Abstract
Raising chickens for eggs in urban areas is becoming increasingly common. Urban chickens may be exposed to lead, a common urban soil contaminant. We measured lead concentrations in chicken eggs from New York City (NYC) community gardens and collected information on factors that might affect those concentrations. Lead was detected between 10 and 167 μg/kg in 48% of NYC eggs. Measures of lead in eggs from a henhouse were significantly associated (p<0.005) with lead concentrations in soil. The association between soil and egg lead has been evaluated only once before, by a study of a rural region in Belgium. In our study, the apparent lead soil-to-egg transfer efficiency was considerably lower than that found in Belgium, suggesting that there may be important geographic differences in this transfer. We developed models that suggested that, for sites like ours, lead concentrations in >50% of eggs from a henhouse would exceed store-bought egg concentrations (<7–13 μg/kg; 3% above detection limit) at soil lead concentrations >120 mg/kg, and that the concentration in one of six eggs from a henhouse would exceed a 100 μg/kg guidance value at soil lead concentrations >410 mg/kg. Our models also suggested that the availability of dietary calcium supplements was another influential factor that reduced egg lead concentrations. Estimates of health risk from consuming eggs with the lead concentrations we measured generally were not significant. However, soil lead concentrations in this study were <600 mg/kg, and considerably higher concentrations are not uncommon. Efforts to reduce lead transfer to chicken eggs and associated exposure are recommended for urban chicken keepers.
Keywords: chicken eggs, lead (Pb) exposure, community gardens, urban agriculture, exposure assessment
1 Introduction
Raising chickens in urban areas of the United States appears to be increasing in popularity, as part of a broader growing interest in local food production, urban agriculture and sustainability (Beam et al. 2013; Pollock et al. 2012); and many cities have reversed earlier bans on raising chickens (Bartling 2010). Urban chickens (hens) are typically raised in residential backyards, urban farms and community gardens for their eggs. In 2010, approximately 4% of 223 New York City (NYC) community gardens surveyed reported having henhouses in their gardens (Gittleman et al. 2010). A number of benefits have been attributed to producing food, including raising chickens, in urban settings, such as reducing environmental impacts of long-distance food transportation and providing opportunities for children to learn about sustainable agriculture (Alaimo et al. 2008; Pollock et al. 2012). Chicken eggs are a nutrient-dense food that can be part of a healthy diet (USDA and HHS 2010), and they may be an important food source for those residing in areas that have poor access to healthy foods.
However, soils in urban yards, and in vacant lots and brownfields often considered as sites for urban community gardens and farms, may contain chemical contaminants. Lead, for example, which has a median background concentration of 23 mg/kg in New York State rural soils (NYSDEC 2006), can be found at concentrations of several hundred or even thousands of mg/kg in soil in NYC and other cities, due to historic sources such as lead-based paint, leaded gasoline combustion emissions, and point sources such as waste incinerators and metal smelters (ATSDR 2007; US EPA 1998). Birds may eat soil to obtain calcium and other minerals for egg shells (Symes et al. 2006), and they can also incidentally ingest soil; Stephens et al. (1995) estimated that soil makes up 10% or more of a free-range chicken’s diet. Therefore, eggs produced by urban chickens may provide a human exposure pathway for lead in urban soils.
Previous studies (Hsu et al. 2010; Kijlstra et al. 2007; Stephens et al. 1995) have investigated soil-to-egg transfer of persistent and bioaccumulative chlorinated organic compounds, such as organochlorine pesticides and dioxins. However, less consideration has been given to transfer of inorganic soil contaminants, such as lead, to eggs, both in research and in risk assessment practice. For example, a widely used US EPA risk assessment guidance document (US EPA 2005) includes biotransfer factors for estimating the transfer of many organic contaminants from soil to chicken eggs, but provides no biotransfer factor for lead. As a result, this potential exposure pathway for lead is excluded.
Nonetheless, studies have found lead in eggs from chickens raised in rural areas in Belgium (Waegeneers et al. 2009a, 2009b) and in chickens in the US observed eating chips of lead paint (Trampel et al. 2003). The Belgian study found lead concentrations in composite samples of eggs in the range of < 2 to 477 μg/kg, with 25–40% of egg samples having concentrations higher than Belgium’s former Maximum Permissible Concentration in chicken eggs of 100 μg/kg, cited by the study as a health-based guidance value. Lead concentrations in eggs were significantly associated with those in soil over a range of 12 to 174 mg/kg, with a Pearson correlation coefficient of 0.49 (Waegeneers et al. 2009b). The authors also developed a model to describe lead transfer into eggs by modifying a dioxin-transfer model. In the lead-transfer model, lead in soil was the major source of lead in eggs in most cases, accounting for up to 92%, of lead in eggs (Waegeneers et al. 2009a).
In general, any exposure to lead is considered to be potentially harmful to human health since no threshold for adverse effects has been identified (Miranda et al. 2007). Studies have shown that elevated blood lead levels are associated with decreased performance in functions of the nervous system, increases in blood pressure, anemia, and reproductive effects (ATSDR 2007), and relatively recent studies (Jusko et al. 2008; Miranda et al. 2007) have reported effects at lower blood lead levels than earlier studies.
The Healthy Soils, Healthy Communities (HSHC) project, a community-based research collaboration between the New York State Department of Health, Cornell University, the NYC Department of Parks and Recreation/GreenThumb and gardeners, has been investigating issues related to soil contamination in urban community gardens. The HSHC project has worked in NYC community gardens in which chickens were kept in areas with lead concentrations in excess of 1000 mg/kg in soil (unpublished data). This suggested the possibility that chicken eggs from community gardens and residential yards in NYC as well as other urban areas of the US may have concentrations of lead that may represent a previously overlooked health risk. This concern was also raised by NYC gardeners, chicken keepers and members of the non-profit organization Just Food who were working with the HSHC project.
In response to these concerns, we initiated a study as part of the HSHC project to assess the concentrations of lead in a sample of eggs from chickens raised in NYC as compared to previously reported concentrations in chicken eggs, to assess associated health risks, and to evaluate environmental and other factors that might contribute to those egg lead concentrations. In keeping with the community focus of the HSHC project, we also sought to develop outreach materials for chicken keepers to explain the results of the project, possible health implications, and, when appropriate, steps that could be taken to reduce exposure to lead in chicken eggs.
2 Materials and Methods
2.1 Study Site Identification, Characterization and Sample Collection
Just Food helped identify chicken-keeping community gardens across NYC as potential participants in the project. Chicken keepers from twelve of the identified gardens in the Bronx, Brooklyn and Queens and one municipally owned farm agreed to participate in an initial phase of our study by helping us gather information about their chicken-keeping practices and allowing us to collect composite surface soil samples from their gardens during the summer of 2011. We collected information about the amount of time chickens were allowed out of the henhouse and whether that time was spent in an fenced-in outdoor “run” or in other parts of the garden, the size of the run, the type of ground cover, whether feed was scattered on the ground, the length of time the chickens had been kept at the garden, the number of eggs laid per day, and other characteristics.
A composite sample of surface soil (0 – 10 cm depth) was collected from the chicken run associated with each henhouse. Additional surface soil samples were collected from other garden areas that were reported to be regularly accessed by chickens. Each sample characterizing an area (e.g., a chicken run) was a composite of 5 to 15 uniformly sized subsamples collected with a clean garden trowel after other materials (bedding, grass, woodchips) had been removed from the soil surface. Subsamples were combined in a resealable plastic freezer bag.
Locations where chickens had been kept for at least three months, were reported to have regular access to an unpaved outdoor run, and had no significant changes recently made to their environment were considered for further participation in the study. An attempt was also made to select gardens for the second phase of the study that represented the range of lead concentrations found in soil from the thirteen gardens participating in the initial phase.
The second phase of the study, in the fall and winter of 2011–2012, included nine NYC henhouses and chicken runs located at six community gardens and the municipally owned farm, along with one residential “rural control garden” in upstate New York, about 220 km north of NYC. Chicken keepers from these sites (hereinafter collectively referred to as “gardens”) helped us confirm information from the previous visit and added information relevant to the three months immediately preceding egg collection.
Chicken keepers at each of the ten participating henhouses were asked to provide six eggs, a sample of the chickens’ commercial layer feed and any other feed materials, and a sample of the chickens’ drinking water. Eggs were collected in standard egg cartons and feed samples in resealable plastic freezer bags. Chicken keepers for three of the henhouses provided more than six eggs (between seven and ten), and three eggs from one henhouse were accidentally broken after collection and were not replaced. Samples of calcium supplements, grit (a dietary supplement), kitchen and garden scraps (such as leafy greens, acorn squash and cabbage), and other feed materials were also collected from a small number of henhouses where chicken keepers reported providing these materials to the chickens. In addition, six eggs were purchased from NYC grocery stores as a “market-basket” sample (two each of three brands, labeled as “free-roaming,” “organic,” or “cage-free”). All egg samples were stored and transported to the laboratory at ambient temperature.
