Abstract
Background
Preclinical evidence implicates the 5-HT1B receptor in cocaine’s effects. This study explores 5-HT1B in humans by examining receptor availability in vivo with primary cocaine-dependent (CD) subjects using positron emission tomography (PET).
Methods
Fourteen medically healthy CD subjects (mean age=41±6 yrs) were compared to 14 age-matched healthy control subjects (41±8 yrs) with no past or current history of cocaine or other illicit substance abuse. Participants received an MRI and then a PET scan with the highly selective 5HT1B tracer, [11C]P943, for purposes of quantifying regional binding potential (BPND). Voxel-based morphometry (VBM) and gray matter masking (GMM) were also employed to control for potential partial volume effects.
Results
[11C]P943 PET imaging data in nine candidate regions (amygdala, anterior cingulate cortex, caudate, frontal cortex, hypothalamus, pallidum, putamen, thalamus and ventral striatum) showed significant or nearly significant reductions of BPND in CD subjects in three regions, including the anterior cingulate (−16%; P<0.01), hypothalamus (−16%, P=0.03) and frontal cortex (−7%, P=0.08). VBM showed significant gray matter reductions in the frontal cortex of CD subjects. After GMM, statistically significant reductions in [11C]P943 BPND were either retained (anterior cingulate, −14%, p=0.01; hypothalamus, −20%, P<0.01) or achieved (frontal cortex, −14%, p<0.01). Whole brain voxel-wise parameter estimation confirmed these results. Secondary analyses were also significant in some regions for years of cocaine and daily tobacco use.
Conclusions
The reductions found in this study suggest that 5-HT1B receptors may contribute to the etiology and/or expression of cocaine dependence and potentially represent a target for medication development.
Keywords: cocaine, 5-HT1B, serotonin, PET, VBM, human
1. Introduction
Cocaine dependence (CD) is a widespread public health problem in the United States and is associated with considerable personal and fiscal costs to both society and the individual. In 2010 the estimated number of current cocaine users in the U.S. was 1.5 million (1). Despite the significant number of users and complications from abuse and dependence, there is no FDA-approved medication treatment for CD. As such, the identification of novel molecular targets that may modulate cocaine’s effects in humans remains a priority. In the current study, we focused on one such molecular target, the serotonin receptor 1B subtype (5-HT 1B).
The 5-HT 1B receptor is an inhibitory G protein coupled metabotropic receptor found primarily as presynaptic autoreceptors on 5-HT neurons and as heteroreceptors on non-serotoninergic neurons (2). Administration of drugs with agonistic or antagonistic properties at 5-HT 1B receptors will typically inhibit or enhance, respectively, 5-HT activity in the brain (3). Based on autoradiographic work focusing on subcortical structures, the basal ganglia, hippocampus, substantia nigra and entorhinal cortex all have significant 5-HT 1B binding, but regional differences in receptor-mediated G-protein activation in these areas have been described (4).
Multiple preclinical studies have investigated the role of the 5-HT 1B receptor in mediating the neurobiological effects of cocaine, but the nature of it’s role in drug reward remains unclear due to inconsistencies across studies (5). Several studies, however, have suggested potentiation of cocaine’s effects by the 5-HT 1B receptor (6). This effect is thought to occur via 5-HT 1B heteroreceptors that have an inhibitory effect on GABA release in the ventral tegmental area (VTA), thereby disinhibiting dopaminergic activity and amplifying drug reward mechanisms. Studies focusing on cocaine administration have similarly shown a reinforcement of stimulant effects via the 5-HT 1B receptor (7, 8). In contrast, 5-HT 1B knock-out mice have shown an increased sensitization to cocaine and stimulants that inhibit reinforcement (9), while pharmacologic activation has paradoxically shown a reduction in stimulant use with 5-HT 1B receptors in the nucleus accumbens (10). More recent studies have focused on potential explanations for seemingly paradoxical effects, implicating variables such as drug dose, brain region and length of time since last use as potential explanations (11, 12). Taken together, the preclinical data indicate that 5-HT 1B receptor function in brain reward circuitry contributes to cocaine use and cocaine responsiveness, albeit in a complex and incompletely understood fashion.
In humans, genetic studies have found associations between 5-HT 1B receptor polymorphisms and substance abuse, suggesting that modified 5-HT 1B receptor activity may be a contributing factor to increased susceptibility to addiction (13). In order to better understand the potential role of 5-HT 1B receptors in CD, we employed the newly available 5-HT 1B PET radioligand [11C]P943 to image receptor availability in CD as compared to healthy control (HC) subjects.
