Abstract
Purpose
As ErbB signaling is a determinant of prolactin synthesis, role of ErbB receptors was tested for prolactinoma outcomes and therapy. The objective of this study was to characterize ErbB receptor expression in prolactinomas and then perform a pilot study treating resistant prolactinomas with a targeted tyrosine kinase inhibitor (TKI).
Methods
Retrospective analysis of prolactinomas and pilot study for dopamine agonist resistant prolactinomas in tertiary referral center. We performed immunofluorescent staining of a tissue array of 29 resected prolactinoma tissues for EGFR, ErbB2, ErbB3, and ErbB4 correlated with clinical features. Two patients with aggressive resistant prolactinomas enrolled and completed trial. They received lapatinib 1250 mg daily for 6 months with tumor and hormone assessments. Main outcome measures were positive tumor staining of respective ErbB receptors, therapeutic reduction of prolactin levels and tumor shrinkage.
Results
Treated PRL levels and tumor volumes were suppressed in both subjects treated with TKI. EGFR expression was positive in 82% of adenomas, ErbB2 in 92%, ErbB3 in 25%, and ErbB4 in 71%, with ErbB2 score > EGFR>ErbB4>ErbB3. Higher ErbB3 expression was associated with optic chiasm compression (p = 0.03), suprasellar extension (p = 0.04), and carotid artery encasement (p= 0.01). Higher DA response rates were observed in tumors with higher ErbB3 expression.
Conclusions
Prolactinoma expression of specific ErbB receptors is associated with tumor invasion, symptoms, and response to dopamine agonists. Targeting ErbB receptors may be effective therapy in patients with resistant prolactinomas.
Keywords: prolactinoma, pituitary, EGFR, ErbB, tyrosine kinase inhibitor, Her2
Introduction
Pituitary tumors are monoclonal adenomas accounting for ~ 15% of primary intracranial neoplasms [1]. Although usually benign, excess hormone secretion may lead to distinct endocrine syndromes and tumoral growth to compressive symptoms [1]. Prolactinomas are usually treated with primary dopamine agonist (DA) therapy which normalizes prolactin levels in 80-90% of patients with microprolactinomas and in 70% of macroprolactinomas. Twenty percent of patients are resistant to medications, failing to normalize prolactin and not experiencing tumor mass shrinkage [2,3]. Increasing DA doses in these patients may not be tolerated, and surgery is then indicated, with 75% cure rate for microprolactinomas and 34% for macroprolactinomas [4,5].
However, 7-50% of prolactinomas recur postoperatively and grow progressively despite antitumor therapy [6,7]. These recurrent tumors may be atypical adenomas, which have been identified in ~ 15% of all resected pituitary tumor tissue [8,9] and may represent an intermediate stage in tumor progression from benign to carcinoma [10].
Alternative treatment options are required for such tumors resistant to available treatments [11,12]. Anecdotal reports of pharmacotherapy for aggressive and/or resistant prolactinomas include somatostatin analogues, which do not inhibit PRL levels, and estrogen receptor modulators, which may mildly inhibit PRL. Temozolomide may reduce tumor size and PRL secretion, though not in all cases nor are effects necessarily maintained over time [13-17].
The epidermal growth factor receptor (EGFR) family comprises EGFR (ErbB1, HER1), p185ErbB2/neu (ErbB2, HER2), ErbB3 (HER3) and ErbB4 (HER4), all transmembrane tyrosine kinase receptors. Ligand binding induces formation of receptor homo- and heterodimers, activation of the intrinsic kinase domain and intracellular signaling [18].
ErbB2 lacks a known direct ligand and acts as a heterodimerization partner for other ErbB receptors, and overexpression can cause transformation without expression of a cognate growth factor [19]. ErbB3 lacks innate kinase function but can form heterodimers with the other three members [20]. ErbB2/ErbB3 heterodimers are the most prevalent and mitogenically potent of ErbB receptor/ligand complexes [20].
Aberrant ErbB receptor expression, mutations, or overexpression lead to abnormal receptor activation of intracellular signaling and is associated with pathogenesis of several human cancers [21-25]. Selective therapeutic targeting particularly of EGFR and p185her2/neu has exhibited therapeutic efficacy [25]. Lapatinib is a TKI that targets both EGFR and ErbB2 and is used in combination for advanced ErbB2-positive breast cancer [26].
ErbB receptors and ligands are expressed in nontumoral lactotrophs and induce PRL [27-29]. Lactosomatotroph derived tumors express ErbB receptors and ligands, and targeting this system regulates PRL gene expression, secretion, and tumor size [30-32]. Models of lactotroph tumor pathways abrogated by tyrosine kinase inhibitors (TKI) support the hypothesis that ErbB receptors activate PRL secretion and lactotroph proliferation [33]. Rationale for use of a dual EGFR/ErbB2 dual TKI was based on gefitinib, an EGFR TKI, decreasing rat GH3 somato-lactotroph cell proliferation and PRL mRNA expression in vitro and tumor xenograft volume and PRL secretion in vivo [34]. Gefitinib also suppressed heregulin-induced ErbB receptor activation and signaling and prevented p185cneu and ErbB3 heterodimerization and PRL secretion in vitro [35]. In stable transfectants of a constitutively active form of ErbB2cDNA (HER2CA), lapatinib suppressed EGF-induced ErbB2 and MAPK phosphorylation, intracellular PRL levels, cell proliferation, and colony formation [33]. Rats implanted with HER2CA cells and treated with lapatinib exhibited smaller tumors and suppressed PRL levels [33]. Estradiol induced rat prolactinoma growth rate and prolactin production were also inhibited by lapatinib. Importantly, lapatinib suppressed PRL mRNA and protein secretion from cultured human prolactinoma cells [33].
We performed a pilot study in 2 subjects to test whether dopamine agonist resistant aggressive prolactinomas respond to lapatinib. We also comprehensively evaluated prolactinoma ErbB receptor expression and assessed their association with clinical tumor features.