A water sample was collected at each henhouse from the chickens’ drinking water source (usually a tap or hydrant). Water was allowed to run for at least 15 seconds, after which samples were collected by filling high density polyethylene bottles pre-cleaned to comply with US EPA recommendations for metals analysis (that were routinely tested by our laboratory for acceptable blank metals levels). Samples were preserved on site with nitric acid (Mallinckrodt ACS reagent grade) and transported to the laboratory in a cooler with ice packs.
2.2 Analytical Methods
Soil samples were air dried, homogenized by hand-mixing in their sealed plastic bags, and screened through a 2 mm sieve. The < 2mm fraction was analyzed for lead in the laboratory with a factory-calibrated Innov-X Alpha 4000 X-ray fluorescence (XRF) analyzer, in general accordance with US EPA SW-846 Method 6200. The instrument’s calibration was checked prior to analysis with two certified soil standards (NIST 2702 and NIST 2781) and a silicon dioxide blank. In addition, a portion of each sieved chicken-run soil sample was analyzed for lead by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; SW-846 Methods 3050B/6010B) at a laboratory certified by New York State’s Environmental Laboratory Approval Program, in order to have certified soil results that could be reported to chicken keepers in accordance with New York State requirements. Water samples were analyzed for total recoverable lead by inductively coupled plasma – mass spectrometry (ICP-MS; CWA Method 200.8) with on-line addition of terbium as an internal standard and a laboratory minimum reporting level of 5 μg/L.
The edible portion only (yolk and albumen) of each egg was homogenized as an individual sample in the laboratory. Eggshells were not analyzed. Egg, feed and supplement samples were digested in a temperature-programmed CEM Mars Express Microwave using a reagent mix of nitric acid and hydrogen peroxide in a closed vessel and analyzed for lead (Pb-208) by ICP-MS (Agilent 7500 CX). Lutetium-175 was used as an internal standard though on-line addition using a connector block. Lead concentrations were calculated using a calibration curve consisting of four standards prepared from a custom AccuTrace™ reference standard solution. All sample sets contained a method reagent blank and spiked matrix. The spike recovery range for all sample sets ranged from 92–110% with an average of 98%. The detection limit of 10 μg/kg fresh weight was based on the method reagent blank.
2.3 Data Analysis and Modeling
Statistical analysis was conducted with SAS 9.3 software (SAS Institute, Inc, Cary, NC, USA) to evaluate relationships between lead concentrations in eggs and a number of independent variables. Because mean and median egg lead concentrations in several henhouses were below the limit of detection, we chose other dependent variables which varied substantially across henhouses to model lead in eggs. Specifically, we considered the fraction of each henhouse’s eggs in which lead was detected (FPbEgg) and the maximum concentration of lead measured in eggs from each henhouse (PbEggMax) as measures of lead in eggs for data analysis.
Several variables were selected or developed to assess the influence of environmental or management factors on our measures of lead in eggs. The lead concentration in chicken-run soil (PbSoilRun) and the maximum lead concentration in chicken-area soil (PbSoilMax) (including samples from the run and other areas accessed by chickens) were selected as two measures of lead in soil from each henhouse. In addition, an “exposure-weighted” soil lead concentration (PbSoilExp) was developed as a variable for each henhouse, based on lead concentrations measured in soil samples from the chicken run and other chicken areas and the fractions of time chickens were reported to spend in those areas. Based on data availability across all ten henhouses, biological plausibility, and previous reports of potentially influential factors, the following variables were also considered: lead concentration in layer feed, number of chickens, run area, run area per chicken, fraction of run area with bare soil, bare soil area per chicken, eggs per laying hen per day, and average chicken residence time in the henhouse. Chicken residence time was selected as a surrogate for chicken age, which has been suggested as influential (Schoeters and Hoogenboom 2006), because there was uncertainty among chicken keepers as to the actual age of some chickens in their henhouses. Three additional factors were considered as dichotomous independent variables: whether feed was scattered on the ground, whether separate calcium supplements were provided, and whether chickens had limited (< 24 h/day) access to the outdoors.
Because the measures of lead in soil and eggs were not normally distributed (Kolmogorov-Smirnoff test), non-parametric tests were used for bivariate analysis to evaluate influence of individual independent variables on both measures of lead in eggs. Spearman rank correlations were used to evaluate associations between FPbEgg and PbEggMax and continuous and ordinal independent variables. Mann-Whitney tests were used to evaluate differences in FPbEgg and PbEggMax with respect to dichotomous independent variables. Because PbSoilExp was theoretically the best estimate of the soil concentration that the chickens were exposed to over time, and because correlations between the other two measures of soil lead contamination and FPbEgg and PbEggMax were no stronger, PbSoilExp was chosen over PbSoilRun and PbSoilMax as the measure of soil lead contamination for multivariable modeling.
After log-transformation, PbSoilExp and PbEggMax were normally distributed, and these transformed data were used for multivariable analysis to simultaneously consider the influence of multiple independent variables on the dependent variables FPbEgg and PbEggMax. The remaining independent variables were also considered for multivariable analysis, even though none of them was determined to be significant in the bivariate analysis.
Two different multivariable regression approaches were used. A model to predict FPbEgg was created using SAS’s GLIMMIX procedure to fit a generalized linear model to the data incorporating a probit link function. Because PbEggMax was left-censored with values for three of the ten henhouses below the detection limit, a Tobit model using the QLIM procedure was fit to log-transformed PbEggMax with predicted values based on the mean functions of the latent and observed variables. Potentially influential variables were introduced into both models and were retained if they predicted the dependent variables with a significance level of p < 0.1. Results of the bivariate and multivariable analysis were considered significant at p < 0.05, and marginally significant at p < 0.1.
We assessed the possible health implications of consuming eggs with elevated lead concentrations in several ways. We compared the lead concentrations we measured in eggs with standards and guidance values for lead in food from the US and the European Union (EC 2006; US FDA 2006). We also estimated the increases in lead intake that would result from daily consumption of eggs with lead concentrations in the range of those we measured. We developed estimates based on two egg consumption rates: the mean egg consumption rates listed in Table 11–7 of the US EPA Child-Specific Exposure Factors Handbook (US EPA 2008), which vary by age group, and a more conservative estimate of one egg per day for children of all ages. We compared those intake estimates with guidance values for dietary lead intake. We then used US EPA’s Integrated Exposure-Uptake-Biokinetic (IEUBK) model (Windows 32-bit version, IEUBKwin v1.1 build 11) to calculate increases in the estimated geometric mean (GM) blood lead concentration of children of various age groups by adding the intake estimates described above to the IEUBK model’s default dietary lead intake values. With the exception of the added lead from egg consumption, all input parameters in the IEUBK model were set to their default values. The default values incorporated lead exposure related to soil, dust, air, water, and dietary sources.
2.4 Outreach
We reported the results of ICP-AES chicken-run soil analysis to chicken keepers from the gardens participating in both phases of the study, along with analytical results for eggs, water, chicken feed and supplements to those participating in the second phase of the study. We also provided guidance to help chicken keepers interpret the results, comparing lead concentrations in their samples with health-based standards and guidance values and offering advice to help reduce exposure to lead in soil and in eggs.
Urban gardeners are often urged to reduce their exposure to contaminants in soil by using raised beds filled with clean soil and implementing other “best practices.” However, advice that focuses on raising and consuming vegetables may not be sufficient to address other urban-agriculture-related exposure pathways, such as those related to keeping chickens and eating chicken eggs. To address this concern, we developed recommendations to help the NYC chicken keepers in this study reduce their exposure to lead in chicken eggs. We considered recommendations found in the literature (California DHS 2004; Kijlstra et al. 2007; Waegeneers et al. 2009a), and we also developed new recommendations based on the findings of this study. We provided these recommendations, along with other, more general recommendations for reducing exposure to contaminants in urban garden soils, in letters transmitting the sample results and interpretations to the participating chicken keepers.
3 Results and Discussion
3.1 Characteristics of henhouses/chicken-keeping practices
With the exception of the municipally owned farm, where henhouses and chicken runs were surrounded by several acres of farmland, the NYC gardens in this study were located on vacant lots in mixed-use residential/commercial neighborhoods. The gardens were typically bounded by city streets and/or multi-story (usually masonry) buildings.
