression occurs by NURF recruitment to STAT binding sites via the transcriptional repressor Ken and Barbie (Ken), which had previously been shown to inhibit STAT targets through competitive binding to STAT responsive DNA elements (Fig. 3B and (Arbouzova et al., 2006; Kwon et al., 2008)). More recent work has revealed that Putzig (Pzg), a component of the TRF2/DREF replication complex, acts in concert with Nurf301 and Ken to repress JAK/STAT target genes (Fig. 3B and (Kugler et al., 2011)). In support of this model, loss of one copy of Nurf301, ken or pzg singly or in combination significantly increases the hopTum-l tumor incidence (Kwon et al., 2008; Kugler et al., 2011).
A genetic screen for hopTum-l modifiers revealed a link between JAK/STAT tumorigenesis and chromatin modifiers. Mutations in Su(var)205 and Su(var)3-9, which encode Heterochromatin Protein 1 (HP1) and a histone methyltransferase, respectively, were identified as enhancers of hopTum-l tumorigenicity (Shi et al., 2006). HP1 and Su(var)3-9 show reduced association with heterochromatin in hopTum-l flies and increased association in animals heterozygous for a hop loss-of-function allele (Shi et al., 2006). Despite the established linear relationship from Hop to Stat92E, they were reported to regulate HP1 in opposite manners (Shi et al., 2008). These results have led to a model of “non-canonical” pathway activity in which unphosphorylated Stat92E is bound to heterochromatin with HP1. This association is disrupted with Stat92E phosphorylation by Hop leading to heterochromatin instability (Shi et al., 2008).
Finally, expression profiling has revealed potential JAK/STAT targets that promote tumorigenesis. eukaryotic initiation factor 1A (eIF-1A) mRNA, which encodes a component of the translation machinery, is significantly upregulated in hopTum-l lymph glands, providing a potential link between increased JAK/STAT activity and growth, but its functional relevance is unclear (Myrick and Dearolf, 2000). The Stat92E target gene chinmo causes melanotic tumors when mis-expressed, but it is not yet known how Chinmo affects blood cells (Flaherty et al., 2010). Transcripts encoding a G protein subunit Gα73B (also called Gαf) are increased in cultured cells treated with Upd (Bina et al., 2010). Decreasing Gα73B suppresses hopTum-l tumorigenesis, while Gα73B over-expression increases the tumor burden (Bausek and Zeidler, 2014). Gα73B likely mediates hemocyte motility and tumor invasion downstream of Stat92E.
Conclusions
Sustained JAK/STAT signaling accounts for the overgrowth and neoplastic appearance caused by loss of several distinct tumor suppressors, including PRC1, ESCRT and scrib. In most cases, mutation of the tumor suppressor results in ectopic expression of Upd. However, overgrowth of the eye disc is also observed with loss of C-terminal Src kinase (Csk) (Read et al., 2004), in which Stat92E protein is activated autonomously without upregulating the ligand Upd (Fig. 3C). Thus, sustained Stat92E activation is sufficient for tissue overgrowth. One emerging theme is that tumorigenesis involves co-operation between oncogenes, such Ras and STAT, in mammals and in flies (Wu et al., 2010; Corcoran et al., 2011; Herranz et al., 2012). Melanotic tumor phenotypes have also been observed with hyperactivation of Ras and Toll pathways (Minakhina and Steward, 2006). One outstanding question is whether the JAK/STAT, Ras, and Toll pathways act in parallel in this process or if there are cooperative interactions between them in melanotic tumor formation in Drosophila. Although the causal link between JAK/STAT activity and oncogenesis is clear both in flies and mammals, the relevant targets that mediate transformation still need to be identified. The lower complexity of the JAK/STAT pathway in Drosophila and the conservation of some genetic relationships (e.g. STAT and Ras) in tumorigenesis in flies and mammals, suggest that research in Drosophila will continue to yield fruitful avenues for understanding tumor formation.
Highlights.
!! JAK/STAT signaling is central to epithelial and hematopoietic tumors in Drosophila.
!! Unpaired is ectopically induced by loss of distinct tumor suppressors.
!! Ras and JAK/STAT pathways cooperate to induce metastatic tumors.
!! Dominant-active JAK mutations cause melanotic tumors, a fly leukemia.
!! Genetic screens have identified new pathway effectors in blood cell tumors.