Materials and Methods
A. Retrospective pathologic study of ErbB receptor expression and correlation with clinical parameters
Tissue array
Pituitary tissue was obtained after written consent by patients participating in the Pituitary Tumor Research Registry (Cedars-Sinai IRB # 2873). Tissue arrays were derived from available tumors after pathology review with 1 core (1.2 mm) collected per tissue block. The prolactinoma array was comprised of tissue derived from 29 prolactinomas operated on at our institution from 1998-2010. Though there were additional prolactinomas in the pathology archives, insufficient tissue was available from these to create a tissue array and therefore only able to create an array of 29 prolactinomas. The following control tissues were included: 2 corticotroph adenomas, 2 GH adenomas, 2 nonfunctioning adenomas, 8 normal pituitaries, 3 tubullovillous adenomas, 1 nonsmall cell lung cancer, 1 lung adenocarcinoma, 1 breast fibroadenoma, and 2 breast cancers (Supplemental Table 1).
Immunofluorescence
Tumor specimens were fixed, embedded, antigen retrieved, blocked, and incubated with rabbit polyclonal anti-EGFR (sc-03; 1:50; Santa Cruz), rabbit polyclonal anti-Neu (C-18) (sc-284, 1:100; Santa Cruz), rabbit polyclonal anti-ErbB3 (C-17) (sc-285, 1:100, Santa Cruz), mouse monoclonal anti-erbB4 (#MS-637-P0, 1:25, Thermoscientific). Slides were incubated with Alexa Fluor goat anti-rabbit (EGFR, ErbB2, ErbB3) or anti-mouse (ErbB4) 488 (H+L) (1:500; Invitrogen) and ToPro3 (Life Technologies/Invitrogen) for 2 h and mounted with Prolong Gold antifade reagent (Life Technologies/Invitrogen). Confocal microscope images were obtained using True Confocal Scanner (TCS-SP) confocal scanner (Leica Microsystems) in a dual-emission mode to separate autofluorescence from specific staining. Positive control tissues were breast cancer for EGFR, ErbB2, ErbB3 and kidney tubules for ErbB4. For nuclear EGFR, positive control tissues were ACTH adenomas and breast fibroadenoma. Negative tissue controls included tubulovillous adenomas and omitting primary antibody during incubation.
Scoring of staining
Slides were manually scored by 2 independent reviewers (SB and OC). For disagreements on positive or negative staining (in 5 cases for EGFR, 2 cases for ErbB2, 4 cases for ErbB3, and 4 cases for ErbB4), a third reviewer decided the score (Supplemental Table 2). The whole tissue core of each tumor was captured on each image and qualitatively assessed for percent positive staining. Intensity was scored on a scale of 0-3 with Score 0 reflecting no staining, score 1 faint/barely perceptible staining; score 2 moderate staining; and score 3 strong staining (Supplemental Fig. 1). The quick score was calculated as a product of percent positive staining and intensity [36,37].
Clinical data
Clinical parameters on the tissue array tumors were retrospectively collected. Cavernous sinus invasion was assessed by Knosp scoring system [38]. Tumor volume was calculated using two methods. The first method calculated volume as a product of each dimension divided by 2. The second method used Open Source OsiriX® Imaging Software for MacOS (analysis performed by OC). The region of interest was defined on each slice and closed polygons manually drawn around the perimeter of the lesion, and the volume was then automatically derived by the program. Biochemical cure was defined as normal prolactin level after dopamine agonists were discontinued at last follow-up visit. Atypical adenomas were defined as Ki-67≥ 3%, increased mitotic index, and positive p53 [39]. The 2011 Endocrine Society clinical practice prolactinoma guidelines define dopamine agonist resistance as “a failure to achieve a normal prolactin level on maximally tolerated doses of dopamine agonist and a failure to achieve a 50% reduction in tumor size” [4]. We defined maximal cabergoline dose as 1 mg daily (or equivalent dose of bromocriptine). Percent tumor size reduction was calculated based on radiologic change in tumor size while receiving preoperative DA. Percent PRL reduction was calculated based on the change in PRL prior to initiating DA to the preoperative value on DA.
B. Prospective patient pilot study
Pilot study design
Informed consent for this investigator-initiated open label, single cohort proof of concept trial (IRB # 18129) was obtained from patients with prolactinomas who demonstrated persistently elevated prolactin levels on maximally tolerated cabergoline therapy (at least 1 mg a day). Exclusion criteria included reduced left ventricular ejection fraction less than 50%, hepatic impairment, and pregnancy. Subjects with visual field deficits were excluded if the deficits had not been stable for at least 6 months. If they were stable for > 6 months, then they were able to be included in the study.
Subjects were administered oral lapatinib 1250 mg daily for 6 months (provided by GlaxoSmithKline, Philadelphia, PA) and maintained on their current dopamine agonist regimen throughout the study..
Primary outcome was normalization of prolactin levels. Secondary outcome was stabilization of tumor size as assessed by the neuroradiologist.
Adverse events were graded by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.02 (US Department of Health and Human Services). Patients demonstrating compromised visual fields or tumor growth on MRI were withdrawn from study and referred for surgery. Subjects were given the option to continue receiving the study drug if they demonstrated response as assessed by MRI and/or prolactin levels.
Statistical analysis
Means and standard deviations were calculated if continuous variables were normally distributed and medians and ranges if not normally distributed. Frequencies and percentages were calculated for categorical variables. For correlation of receptor expression with clinical outcomes, Spearman correlations were used because variables were not normally distributed. For comparison of continuous variables to a binary variable, Rank Sums were calculated with significance testing by Kruskal-Wallis test. Analyses were performed using SAS version 9.3. Statistical significance was set at p <0.05.