Table 1 summarizes information we collected about chicken flocks, living conditions and diet. Chicken keepers reported that their chickens had been living at their henhouses for at least five months, and some as long as three years, at the time of egg sampling. Flock size ranged from five to 147 chickens, with most henhouses having fewer than 40 chickens. Most chicken keepers reported obtaining one egg per chicken approximately every one to two days. Araucana, Rhode Island Red, and Leghorn were the most commonly reported chicken breeds. Other breeds included Ameraucana, Australorp, Bantam Cochin, Silky Bantam, Barred Rock, Buff Orpington, Fayomi, Polish, Red Star, Speckled Sussex, and Wyandotte.
Table 1.
Summary statistics for henhouse characteristics and management practices and analytical results for 9 NYC henhouses and 1 rural henhouse
| NYC Henhouses (n=9 except where noted) | Rural Henhouse | ||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics and Practices | Minimum | Median | Mean | Maximum | |
| Number of Chickens | 5 | 20 | 45 | 147 | 7 |
| Number of Laying Hens | 3 | 20 | 43 | 147 | 2 |
| Number of Chicken Breeds | 1 | 4 | 4 | 6 | 2 |
| Average Chicken Residence Time in Garden (months) | 5 | 12 | 17 | 36 | 36 |
| Average Number of Eggs per Laying Hen per Day | 0.1 | 0.7 | 0.5 | 0.9 | 0.4 |
| Run Area (m2) | 3 | 9 | 115 | 692 | 49 |
| Run Area per Chicken (m2) | 0.2 | 0.6 | 1.2 | 5.6 | 6.9 |
| Run Cover Material - Fraction of Run Covered by | |||||
| Bare Soil | 0% | 90% | 59% | 100% | 100% |
| Grass | 0% | 0% | 22% | 100% | 0% |
| Mulch (e.g., bark, straw, coffee chaff) | 0% | 5% | 19% | 85% | 0% |
| Fraction of time chickens have access to areas outside of henhouse and enclosed run | 0% | 1% | 11% | 50% | 0% |
| Diet - Fraction from | |||||
| Commercial Layer Feed | 20% | 75% | 71% | 90% | 90% |
| Food Scraps | 5% | 15% | 24% | 80% | 0% |
| Other (e.g. corn, grain, seed) | 0% | 1% | 4% | 15% | 10% |
|
| |||||
| Analytical Results
| |||||
| Number of Eggs Analyzed | 3 | 6 | 6 | 10 | 6 |
| Fraction of Eggs with Lead Detected (FPbEgg) a | 0% | 33% | 44% | 100% | 0% |
| Number of Chicken-Area Soil Samples Analyzed | 1 | 2 | 2 | 3 | 1 |
| Lead Concentration in | |||||
| Eggs (μg/kg) | |||||
| Minimum Egg from Henhouse | < 10 | < 10 | < 10 | 18 | < 10 |
| Median Egg from Henhouse | < 10 | < 10 | 13 | 40 | < 10 |
| Maximum Egg from Henhouse (PbEggMax) | < 10 | 26 | 45 | 167 | < 10 |
| Soil (mg/kg) | |||||
| Chicken Run (PbSoilRun) | 20 | 71 | 128 | 351 | 15 |
| Maximum for all Chicken Areas (PbSoilMax) | 51 | 94 | 220 | 631 | 15 |
| Exposure-Weighted (PbSoilExp) | 21 | 71 | 167 | 558 | 15 |
| Water (μg/L) b | < 5 | < 5 | < 5 | < 5 | < 5 |
| Layer Feed (μg/kg) | 111 | 128 | 156 | 272 | 92 |
| Kitchen/Garden Scraps (μg/kg) (n = 2) c,d | 224 | 1020 | 925 | 1530 | - |
| Other Feed (corn, grain, seed) (μg/kg) (n = 3)c | < 10 | 12 | 32 | 80 | < 10 |
| Calcium Supplements (μg/kg) (n = 4) c | 213 | 237 | 335 | 556 | - |
Detection limit for lead in eggs was 10 μg/kg. (For comparison, the US FDA detection limit for eggs is 7 μg/kg [26, 27])
Detection limit for lead in water is 5 ug/L. (For comparison, US EPA’s drinking water action level is 15 μg/L).
Henhouses with no analytical results for these feed/supplement materials were not considered in calculating summary statistics.
Kitchen/garden scraps: Total of three samples from two henhouses; one henhouse provided two samples.
Other feed: Total of three samples from three henhouses; one sample represented two henhouses, and one henhouse provided two samples.
Calcium supplements: Total of three samples from four henhouses; one sample represented two henhouses.
Kitchen scraps included acorn squash and cabbage; garden scraps included garden-grown callaloo (a leafy vegetable) and other vegetable scraps.
The enclosed outdoor chicken runs in the NYC gardens were large enough for the chickens to roam freely, although they were relatively small in comparison to the rural chicken run. The enclosed NYC chicken runs provided 0.2 to 5.6 m2 of space per chicken, with most providing less than 1 m2 per chicken, and the rural chicken run provided 6.9 m2 per chicken. Chickens from six of the nine NYC henhouses were allowed to forage outside their enclosed runs up to 50% of the time, but the median fraction of time spent outside the henhouse and run was only 1%. The ground cover was primarily bare soil in most runs. Several runs were partially covered with mulch (e.g., straw, hay, or coffee chaff), and two were entirely grass-covered.
All chicken keepers reported feeding their chickens commercially produced layer feed, which made up 20% to 90% of chickens’ diets. Kitchen and garden scraps made up five to 30 percent of the NYC chickens’ diets. Five of the nine NYC henhouses reported scattering feed material on the ground.
3.2 Analytical results
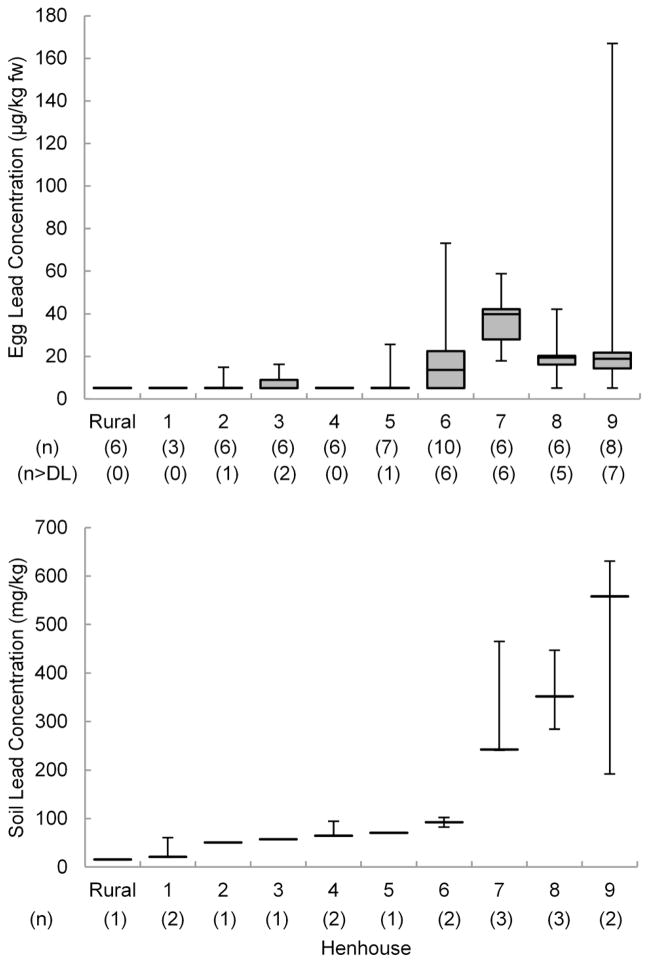
The analytical results by henhouse for lead in eggs, soil, water, food and supplements are summarized in Table 1 (more detailed results are in Online Resource 1, Supplementary Table S1). Lead was detected at concentrations exceeding the 10 μg/kg detection limit in 28 of 58 eggs (48%) collected from the nine NYC henhouses. The highest lead concentration in an egg was 167 μg/kg; the second highest was 73 μg/kg. Lead concentrations in the six eggs from the rural henhouse were below the 10 μg/kg detection limit, as were lead concentrations in the six store-bought eggs. The fraction of eggs from a henhouse with detected concentrations of lead (FPbEgg) varied from none to 100%, and the median was 33%.