Acknowledgements
We apologize to colleagues whose work was not cited due to space constraints. We thank M. Burel for helpful comments on the manuscript. AMA was supported by NIH T32 CA009161 (PI: D. Levy). MA and EAB are supported by NIH R01 GM085075, NYSTEM C028132 and the Hirschl Trust (all to EAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abdel-Wahab O. Genetics of the myeloproliferative neoplasms. Curr Opin Hematol. 2011;18(2):117–23. doi: 10.1097/MOH.0b013e328343998e. [DOI] [PubMed] [Google Scholar]
- Arbouzova NI, Bach EA, Zeidler MP. Ken & barbie selectively regulates the expression of a subset of Jak/STAT pathway target genes. Curr Biol. 2006;16(1):80–8. doi: 10.1016/j.cub.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133(14):2605–16. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Bach EA, Vincent S, Zeidler MP, Perrimon N. A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics. 2003;165(3):1149–66. doi: 10.1093/genetics/165.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhorst P, Voas M, Rebay I, Wu C. Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev. 2002;16(24):3186–98. doi: 10.1101/gad.1032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005;19(16):1861–70. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19(8):754–9. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausek N, Zeidler MP. Galpha73B is a downstream effector of JAK/STAT signalling and a regulator of Rho1 in Drosophila haematopoiesis. J Cell Sci. 2014;127(Pt 1):101–10. doi: 10.1242/jcs.132852. [DOI] [PubMed] [Google Scholar]
- Betz A, Lampen N, Martinek S, Young MW, Darnell JE., Jr. A Drosophila PIAS homologue negatively regulates stat92E. Proc Natl Acad Sci U S A. 2001;98(17):9563–8. doi: 10.1073/pnas.171302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchle D, Struhl G, Muller J. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development. 2001;128(6):993–1004. doi: 10.1242/dev.128.6.993. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18(16):1909–25. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289(5476):113–6. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403(6770):676–80. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Bina S, Wright VM, Fisher KH, Milo M, Zeidler MP. Transcriptional targets of Drosophila JAK/STAT pathway signalling as effectors of haematopoietic tumour formation. EMBO Rep. 2010;11(3):201–7. doi: 10.1038/embor.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. Embo J. 2003;22(21):5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131(16):3839–47. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nat Genet. 2009;41(10):1150–5. doi: 10.1038/ng.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM. Imaginal disc development. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71(14):5020–9. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin HO, Hanratty WP. Characterization of a unique lethal tumorous mutation in Drosophila. Mol Gen Genet. 1976;144(3):345–7. doi: 10.1007/BF00341734. [DOI] [PubMed] [Google Scholar]
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11(6):595–6. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- Devergne O, Ghiglione C, Noselli S. The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci. 2007;120(Pt 19):3457–64. doi: 10.1242/jcs.005926. [DOI] [PubMed] [Google Scholar]
- Djiane A, Krejci A, Bernard F, Fexova S, Millen K, Bray SJ. Dissecting the mechanisms of Notch induced hyperplasia. Embo J. 2013;32(1):60–71. doi: 10.1038/emboj.2012.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekas LA, Baeg GH, Flaherty MS, Ayala-Camargo A, Bach EA. JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development. 2006;133(23):4721–9. doi: 10.1242/dev.02675. [DOI] [PubMed] [Google Scholar]
- Ekas LA, Cardozo TJ, Flaherty MS, McMillan EA, Gonsalves FC, Bach EA. Characterization of a dominant-active STAT that promotes tumorigenesis in Drosophila. Dev Biol. 2010;344(2):621–36. doi: 10.1016/j.ydbio.2010.05.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5(5):673–90. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Feng S, Huang J, Wang J. Loss of the Polycomb group gene polyhomeotic induces non-autonomous cell overproliferation. EMBO Rep. 2011;12(2):157–63. doi: 10.1038/embor.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation and stem cell self-renewal in Drosophila. Developmental Cell. 2010;18(4):556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MS, Zavadil J, Ekas LA, Bach EA. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of notch signaling by the JAK/STAT pathway. Dev Dyn. 2009;238(9):2235–53. doi: 10.1002/dvdy.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Upregulation of the Drosophila Friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol Cell Biol. 2009;29(22):6086–96. doi: 10.1128/MCB.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MM, Beam CK, Robinson BS, Moberg KH. Genetic interactions between the Drosophila tumor suppressor gene ept and the stat92E transcription factor. PLoS One. 2009;4(9):e7083. doi: 10.1371/journal.pone.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I, Simon R, Busturia A. The Polyhomeotic protein induces hyperplastic tissue overgrowth through the activation of the JAK/STAT pathway. Cell Cycle. 2009;8(24):4103–11. doi: 10.4161/cc.8.24.10212. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and four-jointed. EMBO Rep. 2009;10(9):1051–8. doi: 10.1038/embor.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanratty WP, Dearolf CR. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993;238(1-2):33–7. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Hanratty WP, Ryerse JS. A genetic melanotic neoplasm of Drosophila melanogaster. Dev Biol. 1981;83(2):238–49. doi: 10.1016/0012-1606(81)90470-x. [DOI] [PubMed] [Google Scholar]