As animal work has indicated that knocking out 5-HT 1B receptors in mice is associated with increased cocaine self administration (9) and 5-HT 1B receptor over-expression in rats is associated with stress-related stimulant responsiveness (14), there are data to suggest that CD individuals would show either increases or decreases in 5-HT 1B receptors. Given this ambiguity and the unknown effects of cocaine on the 5-HT 1B receptor in humans, the current work could provide evidence for a mechanism in humans and future development by either showing an increase in 5-HT 1B receptor availability in CD, which would support a model of 5-HT 1B sensitization in CD, or a decrease that would support a desensitized model of 5-HT 1B within reward-related brain regions in chronic CD.
2. Methods and Materials
2.1. Subjects
Fourteen medically healthy, non-treatment seeking CD subjects were compared to previously reported age-matched HC subjects (15, 16). All CD and HC scans took place over three years and the mean scan time between groups was 1 year, 4 months. In the 3 months preceding scans, HC subjects were without significant nicotine (with the exception of one subject), alcohol or illicit substance use. Based on our prior work showing statistically significant effects of age (declining) but not sex or race (17) with [11C]P943 availability, CD and HC subjects were matched as a group for age (41± 6.2 vs. 41± 7.8 years, respectively; p=0.73), but not sex (4/10 vs. 5/9 for females/males) or race (3/9/1/1 vs. 10/3/0/1 for Caucasian/African American/Hispanic/other).
Eligibility for the study was confirmed through comprehensive psychiatric histories and clinical semi structured interviews (e.g., the Mini-International Neuropsychiatric Interview or M.I.N.I.) or SCID-1 (Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Axis I disorders), a physical exam with medical history, routine laboratory studies, pregnancy tests, urine toxicology and electrocardiograms (ECGs). Measures of clinical data for secondary analyses included the Hamilton Depression Rating Scale (HAM-D)(18), Barratt Impulsiveness Scale (BIS-11)(19), the State Trait Anxiety Inventory (STAI)(20), and the Childhood Trauma Questionnaire (CTQ)(21).
Individuals were excluded for evidence of a diagnosis of current or lifetime severe Axis I psychiatric disorder (e.g., schizophrenia or bipolar disorder), current or past serious medical or neurological illness (including a history of head injury with loss of consciousness), current pregnancy (as documented by pregnancy testing at screening and on the day of the PET imaging study), breast feeding or general MRI exclusion criteria. All subjects were medication-free for a minimum of 6 weeks at the time of scanning.
CD subjects met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV) criteria for cocaine dependence, were between 18 and 50 years of age, used a high-potency, rapid-onset form of cocaine (i.e., smoked or intravenous), reported a history of regular and recent use and provided objective evidence of current use (i.e., benzoylecgonine positivity) on urine toxicology testing before admission into the study. Clinical characteristics of CD participants are shown in Table 1.
Table 1.
Characteristics for cocaine-dependent (CD) subjects
| CD Subjects (N=14) | Demographics | ||||
|---|---|---|---|---|---|
|
| |||||
| Age | 41 (6) | ||||
| Gender | 4 F/10 M | ||||
| Ethnicity (Caucasian (C), African-American (AA), Hispanic (H) and other | 3 C/9 AA/1 H/1 Other | ||||
|
| |||||
| Clinical Use Characteristics | Mean (S.D.) | ||||
|
| |||||
| Years of cocaine use | 21 (7) | ||||
|
| |||||
| Weekly cocaine use (US Dollars) | 652 (617) | ||||
|
| |||||
| Weekly cocaine use (grams) | 4.6 (4.4) | ||||
|
| |||||
| Weekly ETOH use (drinks) | 16 (13) | ||||
|
| |||||
| Daily nicotine use (cigarettes) | 10 (7) | ||||
|
| |||||
| Cannabis use in the last week (joints) | 3 (7) | ||||
|
| |||||
| Secondary Measures | Mean Scores (S.D.) | ||||
|
| |||||
| STAI (State Trait Anxiety Inventory) State and Trait Subscales | 41 (11) | 45 (12) | |||
|
| |||||
| BIS-11 (Barratt Impulsiveness Scale) | 74 (14) | ||||
|
| |||||
| HAM-D (Hamilton Rating Scale for Depression) | 5 (5) | ||||
|
| |||||
| CTQ (Childhood Trauma Questionnaire) Raw Scores Emotional (EA), Physical (PA) and Sexual (SA) Abuse Emotional (EN) and Physical (PN) Neglect | EA 11 (4) | PA 10 (4) | SA 7 (4) | EN 13 (4) | PN 10 (4) |
The study was performed under protocols approved by the Yale Human Investigation Committee, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Yale University Radiation Safety Committee, the Yale-New Haven Hospital Radiation Safety Committee, and the Yale MRI Safety Committee. Subjects were recruited from New Haven and surrounding areas by advertisement and word of mouth referrals. Written informed consent was obtained from all participants after a full explanation of study procedures (including risks and potential benefits).