Lead concentrations in NYC chicken-run soil samples ranged from 20 to 351 mg/kg. Five gardens provided additional soil samples from areas outside the enclosed run where chickens were reported to forage, which were used in addition to the run samples to calculate exposure-weighted soil lead concentrations (PbSoilExp). Values of PbSoilExp in NYC gardens ranged from 21 to 558 mg/kg. The rural chicken run had a lead concentration of 15 mg/kg in the one soil sample analyzed, slightly below the median NYS rural soil background lead concentration of 23 mg/kg (NYSDEC 2006). The maxima for all chicken areas (run and other foraging areas) (PbSoilMax) for each of the ten henhouses ranged up to 631 mg/kg. The results for chicken run soil samples presented here (measured by XRF analysis) correlated well (r2 = 0.98) with the certified results of ICP-AES analysis for those samples (see Online Resource 1, Supplementary Data Table S2), although the XRF results were biased somewhat low at the highest lead concentrations. The ICP-AES soil results, along with results for lead in the other media, were provided to and interpreted for chicken keepers.
Lead was not detected in any of the chickens’ water samples above the laboratory minimum reporting level of 5 μg/L, well below US EPA’s 15 μg/L drinking water action level. Lead concentrations in commercial layer feed ranged up to 272 μg/kg, while concentrations in three samples of kitchen/garden scraps ranged up to 1,530 μg/kg. Calcium supplements had lead concentrations up to 556 μg/kg, while the one sample of grit we tested had a lead concentration of 1,770 μg/kg, higher than any other feed or supplement samples, but still nearly 10 times lower than the lowest lead concentration in soil (15 mg/kg).
Table 2 compares lead concentrations in eggs from this study (for NYC garden, rural and store-bought eggs) to those reported by others. Our rural and store-bought egg results were consistent with studies of store-bought eggs such as the US FDA Total Diet Study (TDS) (US FDA 2007, 2010). That study tested 64 boiled eggs from US markets between 1991 and 2008 and found lead above the 7 μg/kg detection limit in only two eggs, at 11 and 13 μg/kg. The US FDA TDS considered foods prepared for consumption and therefore did not include raw eggs; however, studies suggest that boiling eggs would not significantly affect metals concentrations. It has been shown, for instance, that cooking had no statistically significant effect on lead concentrations in a variety of foods (Perello et al. 2008) or on concentrations of zinc in eggs (Plaimast et al. 2009). Total Diet Studies from Canada, France, Denmark and the UK have also found low or non-detectable concentrations of lead in prepared store-bought eggs and egg products (including some raw eggs in the French and Danish studies), reporting mean lead concentrations ranging up to 11 μg/kg (Food Standards Agency 2004; Larsen et al. 2002; Leblanc et al. 2005; Ysart et al. 2000). The comparisons in Table 2 show that eggs from urban chickens like those from NYC can have lead concentrations that are higher than store-bought eggs, and possibly also rural eggs, though the number of rural eggs included in this study was small and they were from only a single location.
Table 2.
Summary of lead concentrations in edible portion (yolk and albumen) of eggs in this study and other studies (μg/kg fresh weight)
| Study | 25th | 75th | ||||
|---|---|---|---|---|---|---|
| Minimum | Percentile | Median | Mean | Percentile | Maximum | |
| This study | ||||||
| NYC henhouses (n = 58) | < 10 | < 10 | < 10 | 17a | 20 | 167 |
| Rural (n = 6) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Store-bought (n = 6) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| US FDA Total Diet Study (1991 – 2008) [26,27] | ||||||
| Market basket (boiled) (n = 64) | < 7 | < 7 | < 7 | < 7 | < 7 | 13 |
| Trampel et al. (2003) [17]b | ||||||
| Eggs from hens observed eating lead paint (n = 15) | < 20 | 53 | 75 | 80 | 101 | 163 |
| Eggs from control chickens (n = 9) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Van Overmeire et al. (2006) [34]c | ||||||
| Privately owned chickens in Belgium (n = 22) | 19 | 34 | 49 | 69 | 91 | 240 |
| Commercial farm chickens in Belgium (n = 19) | 1 | 2 | 8 | 9 | 13 | 32 |
| Waegeneers et al. (2009) [16]c | ||||||
| Privately owned chickens in Belgium (fall 2006, n = 40) | 3 | 23 | 80 | 116 | 155 | 471 |
| Privately owned chickens in Belgium (spring 2007, n=58) | < 2 | 15 | 30 | 74 | 105 | 477 |
Concentrations below the detection limit were substituted with one-half the detection limit in calculating mean for eggs from NYC henhouses in this study.
Concentrations have been converted to whole-egg concentrations assuming the yolk is 40% of the edible mass of an egg. Trampel et al. [17] report lead results as μg/kg fresh weight in yolk only. Lead in albumen samples was reported as < 5 μg/kg.
Each egg sample was a composite of 10 – 15 eggs from a single henhouse. Note that Waegeneers et al. [16] are the only others to consider soil lead concentration data.
Overall, lead concentrations in the NYC garden eggs were lower than those reported by other studies of lead-exposed chickens (Trampel et al. 2003; Van Overmeire et al. 2006; Waegeneers et al. 2009b). Median lead concentrations in the edible portions of eggs in those studies ranged from 30 to 80 μg/kg, whereas the median in the NYC eggs in this study was less than 10 μg/kg (Table 2). The maximum concentration in NYC eggs was similar to that found by Trampel et al. (2003) in eggs from hens in Iowa that had eaten chips of lead-based paint, and much lower than that observed by Waegeneers et al. (2009b) in their study of privately owned chickens in Belgium.
3.3 Potentially influential factors and egg lead concentrations
Several studies have reported correlations between concentrations of contaminants, primarily chlorinated organic contaminants, in eggs and soil; it has also been suggested that other factors, such as chicken age (Schoeters and Hoogenboom 2006), chicken run area (Waegeneers et al. 2009a), flock size and time spent outdoors (Kijlstra et al. 2007), may influence contaminant concentrations in free-range chicken eggs. We evaluated associations between these and other potentially influential factors in our study and two measures of lead in eggs: the fraction of eggs with lead detected (FPbEgg) and maximum concentration of lead measured (PbEggMax) in eggs from each henhouse (Online Resource 1, Supplementary Data Tables S3 and S4). Correlations between all three measures of soil lead (run concentration PbSoilRun, maximum concentration PbSoilMax, and exposure-weighted concentration PbSoilExp) and both measures of lead in eggs were significant. Of the three soil lead variables, we considered PbSoilExp to be the most representative of concentrations to which chickens would be exposed. Correlations involving PbSoilExp were among the strongest (rs = 0.83, p = 0.003 for the correlation with FPbEgg and rs = 0.88, p = 0.001 for the correlation with PbEggMax). None of the other variables we considered had a significant association with either FPbEgg or PbEggMax.
Relationships between PbSoilExp and both FPbEgg and PbEggMax are apparent in Figure 1, which compares lead concentrations in soil and in eggs by henhouse. The six henhouses with the lowest lead concentrations in soil had median lead concentrations in eggs below the 10 μg/kg detection limit. The other four henhouses, with higher lead concentrations in soil, had median egg-lead concentrations above the detection limit. The maximum lead concentrations in eggs were also higher at these four henhouses than at the others.
Fig. 1.
25th, 50th and 75th percentiles and ranges of lead concentration in eggs compared to exposure-weighted lead concentrations (PbSoilExp) and ranges of lead concentration in soil by henhouse. Detection limit for lead in eggs is 10 μg/kg. Numbers in parentheses are numbers of egg and soil samples analyzed from a henhouse and number of eggs with lead concentrations greater than the 10 μg/kg detection limit. Eggs with concentrations less than the detection limit were considered equal to one-half the detection limit (5 μg/kg) in calculating summary statistics for this figure.
The multivariable models we developed to predict FPbEgg and log-transformed PbEggMax included egg and soil data from all 10 henhouses. To evaluate the influence of the one henhouse from which only three eggs were analyzed, we excluded it and repeated the analyses with 9 henhouses, with essentially the same results. Log-transformed PbSoilExp was a significant predictor of both measures of lead in eggs.
We considered the other potentially influential factors described in Section 2.3 in our multivariable analysis as well. Of those factors, only one (SCa, representing the availability of dietary calcium supplements in a henhouse, such that SCa = 1 for henhouses that provided calcium supplements and SCa = 0 for those that did not) was a marginally significant predictor (p < 0.1), and was retained in both models. The effect of the SCa term on the models was of interest because it represented a physically plausible scenario, as increased calcium intake has been associated with reduced accumulation of lead in chickens (Bakalli et al. 1995), as well as humans (Mahaffey et al. 1986) and other mammals (Quarterman et al. 1978). However, because SCa was only marginally significant as a predictor, we also developed versions of the models in which log-transformed PbSoilExp was retained as the sole predictor of FPbEgg and log-transformed PbEggMax.