- Hari KL, Cook KR, Karpen GH. The Drosophila Su(var)2-10 locus regulates chromosome structure and function and encodes a member of the PIAS protein family. Genes Dev. 2001;15(11):1334–48. doi: 10.1101/gad.877901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. Embo J. 1995;14(12):2857–65. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz H, Hong X, Hung NT, Voorhoeve PM, Cohen SM. Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes Dev. 2012;26(14):1602–11. doi: 10.1101/gad.192021.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Bergmann A. Genetic analysis of ESCRT function in Drosophila: a tumour model for human Tsg101. Biochem Soc Trans. 2009;37(Pt 1):204–7. doi: 10.1042/BST0370204. [DOI] [PubMed] [Google Scholar]
- Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133(10):1871–80. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84(3):411–9. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16(11):1139–46. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132(11):2521–33. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kallio J, Myllymaki H, Gronholm J, Armstrong M, Vanha-aho LM, Makinen L, Silvennoinen O, Valanne S, Ramet M. Eye transformer is a negative regulator of Drosophila JAK/STAT signaling. FASEB J. 2010;24(11):4467–79. doi: 10.1096/fj.10-162784. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446(7133):325–8. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Kugler SJ, Gehring EM, Wallkamm V, Kruger V, Nagel AC. The Putzig-NURF nucleosome remodeling complex is required for ecdysone receptor signaling and innate immunity in Drosophila melanogaster. Genetics. 2011;188(1):127–39. doi: 10.1534/genetics.111.127795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SY, Xiao H, Glover BP, Tjian R, Wu C, Badenhorst P. The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev Biol. 2008;316(2):538–47. doi: 10.1016/j.ydbio.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230(2):243–57. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lovegrove B, Simoes S, Rivas ML, Sotillos S, Johnson K, Knust E, Jacinto A, Hombria JC. Coordinated control of cell adhesion, polarity, and cytoskeleton underlies Hox-induced organogenesis in Drosophila. Curr Biol. 2006;16(22):2206–16. doi: 10.1016/j.cub.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7(12):1132–9. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Luo H, Asha H, Kockel L, Parke T, Mlodzik M, Dearolf CR. The Drosophila Jak kinase hopscotch is required for multiple developmental processes in the eye. Dev Biol. 1999;213(2):432–41. doi: 10.1006/dbio.1999.9390. [DOI] [PubMed] [Google Scholar]
- Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. Embo J. 1995;14(7):1412–20. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, D’Andrea AD, Dearolf CR. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17(3):1562–71. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki R, Meister M, Pennetier D, Ubeda JM, Braun A, Daburon V, Krzemien J, Bourbon HM, Zhou R, Vincent A, et al. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8(8):e1000441. doi: 10.1371/journal.pbio.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. Embo J. 1999;18(5):1367–77. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menut L, Vaccari T, Dionne H, Hill J, Wu G, Bilder D. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177(3):1667–77. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer. 2010;10(10):669–82. doi: 10.1038/nrc2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Melanotic mutants in Drosophila: pathways and phenotypes. Genetics. 2006;174(1):253–63. doi: 10.1534/genetics.106.061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137(1):27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Tan W, Steward R. JAK/STAT and the GATA factor Pannier control hemocyte maturation and differentiation in Drosophila. Dev Biol. 2011;352(2):308–16. doi: 10.1016/j.ydbio.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9(5):699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147(7):1589–600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Sexl V, Kenner L, Duntsch C, Stangl K, Gingras S, Hoffmeyer A, Bauer A, Piekorz R, Wang D, et al. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7(1):87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;19(10):4167–76. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Hombria JC, Zeidler MP. Opposing roles for Drosophila JAK/STAT signalling during cellular proliferation. Oncogene. 2005a;24(15):2503–11. doi: 10.1038/sj.onc.1208487. [DOI] [PubMed] [Google Scholar]