2.2. Radiochemistry
[11C]P943 (R-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methyl-piperazin-1-yl)benzyl]-pyrrolidin-2-one) was prepared as previously described by N-methylation of the precursor with [11C]methyl triflate, using the PETtrace cyclotron and a TRACERLab™FxC automated synthesizer (GE Healthcare, Chalfont St. Giles, United Kingdom) (22). The GE Microlab® was employed in some of the preparations as a source of the requisite [11C]methyl iodide.
2.3. Scanning and Imaging Procedures
PET imaging was performed with the selective 5-HT 1B receptor antagonist radiotracer [11C]P943. All scans used a High-Resolution Research Tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA), which acquired 207 slices (1.2mm slice separation) with a reconstructed image resolution of ~3mm. A transmission scan with a 137Cs point source was obtained before the emission scan. The PET scans were acquired for 120min at rest following a mean (±SD) single bolus intravenous injection of 660 ± 114 MBq with a specific activity of 148 ± 78MBq/nmol.
Structural magnetic resonance images were performed on a Siemens 3-T Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for each subject to exclude individuals with anatomical abnormalities and for coregistration using an MPRAGE pulse sequence. The dimension and voxel size of MR images were 256 × 256 × 176 and 0.98 × 0.98 × 1.0 mm3, respectively.
Dynamic PET scan data were reconstructed with all corrections (attenuation; normalization; scatter; randoms; deadtime and motion), using the MOLAR algorithm (23) with the following frame timing: 6 × 30sec; 3 × 1min; 2 × 2min; 22 × 5min. Images were smoothed with a Gaussian filter at full width at half maximum (FWHM) of 3 mm and motion was corrected by either an optical detector (Vicra, NDI Systems, Waterloo, Ontario, Canada) or coregistered to each image frame with an early summed image (0–10 min post injection) using a 6-parameter mutual information algorithm (FLIRT, FSL 3.2, Analysis Group, FMRIB, Oxford, UK). The motion correction approach was added as a covariate in all analyses and no significant differences were found in any of the regions studied. As in previous [11C]P943 studies, the multilinear reference tissue model, MRTM2, was used to produce parametric images of BPND based on a cerebellum reference given the negligible levels of 5-HT 1B in this region(22).
A second summed image (0–10 min after injection) was created from the motion-corrected PET data and nonlinearly registered to the subject’s MR image to an MR template (Montreal Neurological Institute or MNI space). All transformations were performed on bioimage suite (version 2.5; http://www.bioimagesuite.com). Regions of interest were based on the Anatomical Automatic Labeling (AAL) template delineated on MR (24) with the exception of a hand-drawn ventral striatum template that was based on guidelines from Mawlawi et al.(25)
Given the exploratory nature of the research (and limited sample sizes and, hence power), nine primary regions of interest (ROIs) were selected a priori (the amygdala, anterior cingulate cortex, caudate, frontal cortex, hypothalamus, pallidum, putamen, thalamus and ventral striatum) based on the known regional densities of 5-HT 1B receptors and brain regions previously implicated as important in mediating cocaine’s effects and addiction processes.(26–28) Results were obtained by applying these template regions to individual parametric images that were nonlinearly resliced into template space using the PET to MR and the MR to template transforms.
In order to account for potential partial volume effects, a binary gray-matter mask (GMM) was also employed. Individual MR images were segmented with FAST (FMRIB’s Automated Segmentation Tool, v3.1) to obtain gray matter, white matter, and CSF masks. The individual GMM images were then applied to the candidate AAL template regions to obtain the mean regional values limited to gray matter voxels.
Voxel-based morphometry (VBM) was performed with structural data analyzed with FSL-VBM, (http://www.fmrib.ox.ac.uk/fslvbm), an optimized VBM protocol carried out with FSL tools. Structural images were first brain-extracted and gray-matter-segmented before being registered to the MNI 152 standard space using non-linear registration. The resulting images were flipped along the x-axis and averaged to create a left-right symmetric, study-specific gray matter template. Second, all native gray-matter images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated gray-matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3mm. Finally, voxelwise GLM was applied using permutation-based non-parametric testing, correcting for multiple comparisons across space.