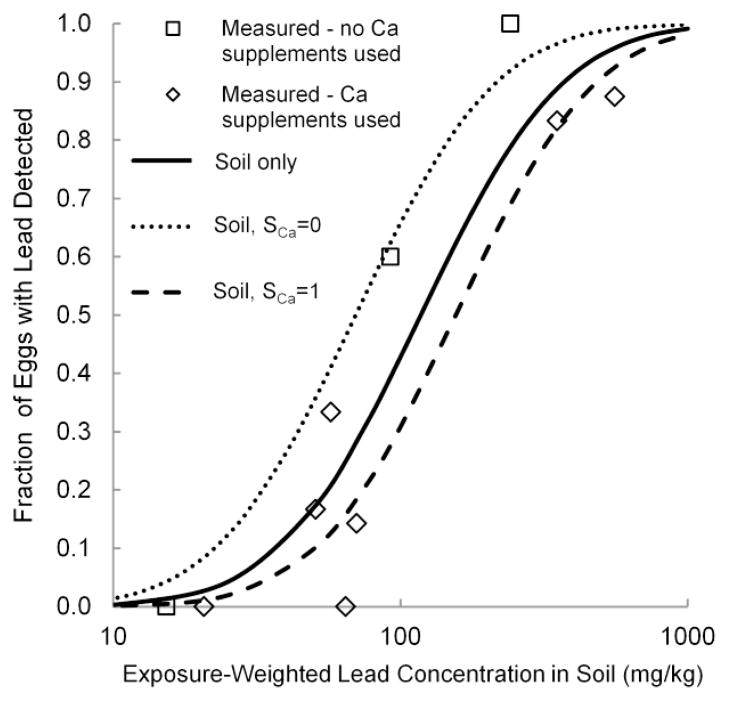
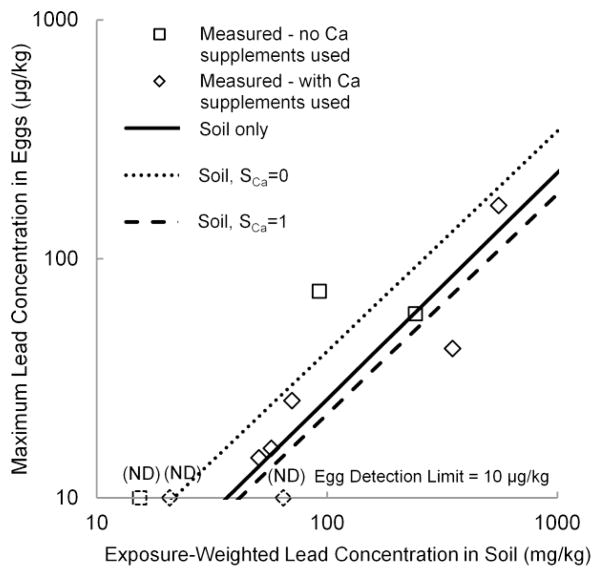
Both versions of the models are illustrated in Figures 2 and 3. The symbols represent the actual values of FPbEgg and PbEggMax at each of the 10 henhouses in this study, the solid lines represent the models considering only log-transformed PbSoilExp as a predictor, and the dashed lines represent the models incorporating the SCa term. The models suggested that the availability of calcium supplements reduced both the likelihood of detecting lead and the maximum concentration of lead that would be expected in eggs from a henhouse with a given concentration of lead in soil.
Fig. 2.
Measured and predicted fractions of eggs with lead detected at each henhouse (FPbEgg), considering lead concentrations in soil alone (solid line) and lead concentrations in soil and use of calcium supplements (SCa) (dashed lines). Regression coefficients are presented in Online Resource 1 (Supplementary Table S5).
Fig. 3.
Measured and predicted maximum lead concentration in eggs at each henhouse (PbEggMax), considering lead concentrations in soil alone (solid line) and lead concentrations in soil and use of calcium supplements (SCa) (dashed lines) (ND = not detected). Regression coefficients are presented in Online Resource 1 (Supplementary Table S5).
Considering soil alone as a predictor, the models indicated that at least one in six eggs would have a detectable (> 10 μg/kg) level of lead (above the 96th percentile of store-bought eggs in the US FDA TDS (US FDA 2007 above the 96th percentile of store-bought eggs in the US FDA TDS (US FDA 2010)) at soil lead concentrations above 50 mg/kg. The maximum lead concentration in eggs would exceed 100 μg/kg at a soil lead concentration of 410 mg/kg. Extrapolating to a soil lead concentration of 1,000 mg/kg – higher than we found in this study, but within the range of concentrations that we have found in NYC gardens in which chickens are kept (unpublished results) – the models suggested that 99% of eggs would have lead detected, with a maximum concentration of 230 μg/kg.
With both lead in soil and calcium supplement availability as predictors, the models predicted a maximum egg lead concentration of 100 μg/kg or greater at soil lead concentrations above 500 mg/kg in gardens where calcium is provided, and at soil lead concentrations above 260 mg/kg in gardens not providing calcium.
While we found a strong correlation between lead concentrations in eggs and in soil, the concentrations we found in eggs were generally lower than those found in a study of Belgian chicken eggs (Waegeneers et al. 2009b). The Belgian study found lead concentrations in eggs as high as 477 μg/kg, while reporting lead concentrations in soil of no more than 174 mg/kg.
It is not apparent why concentrations we found in eggs were lower than those found in the Belgian study. Differences in laboratory analytical methods could contribute some uncertainty. However, laboratory analytical methods were reasonably similar between the studies: both studies used nitric acid digestion of homogenized yolk and albumen samples followed by ICP-MS analysis for eggs, and soil analyses were comparable as well (acid extraction followed by ICP-MS analysis in the Belgian study and XRF in our study, which correlated well with results from acid extraction/ICP-AES analysis of chicken run soil samples). The differences in egg lead concentrations between the two studies were not likely due to differences in calcium supplement use or soil sampling depth, both of which were similar between the two studies (N. Waegeneers, personal communication, Oct. 1, 2012). Compared to the Belgian study, bare soil was more pervasive and there was less area per hen in our study, both conditions that have been reported to be associated with higher contaminant concentrations in eggs. One possible explanation for the difference in egg lead concentrations between Belgium and NYC could be a difference in the bioavailability of lead in soil. Studies (Smith et al. 2011, Zia et al. 2011) have shown that the bioavailability of lead in soil depends on factors such as soil type, pH, the source of the lead contamination and the form in which lead is found. Waegeneers et al. (2009b) report that atmospheric deposition from “non-ferrous industries” is the primary source of elevated levels of trace elements (including lead) in Belgian soils. By contrast, lead in urban soils such as those in NYC may come from a variety of sources, including historical auto emissions (leaded gasoline), building renovation and demolition, weathering and corrosion of building materials (including lead-based paint) and waste incineration (US EPA 1998). This difference in the sources of lead may have resulted in differences in bioavailability of soil lead between Belgium and NYC, which in turn could influence the transfer of lead from soil to chicken eggs.
3.4 Health implications
Because the US does not have health-based standards for lead in eggs, standards and guidance values for lead in other foods from the US and other countries were used to evaluate our study results. These included a US FDA guidance value for lead of 100 μg/kg in candy “likely to be consumed by young children” (US FDA 2006) and European Union standards ranging from 100 to 300 μg/kg in foods such as meat, poultry, and vegetables (EC 2006). The EU currently does not regulate lead concentrations in eggs specifically; however, according to Waegeneers et al. (2009b), a former Maximum Permissible Concentration of 100 μg/kg for lead in eggs in Belgium is used in that country as an action level for commercially produced eggs. All but one of the eggs in our study had less than 100 μg/kg lead, suggesting that, in general, they contained lead at concentrations that were not higher than those in foods considered acceptable for commercial distribution.
Because lead was detected more frequently and at higher concentrations in NYC garden eggs than in store-bought eggs, eating eggs with lead concentrations in the range found in this study would lead to an increase in dietary lead exposure compared to eating store-bought eggs. We estimated the amount of this increase for 1- to 6-year-old children consuming eggs with four different lead concentrations, assuming that dietary lead exposure from store-bought eggs was negligible. The four lead concentrations we considered were 20 μg/kg (slightly above the mean for the NYC garden eggs in our study), 100 μg/kg (the US FDA guideline for candy and the low end of the EU food standards we considered), 167 μg/kg (the maximum concentration in eggs in our study), and 300 μg/kg (the high end of the range of the EU food standards). Our model suggests egg lead concentrations in the range of this highest concentration, while higher than those measured in our study, could be found in eggs from henhouses with soil lead concentrations on the order of 1,000 mg/kg.