Results
A. Retrospective study
Clinical features
Median age of subjects was 37 years (range 16-83), 69% females (n=20), and 31% males (n=9). Median postoperative follow up time was 2 years (0-16) for prolactin levels and 1 year (0-14) for imaging. Preoperative clinical features are summarized in Table 1. Maximal tumor diameter on MRI (n = 20) was a median of 24 mm (4-49), and median tumor volume (n = 13) by the calculation method was 5387 mm3 (86-34937) and by digital volumetrics was 1070 mm3 (60-67880). Initial median prolactin level (n=19) on diagnosis was 300 ng/mL (43-19577). Median duration of DA therapy was 15 months (2-144; n=13). On maximally tolerated DA doses, median percent tumoral reduction was 0% (0-88); n= 15). In eleven subjects, median prolactin percent reduction was by 87% (46-100). Postoperative features are summarized in Table 2. Surgical indications included dopamine agonist resistance in 13/29, dopamine agonist intolerance in 8/29, patient preference in 1/29, preoperative diagnosis of NFA or nonpituitary tumor in 3/29, and undocumented in 4 patients. Median postoperative day 1-2 PRL level (n=22) was 9.9 ng/mL (0.9-2525). Median Ki-67 was 2% (1-5) in 24 subjects.
Table 1.
Preoperative features
| Preoperative features | Number of patients with feature/total number of patients with available data (%) |
|---|---|
| Mass effect symptoms | 11/25 (44) |
| Macroadenomas | 21/27 (78) |
| Suprasellar extension | 11/23 (48) |
| Optic chiasm compression | 9/23 (39) |
| Cavernous sinus invasion | 7/19 (37) |
| Knosp score 0 | 3/14 (21) |
| 1 | 4/14 (29) |
| 2 | 3/14 (21) |
| 3 | 0/14 (0) |
| 4 | 4/14 (29) |
| Carotid encasement | 2/19 (11) |
| Sphenoid sinus invasion | 5/19 (26) |
| Cystic components | 4/29 (14) |
| Tumor hemorrhage | 3/29 (10) |
| Intracranial extension | 2/19 (11) |
| Any hypopituitarism | 22/29 (76) |
| ACTH deficiency | 3/22 (14) |
| TSH deficiency | 4/23 (17) |
| GH deficiency | 3/18 (17) |
| FSH/LH deficiency | 21/23 (91) |
| DA treatment | |
| Use of DA preoperatively | 21/27 (78) |
| Achieved tumor reduction on DA | 2/15 (13) |
| Achieved PRL normalization on DA | 5/16 (31) |
| Achieved PRL reduction on DA | 14/16 (88) |
| Subjects classified as DA intolerant | 12/21 (57) |
| Subjects classified as DA resistant | 13/21 (62) |
Table 2.
Postoperative features
| Outcome | Number of patients with feature/total number of patients with available data (%) |
|---|---|
| Post-operative use of DA | 11/23 (48) |
| Subjects with persistent post-operative PRL hypersecretion | 13/26 (50) |
| Any post-operative hypopituitarism | 6/29 (21) |
| ACTH deficiency | 2/17 (12) |
| TSH deficiency | 4/17 (24) |
| GH deficiency | 1/16 (6) |
| FSH/LH deficiency | 3/11 (27) |
| Tumor histology | |
| Atypical adenomas | 3/26 (12) |
| Positive p53 | 6/12 (50) |
| Increased mitoses | 6/11 (55) |
| Radiologic follow up | |
| Post-operative residual adenoma | 11/20 (55) |
| Growth of post-operative residual on MRI | 4/9 (44) |
| De novo MRI recurrences | 3/8 (38) |
Immunofluorescence staining of tissue array
EGFR immunoreactivity was positive in 23/28 (82%) adenomas, ErbB2 in 24/26 (92%), ErbB3 in 7/28 (25%), and ErbB4 in 20/28 (71%) (Figures 1 and 2) with no gender specificity. While EGFR was localized mostly to the nucleus, the other ErbB receptors were expressed in the membrane/cytoplasm. Normal and tumoral pituitary control tissue also expressed nuclear EGFR (Supplemental Table 1). Median expression scores of percent positive staining in prolactinomas were 75 (0-100), 75 (0-100), 0 (0-100), and 40 (0-100) for EGFR, ErbB2, ErbB3, and ErbB4, respectively. ErbB receptor expression scores (percent positive staining) correlated with the quick scores, r=0.94 (EGFR), 0.92 (ErbB2), 0.998 (ErbB3), 0.97 (ErbB4), p < 0.0001. Higher ErbB3 expression on prolactinomas was associated with higher rates of optic chiasm compression [10 (0- 100) compared to 0 (0-10), p = 0.01, n=23], suprasellar extension [0 (0-100) compared to 0 (0-10), p = 0.04, n=23], and encasement of the carotids [55 (10- 100) compared to 0 (0-80), p=0.01, n=19]. Higher ErbB4 quick score was observed in tumors with sphenoid sinus invasion [100 (0- 200)] compared to those without invasion [25 (0- 90), p = 0.048, n=19]. Two subjects who experienced tumor and PRL reduction on DA therapy had 100% percent positive staining for ErbB3 on their adenomas compared to those without tumor reduction who had 0-10% ErbB3 staining (with one exception). No significant correlations with ErbB2 staining were noted.
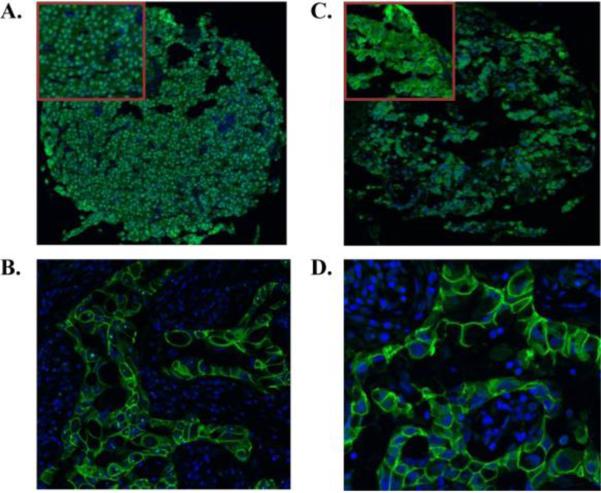
Figure 1.
Representative IHC of EGFR and ErbB2 receptors in prolactinomas on tissue array (20× magnification). Top corner box shows magnification of selected area. EGFR staining was nuclear and ErbB2 staining membranous/cytoplasmic. Positive control tissue is breast cancer A. EGFR staining of prolactinoma B. EGFR staining of breast cancer (63× magnification). C. ErbB2 staining of prolactinoma D. ErbB2 staining of breast cancer (63× magnification).