Voxel wise parameter estimation of binding was generated with MRTM2 parametric images using the cerebellum as the reference region. Normalized BPND maps were statistically investigated to assess significant contrasts between the groups at every voxel, using independent sample t test analysis (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). The threshold for significant clusters was set to p < 0.001 uncorrected. This approach was aimed at confirming a priori differences in BPND at the voxel level without the potential limitations of ROI template placement.
2.5 Statistical Analysis
All outcomes were summarized descriptively and assessed for normality prior to analysis using normal probability plots and Kolmogorov test statistics. All outcomes were approximately normal. Linear mixed models were used to examine the independent and joint effects of group (between-subjects factor) and ROI (within-subjects) on BPND values. Group contrasts within each region were estimated to explain significant interactions. The best-fitting variance-covariance structure was assessed using information criteria. Secondary (i.e., exploratory) analyses included group comparisons of BPND levels in frontal subregions and the potential relationship between imaging and clinical measures (executed without adjustment for multiple tests due to small sample size and the exploratory nature of the comparisons). All analyses were conducted using SAS, version 9.1 (Cary, NC).
3. Results
As seen in Figure S1 (supplemental), results of the initial MRTM2 analysis in nine ROIs showed an overall group-by-region effect (F8,208= 2.91, P=0.004), with BPND reductions in CD individuals in the anterior cingulate (F1,208 = 7.11, P=0.008; −16%), hypothalamus (F1,208 =4.98, P=0.03; −16%) and frontal cortex (F1,208 = 3.05, P =0.08; −7%).
Figure 1 is a structural image of the gray matter differences between CD and HC subjects using a VBM analysis (highlighted is the CD-related decrease in gray matter). Table S1 shows the decreases in gray matter in CD subjects found with VBM (all differences found in frontal regions).
Figure 1.
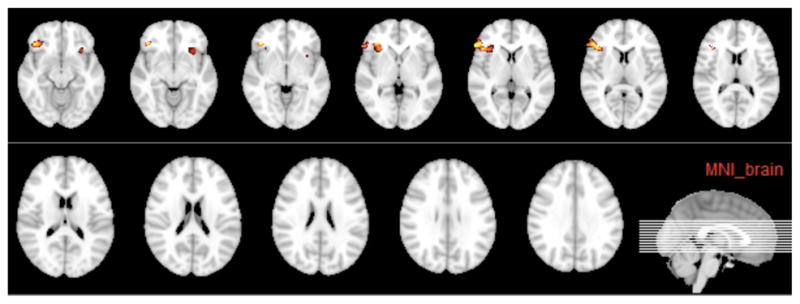
VBM analysis showing reductions in gray-matter volume in CD individuals (P<0.05, corrected).
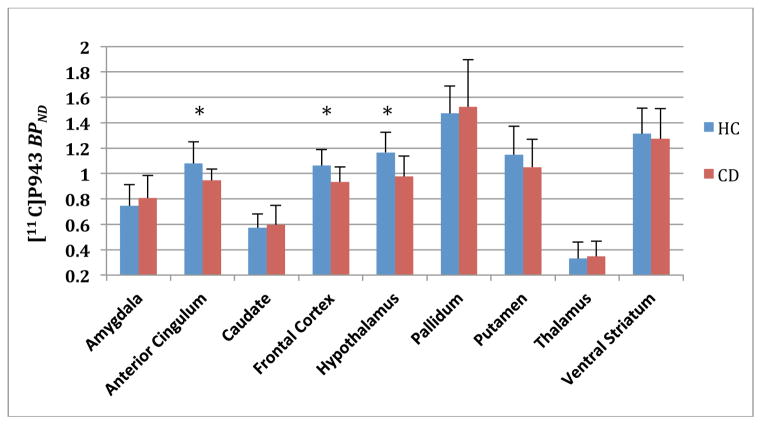
Subsequent analyses aimed at minimizing potential partial volume effects through GMM (Figure 2) resulted in similar group-by-region effects (F8,207 = 2.94, P=0.004) and emergence of statistically significant findings in the frontal cortex (F1,208 = 7.81, P=0.006; −14%) as well as confirmation of a significant difference in the anterior cingulate (F1,207 = 6.43, P=0.01; −14%) and hypothalamus (F1,207 =8.37, P =0.004; −20%).