Our estimates of increases in dietary intake relied on conservative exposure assumptions. For each of the four egg-lead concentrations described above, we assumed that all eggs consumed by a child contained lead at that concentration, even though three of those four concentrations are higher than the concentrations we found in the majority of the NYC garden eggs we tested. We further assumed a child would eat eggs with that lead concentration every day, all year. It is likely that only a fraction of egg intake would come from garden-raised hens (note that Table 13–6 of the US EPA Child-Specific Exposure Factors Handbook (CSEFH) (US EPA 2008) indicates home-produced eggs make up 14.6% of the egg consumption of the average child whose family raises animals, and 21.4% of the egg consumption of a child whose family farms).
We estimated the increase in lead exposure at two representative consumption rates: the mean egg consumption rates reported in Table 11–7 of the CSEFH (US EPA 2008), which vary from 11 – 18 g/day for children between 1 and 7 years of age, and a higher rate of 45 g egg per day – the equivalent of one medium egg every day – a rate 150% to 310% higher than the average. While we are unaware of any data for chicken-egg consumption rates for US chicken keepers and their households, it is possible that they would eat eggs at higher rates than the general population, as has been shown in other locations (for example, Wageneers et al (2009a) reported that Belgian residents who kept chickens consumed eggs at a rate about 60% greater than the general population).
We compared the estimated increases in dietary lead exposure (Table 3) to the Provisional Total Tolerable Intake Level (PTTIL) of 6 μg/day established by the US FDA in 1992 for children six years of age or younger (Carrington and Bolger 1992). At average rates of egg consumption, eggs with lead concentrations like those we found in NYC garden eggs (up to 167 μg/kg) would contribute less than half the PTTIL to a child’s dietary lead intake. Eating one egg a day with a lead concentration of less than 100 μg/kg – which would include all but one of the NYC garden eggs in our study – would increase a child’s dietary lead intake by up to 4.5 μg/day, or 75% of the PTTIL. In more conservative exposure scenarios, eating one egg a day at the highest lead concentration we measured (167 μg/kg) would contribute 7.5 μg of lead to a child’s daily dietary intake, exceeding the PTTIL, and eating one egg with 300 μg/kg lead daily would contribute more than twice the PTTIL to a child’s intake.
Table 3.
IEUBK model-predicted effects of lead in eggs on blood lead levels of children 1 – 6 years of age
| Consumption Rate | Lead Concentration in Eggs (μg/kg) | Increase in Dietary Lead Intake (μg/day)a | Predicted Increase in GM Blood Lead Level (μg/dL)b |
|---|---|---|---|
| Typical | 20 | 0.2 – 0.4 | 0.0 – 0.1 |
| 11 – 18 g egg | 100 | 1.1 – 1.8 | 0.2 – 0.3 |
| per day* | 167 | 1.8 – 3.0 | 0.3 – 0.5 |
| 300 | 3.3 – 5.4 | 0.5 – 0.8 | |
| Frequent | 20 | 0.9 | 0.1 – 0.2 |
| 45 g egg | 100 | 4.5 | 0.6 – 0.8 |
| per day | 167 | 7.5 | 1.0 – 1.4 |
| 300 | 13.5 | 1.8 – 2.4 |
Age-specific consumption rates for children 1 – 6 years of age [23]
Age-specific model predictions for children 1 – 6 years of age based on age-specific model assumptions.
We note that the US FDA derived the PTTIL for infants and children in 1992 using a simple linear model to predict blood lead levels in children that would result from lead exposure. In developing the PTTIL, US FDA considered a lowest observable effects level (LOEL) of 10 μg/dL (which was also CDC’s “level of concern” from 1991–2012), and assumed that a 6 μg/day increase in lead ingestion would translate to a 0.96 μg/dL increase in blood lead level (Carrington and Bolger 1992). More recently, a number of studies have found that blood lead levels below 10 μg/dL are associated with decreased IQ, test scores or cognitive skills (Miranda et al. 2007), with some studies reporting neurobehavioral effects at blood lead levels as low as 2 μg/dL (Jusko et al. 2008). In 2012, CDC announced that it would discontinue use of the term “level of concern” in describing blood lead levels of 10 μg/dL and established a “reference level” of 5 μg/dL to identify children that have blood lead levels much higher than most children, based on the 97.5th percentile blood lead level in US children 1–5 years of age (CDC, 2012b). Given the current understanding of the significance of blood lead levels below 10 μg/dL, the PTTIL may now be considered less conservative than its authors intended.
As another way of assessing the possible effects of consuming eggs with concentrations of lead similar to those we found in NYC garden eggs, we used US EPA’s most recent version of the IEUBK model to predict increases in estimated GM blood-lead concentrations in children based on the conservatively derived estimated increases in lead intake described above and in Table 3. We compared the predicted increases in blood-lead concentrations to a guideline of 1 μg/dL, which is approximately the increase predicted by US FDA’s simple linear model to be associated with lead intake at the PTTIL of 6 μg/day. An incremental increase of 1 μg/dL in a child’s blood lead, with may be associated with a reduction of IQ by up to 1 point, is also equal to a child-specific benchmark established by the California Environmental Protection Agency for use in health risk assessments at school sites in California (CalEPA 2007).
Our IEUBK model predictions suggested that consuming one egg per day with a lead concentration less than 100 μg/kg, in addition to the model’s default lead exposure from diet and all other sources, would result in estimated GM blood-lead concentration increases of less than 1 μg/dL in children. However, daily consumption of one egg with the highest lead concentration we found in eggs from NYC community gardens (167 μg/kg) would increase GM blood-lead concentrations by 1 to 1.4 μg/dL, and daily consumption of one egg with 300 μg/kg lead would increase GM blood-lead concentrations by as much as 2.4 μg/dL, well above the 1 μg/dL guideline.
The IEUBK model output was also used to estimate the number of eggs children could consume without excessively increasing blood-lead concentrations. Among children 1 – 6 years of age, the model output suggested that an increase in dietary lead intake of up to 5.6 μg/day for 1 to 2 year olds (and slightly larger increases – as much as 7.6 μg/day – for older children), would raise GM blood-lead concentrations by less than 1 μg/dL. This intake translates into the consumption of about 6 medium eggs/day at 20 μg/kg lead, 2.5 eggs/day at 50 μg/kg, or 1.2 eggs/day at 100 μg/kg.
These evaluations implied that, overall, the lead concentrations we found in eggs from NYC community gardens were not likely to significantly increase lead exposure or to pose a significant health risk. However, frequent consumption of eggs with the highest lead concentration we found could significantly increase lead exposure, and chickens exposed to higher concentrations of lead in soil are likely to produce eggs with higher concentrations of lead. This exposure pathway could potentially be significant in some gardens, and it should not be ignored.
3.5 Study Limitations
This study had several limitations that should be considered. The number of henhouses that participated in the study and the associated ranges of values for independent variables were limited, and we collected a fairly small number of eggs from each henhouse, limiting our statistical power. Although our detection limit (10 μg/kg) was similar to the detection limit of the US FDA TDS (US FDA 2007, 2010) and other studies (Waegeneers et al. 2009b), lead concentrations in many of our eggs were below the limit of detection, precluding us from modeling henhouse egg lead central tendency concentrations and increasing the uncertainty in our model of maximum henhouse egg lead levels. There was some variation in the number of eggs analyzed from each henhouse, with one henhouse having as few as three eggs analyzed. This may have had some influence over the henhouse fraction of eggs with detectable lead and maximum lead concentration. However, we found that exclusion of the henhouse with only three eggs did not change the results of our regression analyses.
We sampled the top 10 cm of soil, assuming the soils in the areas we sampled were well-mixed, which is often the case in urban community gardens. We saw strong correlations between lead concentrations in eggs and soil samples. However, another soil sampling depth interval may have been even more representative of the soil to which chickens are likely exposed. Had we chosen a different sampling interval, we might have found a stronger or weaker association between soil and egg lead concentrations.
It is also worth noting that the range of lead concentrations in soil was limited. The highest concentration used in our modeling was 558 mg/kg, but considerably higher lead concentrations have been found in some NYC community garden soils, and the applicability of our models at higher lead concentrations is uncertain. Another limitation was the lack of information about actual egg consumption rates among chicken-keepers and their communities, which led us to rely on assumptions and information from the literature regarding egg consumption rates in assessing the potential health implications of our results.