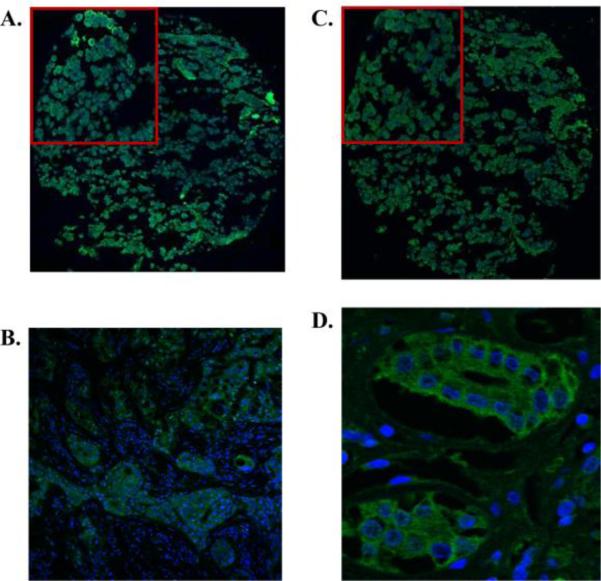
Figure 2.
Representative IHC of ErbB3 and ErbB4 receptors in prolactinomas on tissue array (20× magnification). Top corner box shows magnification of selected area. Positive control tissue is breast cancer for ErbB3 and kidney tubules for ErbB4. A. ErbB3 staining of prolactinoma B. ErbB3 staining of breast cancer (20× magnification) C. ErbB4 staining of prolactinoma D. ErbB4 staining of kidney tubules (63× magnification).
B. Prospective study
Pilot study results
Three subjects with resistant prolactinomas were screened. One was excluded due to unstable visual fields and referred for surgery, and two were enrolled as they did not manifest visual field deficits. They completed the study.
Subject 1
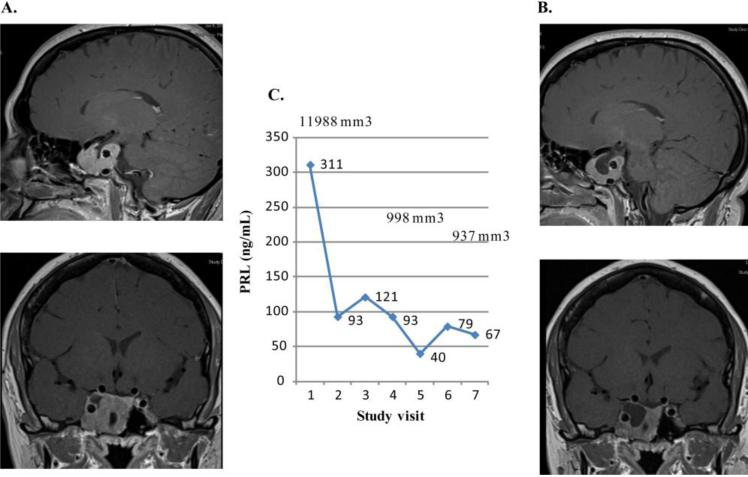
A 42 year old female was diagnosed in 1995 with a prolactinoma, and PRL level 555 ng/mL, and maximum tumor diameter of 40 mm (original images were unable to be obtained to assess in 3 dimensions). She initially was treated with bromocriptine for 4 years which did not reduce the adenoma size, and underwent subtotal transsphenoidal resection with postoperative PRL of 300 ng/mL. Therapy was switched to cabergoline and dose titrated to 1 mg daily, maintained for 13 years. PRL levels remained high in the 100's, and the tumor continued to increase in size. Fatigue and severe headaches were present for the prior 3 years, scoring a 6-7/10 on a pain scale with exacerbations to a 10. Prior to starting study drug, PRL level was 311 ng/mL. Visual fields were normal. MRI dimensions of the adenoma were 23 × 27 × 34 mm (volume 11,988 mm3), extending into the right cavernous sinus and encasing the right carotid (Knosp score 4). PRL level decreased to 93 and 67 ng/mL after 1 month and 6 months of lapatinib treatment, respectively. On MRI, the tumor reduced in size by 22% at 6 months (9,366 mm3) (Figure 3). Headaches decreased to a scale of 5/10 with occasional exacerbations, and fatigue improved. Cabergoline 7 mg/week was maintained throughout the study.
Figure 3.
Prolactin levels and tumor volumes in first lapatinib treated subject. The MRI of subject 1 is depicted. A. Baseline MRI demonstrates homogeneously enhancing mass in right aspect of sella extending into cavernous sinus and encasing the carotid vessel, and abutting inferior aspect of right prechiasmatic optic nerve. Lesion measures 23 × 27 × 34 mm. B. MRI after 6 months on lapatinib shows regression of central portion of the tumor mass. C. Graph shows PRL levels (ng/mL) and tumor volumes (mm 3) at baseline (study visit 1) and at monthly intervals.
Experienced side effects on lapatinib included mild loss of appetite, mild alopecia, a single self-limited episode of diarrhea, and intermittent acneiform rash of the face and back. There were no echocardiogram or liver function changes. Given her clinical response, continued lapatinib was recommended as compassionate therapy in combination with cabergoline.