Figure 2.
Region of interest analysis after gray matter masking (GMM) and associated mean [11C]P943 BPND values for HC (blue) and CD (red) subjects. Asterisks are statistically significant at P=0.01 or better. Error bars denote standard deviation.
Voxel-based results of whole brain analysis are shown in table 2 for each significant region. Whole-brain group-average parametric PET BPND images of HC and CD subjects (Figure S2) are shown for visual comparison.
Table 2.
Voxel based SPM results are shown with brain regions, corresponding Brodmann Areas (BA), T scores of the peak and mean voxels, cluster size (in number of voxels) and Montreal Neurological Institute (MNI) coordinates of the peak voxel for each cluster. Threshold set at P value <0.005 uncorrected and cluster size >50.
| Identified brain region | BA | Peak T Value | Mean T Value | Cluster size (voxels) | Peak voxel MNI coordinate (mm) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Orbitofrontal Cortex | 10,11 | 5.74 | 4.05 | 1454 | 14 | 68 | 0 |
| Superior and Middle Frontal Gyrus | 8,9,10 | 5.03 | 3.94 | 523 | 28 | 42 | 36 |
| Cingulate Gyrus | 31 | 4.81 | 3.90 | 156 | 10 | −44 | 34 |
| Temporal and Occipital Gyrus | 19,39,22,40,18 | 4.60 | 3.80 | 1168 | −58 | −72 | 26 |
| Cingulate Gyrus/Precuneus | 31 | 4.13 | 3.67 | 128 | −14 | −56 | 24 |
| Inferior and Middle Frontal Gyrus | 46,45,10 | 4.06 | 3.64 | 174 | 50 | 34 | 14 |
| Inferior and Middle Frontal Gyrus | 46,45,6,8,9 | 3.81 | 3.55 | 238 | 56 | 22 | 22 |
In exploratory post-hoc analyses (unadjusted p values), significant decreases were seen in CD subjects in multiple frontal cortical subregions with GMM (F1,130 = 7.38, P =0.008) (Figure S3). These results were generalized and non-specific. Within CD individuals, positive associations were observed between years of cocaine use and BPND in the amygdala (r = − 0.56; p =0.04), putamen (r = − 0.54; p =0.05), and thalamus (r = − 0.54; p = 0.05). Weekly cocaine use (in dollars spent) showed a statistical trend in the anterior cingulate (r = − 0.52; p = 0.06.). Daily nicotine use was significant in the frontal cortex (r = − 0.69; p = 0.01), putamen (r = − 0.65; p = 0.01) and ventral striatum (r = − 0.59; p = 0.03) with the anterior cingulate close to significance (r= − 0.51; p = 0.07). There was a correlation with the physical abuse subscale of the CTQ in the pallidum (r = − 0.62; p = 0.02) and the state subscale of STAI (r = − 0.61; p = 0.04) in the thalamus, but no other factors examined in measures of past trauma (CTQ), anxiety (STAI), impulsivity (BIS-11), or depression (HAM-D) were significant. In addition, days since last cocaine use and alcohol use were also not correlated with regional brain BPND availability within this cohort.
4. Discussion
To our knowledge, this study is the first to examine 5-HT 1B receptor availability in human cocaine dependence. The results suggest reductions in 5-HT 1B receptor availability in the hypothalamus, anterior cingulate, and frontal cortex of CD subjects. In two of these regions (i.e., hypothalamus and anterior cingulate), results were significant whether corrected for gray matter volume or not. In the case of the frontal cortex, initial differences in 5-HT 1B availability were non significant (p<0.08) but emerged as significant after efforts to minimize potential partial volume effects (a factor that may pertain to some, but not all frontal cortical subregions). Whole brain voxel-wise parameter analysis confirmed these results in frontal cortex and anterior cingulate.
Given that the CD subjects had not used cocaine on average 6 days before the scans, these results could support a down-regulation (or desensitized) model of 5-HT 1B in early abstinence cocaine dependence. These decreases are likely not in isolation however, as preclinical work has shown that the 5-HT 1B system is largely dependent on the stage of the addiction cycle with cocaine administration and abstinence increasing and reducing 5-HT 1B mRNA expression respectively.(29, 30) Mechanistic explanations for these changes are not fully known, but reductions in 5-HT 1B receptor availability in CD could be due to 5-HT 1B down regulation or also preexisting differences or higher levels of extracellular 5-HT in CD. Any possible preexisting differences in CD could be supported by a host of genetic studies in addiction (13, 31) and inherent 5-HT 1B heterogeneity could potentially alter dopamine activity in reward areas by reducing inhibition of GABA release. (30) Potential extracellular 5-HT affects on 5-HT 1B are also an important consideration and given that 5-HT levels were increased in a human post-mortem CD sample (32) and [11C]P943 has been shown to be sensitive to endogenous 5-HT in the nonhuman primate (33), elucidation of this possible affect should be made to further interpretations of 5-HT 1B in CD.