3.6 Recommendations to Reduce Exposure
Although the lead concentrations we found in most eggs from NYC community gardens would not be expected to significantly increase lead exposure, chickens exposed to greater lead concentrations in soil could produce eggs with higher lead concentrations that could significantly increase exposure for those who consume the eggs. Even at the lead concentrations we found in NYC garden eggs, chicken keepers may wish to consider reducing their exposure to lead in eggs. Chicken keepers may consider modifying chicken-keeping practices to reduce the amount of lead that is transferred from soil to eggs.
We reviewed existing literature on measures to reduce contaminant levels in eggs (California DHS 2004; De Vries et al. 2006; Waegeneers et al. 2009a) to help develop recommendations that would be practical and feasible for NYC community gardeners. Some recommendations, such as keeping chickens indoors or in cages above the ground, were incompatible with the preferred practices of the chicken keepers participating in this study. Others, such as the suggestions of Waegeneers et al. (2009a) to provide larger runs (they recommend 10 to 25 m2/chicken) and encourage grass growth in chicken runs, may be appropriate for rural areas like those in the Belgian study, where the average run provided 10 m2/chicken, but are not practical given the space constraints of an urban community garden (most of the NYC runs in our study provided less than 1 m2 per chicken).
Based on our findings and our review of the literature, we developed the following recommendations to help NYC chicken keepers reduce lead concentrations in eggs:
Add clean soil, mulch, or other clean cover material to existing chicken runs to help reduce chickens’ contact with and ingestion of contaminated soil. Use clean soil when constructing new chicken runs. Inspect the clean cover material regularly, and add or maintain material as needed to help keep chickens from coming in contact with underlying soil that may have higher concentrations of lead.
Provide chickens’ regular feed in feeders, and avoid scattering feed, including scratch grains and food scraps, on bare ground in areas where soil has higher concentrations of lead, or where lead concentrations are not well characterized.
Evaluate gardens for potential sources of lead. Do not allow chickens to forage near these sources. For example, keep chickens away from structures painted with lead-based paint and out of areas where the soil has higher concentrations of lead.
Avoid feeding chickens unwashed garden scraps from areas where the soil has higher concentrations of lead.
Consider providing a calcium supplement, which may help to reduce the amount of lead that gets into chickens’ eggs.
Some studies (Trampel et al. 2003) have found much lower lead concentrations in egg whites than in egg yolks, which suggests that serving fewer egg yolks may also be a step chicken keepers can take to reduce exposure to lead in eggs.
We provided these recommendations to chicken keepers, along with lead results and interpretation for their egg and chicken run soil samples. In the letters, we also provided more general advice developed by our HSHC project team to help address other potential exposure pathways to lead and other contaminants in urban garden soils (e.g., wear gloves; wash produce; use raised growing beds; mulch beds to reduce soil splash onto vegetables; cover or mulch walkways to reduce soil tracking and resuspension; install and maintain a cover layer in children’s play areas).
4 Conclusions
To our knowledge this is the first study to present urban chicken egg lead concentrations from the US with an evaluation of potentially influential factors and public health implications. Even with a relatively small number of samples (58 urban eggs), our results clearly demonstrate that the concentration of lead in soil to which chickens are exposed is an important determinant of lead concentrations in eggs (p = 0.003). It also appears that the use of calcium supplements may help reduce transfer of lead from soil to eggs. Lead concentrations in chicken eggs measured in our study tended to be lower than those previously reported for chickens in Belgium (the only other published study relating lead concentrations in soil and eggs), even though soil concentrations in our study were higher. This suggests that the relationship between soil lead and egg lead can vary considerably by geographic region, perhaps as a function of a factor that was not measured, such as lead bioavailability.
Lead was detected more frequently (28 of 58 eggs) and at higher concentrations in eggs from chickens raised in NYC gardens than in store-bought eggs (0 of 6 in our study; 2 of 64 by US FDA (2007, 2010)) and rural eggs (0 of 6) used for reference. Based on comparison to health-based standards and guidance values, and exposure and blood-lead modeling, consumption of eggs with the lead concentrations we found does not appear to pose a significant lead exposure and associated health risk. However, although based on limited data and conservative exposure assumptions, our modeling suggests that at soil lead concentrations higher than those found in this study, which are not uncommon in NYC and other urban centers, consumption of urban chicken eggs may pose a more significant risk. Our models suggest that soil lead concentrations between 260 and 500 mg/kg would be associated with maximum egg lead concentrations at the 100 μg/kg guidance value. Considering soil lead alone as a predictor (without considering calcium supplementation), we estimate that lead concentrations in more than half of eggs from a henhouse would exceed store-bought egg levels (<7 – 13 μg/kg; 3% above detection limit) at soil lead concentrations greater than 120 mg/kg, and the maximum concentration for approximately six eggs would exceed 100 μg/kg at soil lead concentrations above 410 mg/kg. Urban chicken keepers should be made aware that raising chickens in areas with elevated concentrations of lead in soil may increase lead exposure of those consuming the eggs compared with store-bought alternatives. Urban gardeners also should be aware of the potential for exposure to soil contaminants from multiple pathways, including raising chickens for eggs. Based on our findings, some urban chicken keepers should take steps to reduce chickens’ exposure to lead, including limiting chickens’ access to areas with known or suspected soil contamination or importing clean soil.
A larger study is needed to examine lead concentrations in eggs from urban chickens, including those exposed to higher concentrations of lead in soil, to further assess potential public health risks. Further study of lead concentrations in chicken eggs should make use of more sensitive analytical methods to reduce the proportion of eggs with lead concentrations below the detection limit. Also, differences in the bioavailability of lead in soil and their effect on lead concentrations in chicken eggs should be examined if appropriate measures of bioaccessibility could be identified. Finally, effectiveness of exposure mitigation strategies, including calcium supplement use, should be examined.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the assistance of colleagues from the Healthy Soils, Healthy Communities project partner organizations, particularly Stephen Shost and Jan Storm of the New York State Department of Health and Edie Stone of New York City’s GreenThumb program. We are also grateful to Just Food for their contributions to this project, including the time and energy of Gregory Anderson and “chicken interns” Meredith Wells, Marlie Wilson, L. Reed, and Rafael Aponte. Most importantly, we thank the many community gardeners and chicken-keepers who have participated in this project. Funding for this research was provided by the National Institutes of Health/National Institute of Environmental Health Sciences, Award Number R21ES017921 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or the National Institutes of Health.
References
- Alaimo K, Packnett E, Miles RA, Kruger DJ. Fruit and Vegetable Intake among Urban Community Gardeners. Journal of Nutrition Education and Behavior. 2008;40(2):94–101. doi: 10.1016/j.jneb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Lead. Atlanta, GA: Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services; 2007. [Google Scholar]
- Bakalli RI, Pesti GM, Ragland WL. The magnitude of lead toxicity in broiler chickens. Veterinary and human toxicology. 1995;37(1):15–19. [PubMed] [Google Scholar]
- Bartling H. Chicken Ordinance Survey. Chicago, IL: DePaul University; 2010. Retrieved from http://www.cityofbatavia.net/content/articlefiles/7186-Suburban%20Chickens.pdf. [Google Scholar]
- Beam A, Garber L, Sakugawa J, Kopral C. Salmonella awareness and related management practices in U.S. urban backyard chicken flocks. Preventive Veterinary Medicine. 2013 doi: 10.1016/j.prevetmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- CalEPA. Development of Health Criteria for School Site Risk Assessment Pursuant to Health and Safety Code Section 901(g): Child-Specific Benchmark Change in Blood Lead Concentration for School Site Risk Assessment. Integrated Risk Assessment Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency; 2007. Retrieved from http://www.oehha.org/public_info/public/kids/pdf/PbHGV041307.pdf. [Google Scholar]
- California DHS. Backyard chicken eggs in California: reducing risks questions and answers. Environmental Health Investigations Branch, California Department of Health Services; 2004. Retrieved from http://www.ehib.org/papers/caeggs8by11c.pdf. [Google Scholar]
- Carrington CD, Bolger PM. An assessment of the hazards of lead in food. Regulatory toxicology and pharmacology: RTP. 1992;16(3):265–272. doi: 10.1016/0273-2300(92)90006-u. [DOI] [PubMed] [Google Scholar]
- De Vries MD, Kwakkel RP, Kijlstra A. Dioxins in organic eggs: a review. NJAS-Wageningen Journal of Life Sciences. 2006;54(2):207–221. [Google Scholar]
- EC. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L. 2006;364:06–24. [Google Scholar]
- Food Standards Agency. 2000 Total Diet Study of 12 elements – aluminium, arsenic, cadmium, chromium, copper, lead, manganese, mercury, nickel, selenium, tin and zinc (No. FSIS 48/04) UK: Food Standards Agency; 2004. [DOI] [PubMed] [Google Scholar]
- Gittleman M, Librizzi L, Stone E. Community Garden Survey New York City Results 2009/2010. GrowNYC. 2010 Retrieved from http://www.greenthumbnyc.org/pdf/GrowNYC_community_garden_report.pdf.