Subject 2
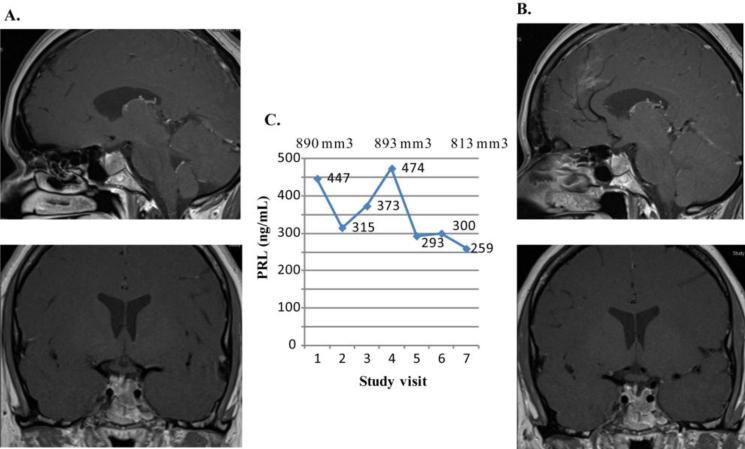
A 31 year old female was diagnosed in 2006 with a prolactinoma, initial PRL level of 616 ng/mL, and MRI maximal diameter of 16 mm. Cabergoline was initiated and switched to bromocriptine due to concerns about valvulopathy, as suggested by presence of mild tricuspid valve regurgitation on an echocardiogram performed after one year of cabergoline use. PRL remained persistently elevated up to 1,139 ng/mL on bromocriptine 5 mg/day. She underwent subtotal transsphenoidal resection, of a densely granulated prolactinoma with postoperative PRL level of 286 ng/mL. Ki-67 was 3%, weakly positive p53 to 2%, and no increased mitotic activity. Bromocriptine was re-initiated at 5 mg/day with subsequent PRL level of 263 ng/mL. Dose was further titrated to 10 mg/day, and PRL remained at 277 ng/mL, and then titrated up to 12.5 mg a day at which PRL levels continued to increase up to 692 ng/mL. At that time, she was switched to cabergoline 3 mg/week, and PRL was initially reduced to 373 ng/mL. However, as the dose was titrated up to 7 mg/week, PRL level remained at 383-472 ng/mL with persistent tumor growth, at which point the subject was referred for evaluation in the lapatinib study. Headache occurred twice a month with mild hair loss. At baseline, PRL level was 447 ng/mL, the adenoma measured 9.9 × 6.8 × 13.1 mm (volume of 890 mm3) and had invaded the left cavernous sinus (Knosp score 1). Lapatinib 1250 mg was added in 2012, and PRL levels decreased to 315 ng/mL and 259 ng/mL, after 1 and 6 months of treatment, respectively (Figure 4). On MRI, the adenoma size was unchanged (volume of 813 mm3) with improvement of the deviated infundibulum and improved headaches.
Figure 4.
Prolactin levels and tumor volumes in second lapatinib treated subject. The MRI of subject 2 is depicted. A. Baseline MRI demonstrates a hypoenhancing intrasellar mass inferolateral on the left side growing into the left cavernous sinus. The mass measures 9.9 mm transversely, 6.8 mm cephalocaudad, and 13.1 mm anteroposteriorly. B. MRI after 6 months on lapatinib shows no change. C. Graph shows PRL levels (ng/mL) and tumor volumes (mm3) at baseline (study visit 1) and at monthly intervals.
Reported drug side effects included mild alopecia and a pruritic, acneiform rash on the face, chest and back, few episodes of self-limiting diarrhea, and no cardiac changes. The subject continues to receive cabergoline (1 mg daily) and will be undergoing surgical resection of the adenoma.
Discussion
We report for the first time a beneficial effect of ErbB antagonism, specifically with lapatinib, in the treatment of 2 patients with DA resistant prolactinomas. We support our clinical observation with a comprehensive assessment of prolactinomas ErbB receptor subtype expression profiles in association with clinical features.
Translation of our pre-clinical findings was implemented by our pilot study in DA resistant prolactinomas. The enrolled subjects had received cabergoline (1 mg daily) for several years, one prior surgical resection, and they exhibited persistent hyperprolactinemia and tumor growth despite continued DA therapy. Lapatinib was added in combination with cabergoline and responses monitored over 6 months. Both subjects exhibited PRL reductions with one subject having a 78% reduction and near normalization of PRL level. This subject also experienced significant tumoral reduction in a tumor that had persistently grown while on 13 years of cabergoline monotherapy. After 6 years of DA therapy with suboptimal response, the second subject had a 42% reduction in PRL levels with stabilization of tumor size with the addition of lapatinib to cabergoline, in contrast to prior growth noted while receiving cabergoline monotherapy. Lapatinib was well tolerated in both subjects with minimal side effects which did not lead to premature discontinuation of the study drug, unlike experience with higher DA doses. Subjects noted improved clinical symptoms, including the first subject who had years of severe headaches requiring analgesic therapy several times a day, but during the course of the lapatinib trial noted marked headache improvement with decreased dose and frequency of required analgesics. The second subject also experienced headache improvement.
Lapatinib effect is mediated by binding the ATP binding pocket of both EGFR and ErbB2, preventing autophosphorylation. In breast cancer models, overexpression of ErbB2 leads to downstream activation of MAPKErk signaling which is inhibited by lapatinib. PI3K-Akt is also activated in ErbB2 positive breast cancers where it promotes tumor cell survival and resistance to cancer therapies, and lapatinib blocks these effects, leading to increased apoptosis and decreased cell proliferation. Lapatinib also down-regulates survivin and XIAP, inhibitors of apoptosis (reviewed in [40]). Lapatinib blocks formation of neuregulin induced ErbB2-ErbB3 heterodimers in MCF7 breast cells [41], stabilizes ErbB2-ErbB3 heterodimers [42] and upregulates ErbB3 [43]. Potentially, higher expression of ErbB3 in patients treated with lapatinib could improve DA responsiveness as suggested by our IHC results which demonstrate that patients with higher tumoral ErbB3 expression are more sensitive to DA. It is unknown how lapatinib affects EGFR-ErbB3 heterodimers.
There has been concern on long term effects of cabergoline on cardiac valves. While lapatinib has a reported rare adverse event of decreasing systolic function, it does not affect the heart valves. Combining lapatinib with cabergoline should not exacerbate a potential valvulopathy, and the combined treatment could help decrease the dose of cabergoline required over time, thereby limiting cumulative dose effects over many years of therapy facing patients with resistant prolactinomas. During the course of the trial, subjects were able to postpone additional therapy, namely surgery or radiotherapy. These were young women who would face long term sequelae from such invasive procedures, especially as they did not exhibit panhypopituitarism nor visual deficits. The first subject is indeed continuing to receive lapatinib and cabergoline and hopes to continue delaying further interventions.