Clinical work using this same radiotracer, interestingly, found an increase in 5-HT 1B receptor availability in the ventral striatum/pallidum in alcohol-dependent subjects (34). Additionally, 5-HT 1B receptor availability in the ventral striatum/pallidum, putamen and anterior cingulum correlated positively with problem-gambling severity in pathological gambling (35). These findings are consistent with 5-HT 1B receptor involvement in the mesocorticolimbic pathway in addiction, and the ventral striatum, a key area in this pathway, has generally been found to be altered with cocaine and 5-HT 1B receptor function (12). Given prior findings, our failure to find differences in the ventral striatum in our CD cohort was surprising. In fact, because of potential limitations of the AAL template (globus pallidus/nucleus accumbens) with respect to the precise delineation of ventral striatal regions, we specifically applied a hand-drawn ventral striatum template using methods previously validated by other groups for PET radiotracer analyses. Findings were nonetheless negative despite such specialized approaches.
The differences in CD that were found in the current study (i.e. hypothalamus, anterior cingulate and frontal cortex) all showed a decreased BPND, while the previous studies of alcohol dependence and pathological gambling using the [11C]P943 radiotracer (34, 35) found an increase in 5-HT 1B receptor availability in the ventral striatum. These findings are important for multiple reasons. First, It is possible that apparent differences in the studies reflect the time of abstinence (while no differences were found within group with the last day of cocaine use, this study had a short window of abstinence of 6 days vs. 4 weeks for the alcohol-dependence study). Secondly, the current findings may suggest that 5-HT 1B receptors contribute uniquely to different addictions, perhaps in a manner related to the specific effects of each drug of abuse. The apparent 5-HT 1B- receptor-related differences in CD and alcohol-dependent subjects suggest that the findings are not attributable to alcohol use in the CD group, an idea supported by the lack of correlation between ETOH use and 5-HT 1B BPND in this study (it is interesting to note, however, the correlation of active daily nicotine use across multiple brain regions, which could be a proxy for cocaine use or alternatively may provide preliminary evidence that nicotine may be have similar effects on 5-HT 1B as cocaine.) Lastly, 5-HT 1B receptors may contribute to clinically relevant states relating to cocaine use. For example, given the reductions in 5-HT 1B receptor availability observed in major depression (16), 5-HT 1B- receptor function may contribute to negative affective states observed in cocaine dependence (although we found little evidence for 5-HT 1B correlations with clinically based depression, anxiety and trauma measures in this cohort). These factors, alone or in sum, are similar to the differences found to be important in 5-HT 1B animal addiction models and could account for the seemingly divergent results of the clinical addiction imaging studies of 5-HT 1B.
Observations of reduction in 5-HT 1B receptor binding in the hypothalamus is a potentially very interesting finding, given the increasing appreciation of this region’s role in the neurobiology of addiction. The hypothalamus has high levels of 5-HT 1B receptor binding in rats (36), and is also associated with alterations in 5-HT during cocaine self-administration (37) as well as reduced 5-HT 1A receptors following cocaine binge behavior (38). Our findings are intriguing given that 5-HT 1B knock-out mice have also been found to be hyperreactive to mild stress (39), which is important in cocaine dependence considering the role stress and the hypothalamic–pituitary–adrenal (HPA) axis have in addiction and relapse (40).
Studies have increasingly identified abnormalities in frontal cortical functioning in CD individuals, including abnormalities identified by both functional (e.g., PET) (41) and structural neuroimaging methods. In regards to the latter, structural MRI studies have previously produced contradictory results in regards to gray matter changes, with some suggesting generalized reductions (42), others noting reductions in frontal cortex alone (43, 44) and others failing to find changes altogether (45). Given inconsistencies in the literature, we conducted VBM analyses directly in the current cohort as a prerequisite to potential partial volume corrections. Our VBM results demonstrated significant and moderate reductions of gray matter in the frontal cortex (including the right superior and inferior gyri, and left inferior gyrus) of CD subjects (in accordance with prior findings (43, 44)).