- Mukherjee T, Schaefer U, Zeidler MP. Identification of Drosophila genes modulating JAK/STAT signal transduction. Genetics. 2005b doi: 10.1534/genetics.105.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436(7052):871–5. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- Myrick KV, Dearolf CR. Hyperactivation of the Drosophila Hop jak kinase causes the preferential overexpression of eIF1A transcripts in larval blood cells. Gene. 2000;244(1-2):119–25. doi: 10.1016/s0378-1119(99)00568-5. [DOI] [PubMed] [Google Scholar]
- Oktaba K, Gutierrez L, Gagneur J, Girardot C, Sengupta AK, Furlong EE, Muller J. Dynamic regulation by polycomb group protein complexes controls pattern formation and the cell cycle in Drosophila. Dev Cell. 2008;15(6):877–89. doi: 10.1016/j.devcel.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302(5648):1227–31. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1(2-3):144–54. doi: 10.1242/dmm.000950. discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RD, Bach EA, Cagan RL. Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol Cell Biol. 2004;24(15):6676–89. doi: 10.1128/MCB.24.15.6676-6689.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol. 2005;285(1):38–48. doi: 10.1016/j.ydbio.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Rodahl LM, Haglund K, Sem-Jacobsen C, Wendler F, Vincent JP, Lindmo K, Rusten TE, Stenmark H. Disruption of Vps4 and JNK function in Drosophila causes tumour growth. PLoS One. 2009;4(2):e4354. doi: 10.1371/journal.pone.0004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AB, Zoranovic T, Ayala-Camargo A, Grewal S, Reyes-Robles T, Krasny M, Wu DC, Johnston LA, Bach EA. Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development. 2012;139(21):4051–61. doi: 10.1242/dev.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Vaccari T, Stenmark H. Shaping development with ESCRTs. Nat Cell Biol. 2012;14(1):38–45. doi: 10.1038/ncb2381. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8(1):9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- Shi S, Calhoun HC, Xia F, Li J, Le L, Li WX. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet. 2006;38(9):1071–6. doi: 10.1038/ng1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Larson K, Guo D, Lim SJ, Dutta P, Yan SJ, Li WX. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat Cell Biol. 2008;10(4):489–96. doi: 10.1038/ncb1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers M, Hanratty WP. Alterations in the production of hemocytes due to a neoplastic mutation of Drosophila melanogaster. J Invertebr Pathol. 1984;44(3):324–8. doi: 10.1016/0022-2011(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243(1):65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Tokusumi T, Schulz RA. The Friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev Biol. 2007;311(2):311–23. doi: 10.1016/j.ydbio.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Thomas C, Strutt D. Rabaptin-5 and Rabex-5 are neoplastic tumour suppressor genes that interact to modulate Rab5 dynamics in Drosophila melanogaster. Dev Biol. 2014;385(1):107–21. doi: 10.1016/j.ydbio.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9(5):711–20. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Tsai YC, J.G. Y, P.H. C, Posakony JW, Barolo S, Kim J, Henry Sun Y. Upd/Jak/STAT signaling represses wg transcription to allow initiation of morphogenetic furrow in Drosophila eye development. Dev Biol. 2007;306(2):760–71. doi: 10.1016/j.ydbio.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Sun YH. Long-range effect of upd, a ligand for Jak/STAT pathway, on cell cycle in Drosophila eye development. Genesis. 2004;39(2):141–53. doi: 10.1002/gene.20035. [DOI] [PubMed] [Google Scholar]
- Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, Xu CF, Neubert TA, Skoda RC, Hubbard SR, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18(9):971–6. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9(5):687–98. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. At the crossroads of polarity, proliferation and apoptosis: the use of Drosophila to unravel the multifaceted role of endocytosis in tumor suppression. Mol Oncol. 2009;3(4):354–65. doi: 10.1016/j.molonc.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180(4):755–62. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, Bilder D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci. 2009;122(Pt 14):2413–23. doi: 10.1242/jcs.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal OM, Stec W, Bausek N, Smythe E, Zeidler MP. Negative regulation of Drosophila JAK-STAT signalling by endocytic trafficking. J Cell Sci. 2010;123(Pt 20):3457–66. doi: 10.1242/jcs.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfield SE, Graves HK, Hernandez JA, Bergmann A. De-regulation of JNK and JAK/STAT signaling in ESCRT-II mutant tissues cooperatively contributes to neoplastic tumorigenesis. PLoS One. 2013;8(2):e56021. doi: 10.1371/journal.pone.0056021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463(7280):545–8. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Small S, Desplan C, Dearolf CR, Darnell JE., Jr. Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84(3):421–30. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101(39):14192–7. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]