In light of the clinical response to EGFR targeted therapy, we examined receptor expression in available prolactinomas, demonstrating that majority of prolactinomas exhibit variable ErbB expression, similar to previous reports [44-47,35,48-51] . We also demonstrate, as we previously showed for ACTH-secreting adenomas and prolactinomas [33,52], nuclear localization of EGFR in human prolactinoma tumor cells. We now report associations between selective ErbB receptor expression and tumoral invasion and response to DA.
ErbB3 expression was positively associated with tumoral invasion and also better response to DA treatment. ErbB3 mRNA was previously reported to be ~40-fold higher in more aggressive prolactinoma specimens [35]. Interaction of ErbB3 and dopamine receptors in prolactinomas is unknown but may be a potential mechanism to explain this association. Heregulin, the ligand for ErbB3 and ErbB4, was shown to induce rapid ErbB2 and ErbB3 phosphorylation in GH4C1 cells, leading to increasing PRL mRNA expression and secretion and associated with formation of ErbB2/ErbB3 heterodimers [35]. However, as GH4C1 cells do not express D2 receptors, these findings may not reflect effects of ErbB3 receptors in human prolactinomas.
Several limitations to the pilot study render the results biased. While one subject had both a radiological and biochemical response to lapatinib, the other subject had a minimal reduction of PRL on the study drug with no change in tumor size. This may be related to differential adenoma expression of ErbB receptors, but this was unable to be assessed. It is possible that the reductions in PRL observed in the subjects were from continued use of dopamine agonists. In addition, additive or synergistic effect between lapatinib and DA cannot be excluded, however, DA treatment could not be discontinued because of potential rebound tumoral growth and PRL elevation, and there were also ethical concerns not to discontinue an approved therapy for prolactinomas in order to start an unproven monotherapy, namely lapatinib. It is unknown at this time whether these medications directly interact. However, it has been shown that EGF induces endogenous D2 receptor gene expression in GH3 cells leading to a 10 fold increase in Gi3α subunit and increased PRL secretion [53]. TKIs may affect D2 receptors on prolactinomas which may account for reduced PRL levels observed in lapatinib treated subjects. Moreover, as shown here, ErbB3, which is not targeted by lapatinib, may be associated with tumoral invasion and response to DA, and, as ErbB3 heterodimerizes with ErbB2, may be a strong oncogenic stimulus. ErbB3 may exhibit different functions in pituitary adenomas. On the one hand, ErbB3 receptor expression promotes epithelial to mesenchymal transition which leads to invasion and metastases in breast cancer [54], supporting our findings in the retrospective study. On the other hand, ErbB3 may have a synergistic function in relation to dopamine 2 receptors expressed on prolactinomas as suggested by the response of our subjects to lapatinib in combination with cabergoline. ErbB2/ErbB3 inhibitor effect on prolactinomas to suppress effects of ErbB3 receptor signaling requires study.
Caveats which may confound interpretation of the results of the retrospective study include incomplete subject data, different interpretation of “resistant” prolactinomas by treating physicians, and relatively short follow-up durations. The classification of dopamine agonist resistant patients is difficult as it may be limited to subjective interpretation by the treating physician and may have been due to use of standard doses of dopamine agonists without full escalation to higher doses. Further, the significance value of p=0.05 was chosen to determine statistical significance though some retrospective analyses use p values of < 0.01. While in larger studies, a smaller p value may be helpful to discriminate when multiple results show significance, given that the current study had a relatively small sample size, a p value of 0.05 was deemed adequate to assess for statistical significance. ErbB receptor scoring technique is limited by the subjective evaluation of the reviewers. Digital slide scanning potentially could improve accuracy of the scoring through quantitative assessments though these technologies were not available at the time of this study. While ErbB receptor expression is strongly associated with specific clinical features, this does not necessarily translate to causality
In summary, this is the first pilot study evaluating the ErbB antagonist lapatinib in conjunction with a dopamine agonist in patients with DA-resistant prolactinomas, demonstrating a beneficial effect of lapatinib on clinical outcome including reducing prolactin levels and tumor shrinkage in two subjects. Lapatinib was well tolerated and improved clinical symptoms, providing an alternative to more invasive interventions such as surgery and radiotherapy. Complementary assessment of ErbB receptors demonstrated expression of all four EGFR family receptors subtypes, including ErbB3 and ErbB4 which have rarely been described in pituitary tissue [35,55]. Moreover, associations between ErbB receptor expression and clinical manifestations were observed, specifically tumoral invasion and symptoms, and response to dopamine agonists. Therefore, this study provides a rationale for comprehensive clinical trials using targeted ErbB therapies as combination therapy with dopamine agonists for resistant prolactinomas.
Supplementary Material
Supplemental Figure 1: Representative samples of intensity scores 0-3 in prolactinoma tissues (stained for ErbB2) 20× magnification. A. Intensity score 0; B. Intensity score 1; C. Intensity score 2; D. Intensity score 3
Acknowledgements
Grant support: This work was supported by NIH grant K23DK085148 (O. Cooper).
Dr. Shlomo Melmed for continuing mentorship, guidance, and patient consultations. GlaxoSmithKline for provision of lapatinib, Kolja Wawrowsky for confocal imaging of TMA and advise, Grace Labrado and Karen Geiser for assistance with preparation of the manuscript, Lori Korsakoff and Billy Gellepis for coordinating the research study.
Footnotes
Disclosures: The authors have no disclosures.
Ethical standards: The studies in this manuscript were approved and monitored by the Institutional Review Board.