Based on these results, we applied GMM (using a segmented MRI) to control for between-group differences in frontal cortical gray matter volume (using analyses of unaffected regions as a negative control). Consistent with predictions, BPND differences emerged as statistically significant in the frontal cortex following GMM (and as importantly, findings in other regions remained unchanged). The whole brain voxel-wise analysis was performed as a confirmatory step and with this and the VBM analyses, we conducted an exploratory post-hoc analysis of frontal cortical subregions (including, inferior, middle, and superior left/right frontal ROIs) in an effort to determine whether 5-HT 1B reductions might be confined to areas of gray-matter change. However, results failed to suggest subregional selectivity to these differences (i.e., differences in all frontal subregions were significant post-hoc).
The implications of the anterior cingulate and frontal 5HT1B reductions are largely speculative because previous preclinical work has been noticeably silent as to whether cortical 5-HT 1B receptor differences exist in CD. Interestingly, however, 5-HT 1B receptors have a relatively high density in the frontal brain regions, and a 5-HT 1B agonist has been found to reduce aggression and impulsivity in mice when injected into the prefrontal cortex (46). Chronic cocaine users have been shown to have impairments in the cognitive control of these and other executive functions (e.g., error and conflict monitoring, response inhibition, outcome expectancies) and numerous neuroimaging studies have implicated the anterior cingulum and frontal cortex with these deficits in cocaine and stimulant dependence (47).
Lastly, while the current work focused on 5-HT 1B, this is the first human PET data to investigate serotonin dysfunction in CD of any subtype and these differences should not be interpreted as necessarily exclusive and specific in regards to serotonin. Indeed, the potential importance of other serotonergic mechanisms (e.g., 5-HT 2C receptor) in CD and related risk factors (48) corroborates the potential importance of our findings in a growing body of cocaine research focused on serotonin. Future studies could improve upon the current limitations by having prospectively matched controls with corresponding amounts of nicotine use (given the possibility of this confound in the current study) and larger sample sizes. It also remains to be determined whether cognitive differences may be attributable to 5-HT 1B receptor function, and future investigations could focus on neuropsychological measures of executive function as well as exploring whether 5-HT 1B-receptor agonists have an influence on cognitive function. Thus, future studies will be required to more definitively address the nature of potential changes in 5-HT 1B regional availability and to directly examine the extent to which the 5-HT 1B receptor represents a new and potentially clinically relevant molecular target for the treatment of cocaine dependence.
Supplementary Material
Footnotes
Conflicts of Interest and Disclosures: Dr. Bhagwagar is currently an employee of Bristol-Myers Squibb, but this research predated his employment there. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Lundbeck and Ironwood; has received research support from the National Institutes of Health, Mohegan Sun Casino, the National Center for Responsible Gaming, and Psyadon pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling and legal entities on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program and The Connection; has performed grant reviews for the National Institutes of Health and other agencies; has guest edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. All other authors report no biomedical financial interests or potential conflicts of interest related to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Administration SAaMHS; Administration SAaMHS, editor. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: NSDUH; 2011. [Google Scholar]
- 2.Ruf BM, Bhagwagar Z. The 5-HT 1B receptor: a novel target for the pathophysiology of depression. Current drug targets. 2009;10:1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- 3.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Mostany R, Pazos A, Castro ME. Autoradiographic characterisation of [35S]GTPgammaS binding stimulation mediated by 5-HT 1B receptor in postmortem human brain. Neuropharmacology. 2005;48:25–33. doi: 10.1016/j.neuropharm.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Miszkiel J, Filip M, Przegalinski E. Role of serotonin (5-HT)1B receptors in psychostimulant addiction. Pharmacological reports: PR. 2011;63:1310–1315. doi: 10.1016/s1734-1140(11)70695-8. [DOI] [PubMed] [Google Scholar]