- Hsu JF, Chen C, Liao PC. Elevated PCDD/F levels and distinctive PCDD/F congener profiles in free range eggs. Journal of Agricultural and Food Chemistry. 2010;58(13):7708–7714. doi: 10.1021/jf100456b. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentration <10 μg/dL and child intelligence at 6 years of age. Environmental Health Perspectives. 2008;116(2):243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A, Traag WA, Hoogenboom LAP. Effect of flock size on dioxin levels in eggs from chickens kept outside. Poultry Science. 2007;86(9):2042–2048. doi: 10.1093/ps/86.9.2042. [DOI] [PubMed] [Google Scholar]
- Larsen EH, Andersen NL, Møller A, Petersen A, Mortensen GK, Petersen J. Monitoring the content and intake of trace elements from food in Denmark. Food Additives and Contaminants. 2002;19(1):33–46. doi: 10.1080/02652030110087447. [DOI] [PubMed] [Google Scholar]
- Leblanc JC, Guérin T, Noël L, Calamassi-Tran G, Volatier JL, Verger P. Dietary exposure estimates of 18 elements from the 1st French Total Diet Study. Food Additives and Contaminants. 2005;22(7):624–641. doi: 10.1080/02652030500135367. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Gartside PS, Glueck CJ. Blood lead levels and dietary calcium intake in 1- to 11-year-old children: The Second National Health and Nutrition Examination Survey, 1976 to 1980. Pediatrics. 1986;78(2):257–262. [PubMed] [Google Scholar]
- Miranda ML, Kim D, Galeano MAO, Paul CJ, Hull AP, Morgan SP. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environmental Health Perspectives. 2007;115(8):1242–1247. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSDEC. New York State Brownfield Cleanup Program Development of Soil Cleanup Objectives Technical Support Document. Appendix D. New York State Department of Environmental Conservation; 2006. Retrieved from http://www.dec.ny.gov/docs/remediation_hudson_pdf/appendixde.pdf. [Google Scholar]
- Perello G, Martí-Cid R, Llobet JM, Domingo JL. Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. Journal of Agricultural and Food Chemistry. 2008;56(23):11262–11269. doi: 10.1021/jf802411q. [DOI] [PubMed] [Google Scholar]
- Plaimast H, Sirichakwal PP, Puwastien P, Judprasong K, Wasantwisut E. In vitro bioaccessibility of intrinsically zinc-enriched egg and effect of cooking. Journal of Food Composition and Analysis. 2009;22(7–8):627–631. doi: 10.1016/j.jfca.2009.04.007. [DOI] [Google Scholar]
- Pollock SL, Stephen C, Skuridina N, Kosatsky T. Raising chickens in city backyards: The public health role. Journal of Community Health. 2012;37(3):734–742. doi: 10.1007/s10900-011-9504-1. [DOI] [PubMed] [Google Scholar]
- Quarterman J, Morrison JN, Humphries WR. The influence of high dietary calcium and phosphate on lead uptake and release. Environmental Research. 1978;17(1):60–67. doi: 10.1016/0013-9351(78)90061-0. [DOI] [PubMed] [Google Scholar]
- Schoeters G, Hoogenboom R. Contamination of free-range chicken eggs with dioxins and dioxin-like polychlorinated biphenyls. Molecular Nutrition and Food Research. 2006;50(10):908–914. doi: 10.1002/mnfr.200500201. [DOI] [PubMed] [Google Scholar]
- Smith E, Kempson IM, Juhasz AL, Weber J, Rofe A, Gancarz D, et al. In vivo-in vitro and XANES spectroscopy assessments of lead bioavailability in contaminated periurban soils. Environmental Science and Technology. 2011;45(14):6145–6152. doi: 10.1021/es200653k. [DOI] [PubMed] [Google Scholar]
- Stephens RD, Petreas MX, Hayward DG. Biotransfer and bioaccumulation of dioxins and furans from soil: Chickens as a model for foraging animals. Science of the Total Environment. 1995;175(3):253–273. doi: 10.1016/0048-9697(95)04925-8. [DOI] [PubMed] [Google Scholar]
- Symes CT, Hughes JC, Mack AL, Marsden SJ. Geophagy in birds of Crater Mountain Wildlife Management Area, Papua New Guinea. Journal of Zoology. 2006;268(1):87–96. doi: 10.1111/j.1469-7998.2005.00002.x. [DOI] [Google Scholar]
- Trampel DW, Imerman PM, Carson TL, Kinker JA, Ensley SM. Lead contamination of chicken eggs and tissues from a small farm flock. Journal of Veterinary Diagnostic Investigation. 2003;15(5):418–422. doi: 10.1177/104063870301500503. [DOI] [PubMed] [Google Scholar]
- US EPA. Final Report Sources of Lead in Soil: A literature Review EPA 747-R-98-001a. Washington, DC: National Center for Environmental Assessment, US Environmental Protection Agency; 1998. [Google Scholar]
- US EPA. Human Health Risk Assessment Protocol for Hazardous Waste Combustion Facilities EPA530-R-05-006. Washington, DC: Office of Solid Waste and Emergency Response, US Environmental Protection Agency; 2005. [Google Scholar]
- US EPA. Child-Specific Exposure Factors Handbook EPA/600/R-06/096F. National Center for Environmental Assessment, US Environmental Protection Agency; Washingon, DC: 2008. [Google Scholar]
- US FDA. Supporting Document for Recommended Maximum Level for Lead in Candy Likely To Be Consumed Frequently by Small Children [Docket No. 2005D-0481] (WebContent) US Food and Drug Administration; 2006. Retrieved from http://www.fda.gov/food/foodsafety/foodcontaminantsadulteration/metals/lead/ucm172050.htm. [Google Scholar]
- US FDA. Total Diet Study Statistics on Element Results Revision 4.1, Market Baskets 1991-3 through 2005-4. College Park, MD: US Food and Drug Administration; 2007. Retrieved from http://www.fda.gov/downloads/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/UCM243059.pdf. [Google Scholar]
- US FDA. Total Diet Study Statistics on Element Results Market Baskets 2006-1 through 2008-4. College Park, MD: US Food and Drug Administration, Center for Food Safety and Applied Nutrition; 2010. Retrieved from http://www.fda.gov/downloads/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/UCM184301.pdf. [Google Scholar]
- USDA & HHS. Dietary Guidelines for Americans 2010. Washington, DC: US Government Printing Office: US Department of Agriculture and US Department of Health and Human Services; 2010. [Google Scholar]
- Van Overmeire I, Pussemier L, Hanot V, De Temmerman L, Hoenig M, Goeyens L. Chemical contamination of free-range eggs from Belgium. Food Additives and Contaminants. 2006;23(11):1109–1122. doi: 10.1080/02652030600699320. [DOI] [PubMed] [Google Scholar]
- Waegeneers N, De Steur H, De Temmerman L, Van Steenwinkel S, Gellynck X, Viaene J. Transfer of soil contaminants to home-produced eggs and preventive measures to reduce contamination. Science of the Total Environment. 2009a;407(15):4438–4446. doi: 10.1016/j.scitotenv.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Waegeneers N, Hoenig M, Goeyens L, De Temmerman L. Trace elements in home-produced eggs in Belgium: Levels and spatiotemporal distribution. Science of the Total Environment. 2009b;407(15):4397–4402. doi: 10.1016/j.scitotenv.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Ysart G, Miller P, Croasdale M, Crews H, Robb P, Baxter M, et al. 1997 UK total diet study - Dietary exposures to aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Additives and Contaminants. 2000;17(9):775–786. doi: 10.1080/026520300415327. [DOI] [PubMed] [Google Scholar]
- Zia MH, Codling EE, Scheckel KG, Chaney RL. In vitro and in vivo approaches for the measurement of oral bioavailability of lead (Pb) in contaminated soils: A review. Environmental Pollution. 2011;159(10):2320–2327. doi: 10.1016/j.envpol.2011.04.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.