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- 1.Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112(11):1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, Di Somma C, Faggiano A, Lombardi G, Colao A. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. The Journal of clinical endocrinology and metabolism. 2001;86(11):5256–5261. doi: 10.1210/jcem.86.11.8054. [DOI] [PubMed] [Google Scholar]
- 3.Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinology and metabolism clinics of North America. 2008;37(1):67–99. viii. doi: 10.1016/j.ecl.2007.10.013. doi:10.1016/j.ecl.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–288. doi: 10.1210/jc.2010-1692. doi:10.1210/jc.2010-1692 96/2/273 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clinical endocrinology. 2006;65(2):265–273. doi: 10.1111/j.1365-2265.2006.02562.x. doi:10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 6.Losa M, Mortini P, Barzaghi R, Gioia L, Giovanelli M. Surgical treatment of prolactin-secreting pituitary adenomas: early results and long-term outcome. J Clin Endocrinol Metab. 2002;87(7):3180–3186. doi: 10.1210/jcem.87.7.8645. [DOI] [PubMed] [Google Scholar]
- 7.Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983;309(5):280–283. doi: 10.1056/NEJM198308043090505. doi:10.1056/NEJM198308043090505. [DOI] [PubMed] [Google Scholar]
- 8.Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER., Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg. 2011;114(2):336–344. doi: 10.3171/2010.8.JNS10290. doi:10.3171/2010.8.JNS10290. [DOI] [PubMed] [Google Scholar]
- 9.Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, Brue T, Figarella-Branger D, Roy P, Jouanneau E, Jan M, Lachuer J, Trouillas J. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab. 2010;95(4):1708–1716. doi: 10.1210/jc.2009-1191. doi:10.1210/jc.2009-1191jc.2009-1191 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7(5):257–266. doi: 10.1038/nrendo.2011.40. doi:10.1038/nrendo.2011.40nrendo.2011.40 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Kaltsas GA, Nomikos P, Kontogeorgos G, Buchfelder M, Grossman AB. Clinical review: Diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab. 2005;90(5):3089–3099. doi: 10.1210/jc.2004-2231. [DOI] [PubMed] [Google Scholar]
- 12.Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, Young WF, Jr., Lloyd RV. Pituitary carcinoma: a clinicopathological review. Neurosurgery. 2005;56(5):1066–1074. discussion 1066-1074. [PubMed] [Google Scholar]
- 13.Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27(5):485–534. doi: 10.1210/er.2005-9998. [DOI] [PubMed] [Google Scholar]
- 14.Bronstein MD, Knoepfelmacher M, Liberman B, Marino R, Jr., Germek OA, Schally AV. Absence of suppressive effect of somatostatin on prolactin levels in patients with hyperprolactinemia. Horm Metab Res. 1987;19(6):271–274. doi: 10.1055/s-2007-1011796. doi:10.1055/s-2007-1011796. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts SW, Verleun T, Oosterom R. Effect of tamoxifen administration on prolactin release by invasive prolactin-secreting pituitary adenomas. Neuroendocrinology. 1982;34(5):339–342. doi: 10.1159/000123324. [DOI] [PubMed] [Google Scholar]
- 16.Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C, Assaker R, Dufour H, Gaillard S, Francois P, Jouanneau E, Passagia JG, Bernier M, Cornelius A, Figarella-Branger D, Trouillas J, Borson-Chazot F, Brue T. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab. 2010;95(10):4592–4599. doi: 10.1210/jc.2010-0644. doi:10.1210/jc.2010-0644jc.2010-0644 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Losa M, Mazza E, Terreni MR, McCormack A, Gill AJ, Motta M, Cangi MG, Talarico A, Mortini P, Reni M. Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. Eur J Endocrinol. 2010;163(6):843–851. doi: 10.1530/EJE-10-0629. doi:10.1530/EJE-10-0629EJE-10-0629 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 19.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 20.Roskoski R., Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319(1):1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 21.Gorgoulis V, Aninos D, Mikou P, Kanavaros P, Karameris A, Joardanoglou J, Rasidakis A, Veslemes M, Ozanne B, Spandidos DA. Expression of EGF, TGF-alpha and EGFR in squamous cell lung carcinomas. Anticancer Res. 1992;12(4):1183–1187. [PubMed] [Google Scholar]
- 22.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16(6):413–428. doi: 10.1002/stem.160413. doi:10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Lau YK, Zhang HZ, Xiao FY, Johnston DA, Liu AR, Li L, Katz RL, Hung MC. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5(12):4164–4174. [PubMed] [Google Scholar]
- 24.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57(15):3272–3280. [PubMed] [Google Scholar]
- 25.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2--clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008;5(9):512–520. doi: 10.1038/ncponc1156. doi:10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 27.Murdoch GH, Potter E, Nicolaisen AK, Evans RM, Rosenfeld MG. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- 28.Mouihate A, Lestage J. Epidermal growth factor: a potential paracrine and autocrine system within the pituitary. Neuroreport. 1995;6(10):1401–1404. [PubMed] [Google Scholar]
- 29.Armstrong J, Childs GV. Changes in expression of epidermal growth factor receptors by anterior pituitary cells during the estrous cycle: cyclic expression by gonadotropes. Endocrinology. 1997;138(5):1903–1908. doi: 10.1210/endo.138.5.5118. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Kulig E, Jin L, Lloyd RV. Effects of estrogen and epidermal growth factor on prolactin and Pit-1 mRNA in GH3 cells. Proc Soc Exp Biol Med. 1993;202(2):193–200. doi: 10.3181/00379727-202-43526. [DOI] [PubMed] [Google Scholar]
- 31.Pickett CA, Manning N, Akita Y, Gutierrez-Hartmann A. Role of specific protein kinase C isozymes in mediating epidermal growth factor, thyrotropin-releasing hormone, and phorbol ester regulation of the rat prolactin promoter in GH4/GH4C1 pituitary cells. Mol Endocrinol. 2002;16(12):2840–2852. doi: 10.1210/me.2001-0305. [DOI] [PubMed] [Google Scholar]