- 6.O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. The Journal of pharmacology and experimental therapeutics. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- 7.Parsons LH, Weiss F, Koob GF. Serotonin1b receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology. 1996;128:150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- 8.Przegalinski E, Filip M, Papla I, Siwanowicz J. Effect of serotonin (5-HT)1B receptor ligands on cocaine sensitization in rats. Behavioural pharmacology. 2001;12:109–116. doi: 10.1097/00008877-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, et al. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher PJ, Azampanah A, Korth KM. Activation of 5-HT (1B) receptors in the nucleus accumbens reduces self-administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav. 2002;71:717–725. doi: 10.1016/s0091-3057(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 11.Barot SK, Ferguson SM, Neumaier JF. 5-HT (1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. The European journal of neuroscience. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- 12.Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL. Protracted Withdrawal from Cocaine Self-Administration Flips the Switch on 5-HT (1B) Receptor Modulation of Cocaine Abuse-Related Behaviors. Biological psychiatry. 2012;72:396–404. doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, et al. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenetics and genomics. 2006;16:25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson SM, Sandygren NA, Neumaier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptibility to amphetamine. The European journal of neuroscience. 2009;30:1576–1584. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Archives of general psychiatry. 2011;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology. 2011;213:547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matuskey D, Pittman B, Planeta-Wilson B, Walderhaug E, Henry S, Gallezot JD, et al. Age Effects on Serotonin Receptor 1B as Assessed by PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2012;53:1411–1414. doi: 10.2967/jnumed.112.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 21.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 22.Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, et al. Kinetic modeling of the serotonin 5-HT (1B) receptor radioligand [(11)C]P943 in humans. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson REBW, Liow J-S, Adler S, Johnson CA. Design of a motion-compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction of the HRRT. Conf Record IEEE Nuclear Science Symposium and Medical Imaging Conference; Portland, OR. 2003. pp. 3281–3285. [Google Scholar]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 28.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 29.Neisewander JL, Cheung TH, Pentkowski NS. Dopamine D3 and 5-HT receptor dysregulation as a result of psychostimulant intake and forced abstinence: Implications for medications development. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumaier JF, McDevitt RA, Polis IY, Parsons LH. Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. J Neurochem. 2009;111:217–227. doi: 10.1111/j.1471-4159.2009.06313.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, et al. Association study of novel human serotonin 5-HT (1B) polymorphisms with alcohol dependence in Taiwanese Han. Biological psychiatry. 2002;51:896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- 32.Little KY, Patel UN, Clark TB, Butts JD. Alteration of brain dopamine and serotonin levels in cocaine users: a preliminary report. The American journal of psychiatry. 1996;153:1216–1218. doi: 10.1176/ajp.153.9.1216. [DOI] [PubMed] [Google Scholar]
- 33.Cosgrove KP, Kloczynski T, Nabulsi N, Weinzimmer D, Lin SF, Staley JK, et al. Assessing the sensitivity of [(1)(1)C]p943, a novel 5-HT 1B radioligand, to endogenous serotonin release. Synapse. 2011;65:1113–1117. doi: 10.1002/syn.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, et al. Serotonin 1B receptor imaging in alcohol dependence. Biological psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potenza MN, Walderhaug E, Henry S, Gallezot JD, Planeta-Wilson B, Ropchan J, et al. Serotonin 1B receptor imaging in pathological gambling. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2011 doi: 10.3109/15622975.2011.598559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makarenko IG, Meguid MM, Ugrumov MV. Distribution of serotonin 5-hydroxytriptamine 1B (5-HT (1B)) receptors in the normal rat hypothalamus. Neuroscience letters. 2002;328:155–159. doi: 10.1016/s0304-3940(02)00345-2. [DOI] [PubMed] [Google Scholar]
- 37.Smith JE, Koves TR, Co C. Brain neurotransmitter turnover rates during rat intravenous cocaine self-administration. Neuroscience. 2003;117:461–475. doi: 10.1016/s0306-4522(02)00819-9. [DOI] [PubMed] [Google Scholar]
- 38.Perret G, Schluger JH, Unterwald EM, Kreuter J, Ho A, Kreek MJ. Downregulation of 5-HT 1A receptors in rat hypothalamus and dentate gyrus after “binge” pattern cocaine administration. Synapse. 1998;30:166–171. doi: 10.1002/(SICI)1098-2396(199810)30:2<166::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Corticosterone responses in 5-HT 1B receptor knockout mice to stress or 5-HT 1A receptor activation are normal. Psychopharmacology. 2001;153:484–490. doi: 10.1007/s002130000598. [DOI] [PubMed] [Google Scholar]
- 40.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, et al. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PloS one. 2011;6:e16573. doi: 10.1371/journal.pone.0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 43.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 44.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayana PA, Datta S, Tao G, Steinberg JL, Moeller FG. Effect of cocaine on structural changes in brain: MRI volumetry using tensor-based morphometry. Drug Alcohol Depend. 2010;111:191–199. doi: 10.1016/j.drugalcdep.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT (1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology. 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- 47.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, et al. Synergism between a serotonin 5-HT 2A receptor (5-HT 2AR) antagonist and 5-HT 2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS chemical neuroscience. 2013;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.