- 32.Hapgood J, Libermann TA, Lax I, Yarden Y, Schreiber AB, Naor Z, Schlessinger J. Monoclonal antibodies against epidermal growth factor receptor induce prolactin synthesis in cultured rat pituitary cells (GH3). Proc Natl Acad Sci U S A. 1983;80(21):6451–6455. doi: 10.1073/pnas.80.21.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuoka H, Cooper O, Mizutani J, Tong Y, Ren SG, Bannykh S, Melmed S. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol. 2011;25(1):92–103. doi: 10.1210/me.2010-0353. doi:10.1210/me.2010-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008;68(15):6377–6386. doi: 10.1158/0008-5472.CAN-08-0508. doi:68/15/6377 [pii] 10.1158/0008-5472.CAN-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlotides G, Cooper O, Chen YH, Ren SG, Greenman Y, Melmed S. Heregulin regulates prolactinoma gene expression. Cancer Res. 2009;69(10):4209–4216. doi: 10.1158/0008-5472.CAN-08-4934. doi:0008-5472.CAN-08-4934 [pii] 10.1158/0008-5472.CAN-08-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, Adamson R, Rhodes T, Miller K, Walker R. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol. 2000;53(8):634–635. doi: 10.1136/jcp.53.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, Puig B, Geneix J, Hassoun J, Birnbaum D, Jacquemier J, Viens P. Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J Pathol. 2004;202(3):265–273. doi: 10.1002/path.1515. doi:10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- 38.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610–617. doi: 10.1227/00006123-199310000-00008. discussion 617-618. [DOI] [PubMed] [Google Scholar]
- 39.Al-Shraim M, Asa SL. The 2004 World Health Organization classification of pituitary tumors: what is new? Acta Neuropathol. 2006;111(1):1–7. doi: 10.1007/s00401-005-1093-6. doi:10.1007/s00401-005-1093-6. [DOI] [PubMed] [Google Scholar]
- 40.Chen FL, Xia W, Spector NL. Acquired resistance to small molecule ErbB2 tyrosine kinase inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(21):6730–6734. doi: 10.1158/1078-0432.CCR-08-0581. doi:10.1158/1078-0432.CCR-08-0581. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Martin M, Pandiella A. Differential action of small molecule HER kinase inhibitors on receptor heterodimerization: therapeutic implications. International journal of cancer. Journal international du cancer. 2012;131(1):244–252. doi: 10.1002/ijc.26358. doi:10.1002/ijc.26358. [DOI] [PubMed] [Google Scholar]
- 42.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, Baselga J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–814. doi: 10.1038/onc.2008.432. doi:10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 43.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5021–5026. doi: 10.1073/pnas.1016140108. doi:10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontogeorgos G, Stefaneanu L, Kovacs K, Cheng Z. Localization of Epidermal Growth Factor (EGF) and Epidermal Growth Factor Receptor (EGFr) in Human Pituitary Adenomas and Nontumorous Pituitaries: An Immunocytochemical Study. Endocr Pathol. 1996;7(1):63–70. doi: 10.1007/BF02739916. [DOI] [PubMed] [Google Scholar]
- 45.Jaffrain-Rea ML, Petrangeli E, Lubrano C, Minniti G, Di Stefano D, Sciarra F, Frati L, Tamburrano G, Cantore G, Gulino A. Epidermal growth factor binding sites in human pituitary macroadenomas. J Endocrinol. 1998;158(3):425–433. doi: 10.1677/joe.0.1580425. [DOI] [PubMed] [Google Scholar]
- 46.Theodoropoulou M, Arzberger T, Gruebler Y, Jaffrain-Rea ML, Schlegel J, Schaaf L, Petrangeli E, Losa M, Stalla GK, Pagotto U. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J Endocrinol. 2004;183(2):385–394. doi: 10.1677/joe.1.05616. [DOI] [PubMed] [Google Scholar]
- 47.Onguru O, Scheithauer BW, Kovacs K, Vidal S, Jin L, Zhang S, Ruebel KH, Lloyd RV. Analysis of epidermal growth factor receptor and activated epidermal growth factor receptor expression in pituitary adenomas and carcinomas. Mod Pathol. 2004;17(7):772–780. doi: 10.1038/modpathol.3800118. [DOI] [PubMed] [Google Scholar]
- 48.Chaidarun SS, Eggo MC, Sheppard MC, Stewart PM. Expression of epidermal growth factor (EGF), its receptor, and related oncoprotein (erbB-2) in human pituitary tumors and response to EGF in vitro. Endocrinology. 1994;135(5):2012–2021. doi: 10.1210/endo.135.5.7956924. [DOI] [PubMed] [Google Scholar]
- 49.Ezzat S, Zheng L, Smyth HS, Asa SL. The c-erbB-2/neu proto-oncogene in human pituitary tumours. Clin Endocrinol (Oxf) 1997;46(5):599–606. doi: 10.1046/j.1365-2265.1997.1921003.x. [DOI] [PubMed] [Google Scholar]
- 50.Nose-Alberti V, Mesquita MI, Martin LC, Kayath MJ. Adrenocorticotropin-Producing Pituitary Carcinoma with Expression of c-erbB-2 and High PCNA Index: A Comparative Study with Pituitary Adenomas and Normal Pituitary Tissues. Endocr Pathol. 1998;9(1):53–62. doi: 10.1007/BF02739952. [DOI] [PubMed] [Google Scholar]
- 51.Botelho CH, Magalhaes AV, Mello PA, Schmitt FC, Casulari LA. Expression of p53, Ki-67 and c-erb B2 in growth hormone-and/or prolactin-secreting pituitary adenomas. Arq Neuropsiquiatr. 2006;64(1):60–66. doi: 10.1590/s0004-282x2006000100013. [DOI] [PubMed] [Google Scholar]
- 52.Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712–4721. doi: 10.1172/JCI60417. doi:10.1172/JCI6041760417 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Missale C, Castelletti L, Boroni F, Memo M, Spano P. Epidermal growth factor induces the functional expression of dopamine receptors in the GH3 cell line. Endocrinology. 1991;128(1):13–20. doi: 10.1210/endo-128-1-13. [DOI] [PubMed] [Google Scholar]
- 54.Kim J, Jeong H, Lee Y, Kim C, Kim H, Kim A. HRG-beta1-driven ErbB3 signaling induces epithelial-mesenchymal transition in breast cancer cells. BMC cancer. 2013;13:383. doi: 10.1186/1471-2407-13-383. doi:10.1186/1471-2407-13-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Representative samples of intensity scores 0-3 in prolactinoma tissues (stained for ErbB2) 20× magnification. A. Intensity score 0; B. Intensity score 1; C. Intensity score 2; D. Intensity score 3