Abstract
Summary
The Hajdu–Cheney syndrome is a very rare disease that affects several organ system, leading to severe osteoporosis and other abnormalities. We describe clinical and genetic findings of nine patients with this disease.
Introduction
The Hajdu–Cheney syndrome (HCS) is a rare autosomal dominant disorder characterized by severe osteoporosis, acroosteolysis of the distal phalanges, renal cysts, and other abnormalities. Recently, heterozygous mutations in NOTCH2 were identified as the cause of HCS.
Methods
Nine patients with typical presentations of HCS took part in this study: five affected patients from two small families and four sporadic cases. Peripheral blood DNA was obtained and exome sequencing performed in one affected individual per family and in all four sporadic cases. Sanger sequencing confirmed mutations in all patients.
Results
One of the identified mutations was introduced in a plasmid encoding NOTCH2. Wild-type and mutant NOTCH2 were transiently expressed in HEK293 cells to assess intracellular localization after ligand activation. Deleterious heterozygous mutations in the last NOTCH2 exon were identified in all patients; five of the six mutations were novel.
Conclusion
Consistent with previous reports, all mutations are predicted to result in a loss of the proline/glutamic acid/serine/threonine sequence, which harbors signals for degradation, therefore suggesting activating mutations. One of the six mutations furthermore predicted disruption of the second nuclear localization signal of NOTCH2, but the mutant revealed normal nuclear localization after transfection, which is consistent with the proposed gain-of-function mechanism as the cause of this autosomal dominant disease. Our findings confirm that heterozygous NOTCH2 mutations are the cause of HCS and expand the mutational spectrum of this disorder.
Keywords: Acroosteolysis, Exome sequencing, Hajdu–Cheney syndrome, NOTCH2, Osteoporosis
Introduction
The Hajdu–Cheney syndrome (HCS) was first described in 1948 by Hajdu and Kauntze [1] and later by Cheney in 1965 [2]. This rare disorder is characterized by acroosteolysis of the distal phalanges, severe osteoporosis often leading to multiple fractures, craniofacial and dental abnormalities, and a variety of other findings including renal cysts. Most cases of HCS are sporadic, but rare familial forms with autosomal dominant mode of inheritance have been described [3]. Attempts to determine the underlying genetic defect through positional cloning were hampered by the lack of large families with multiple members affected by HCS.
Recently, disease-causing mutations in the gene encoding NOTCH2, located on chromosome 1, were discovered through exome sequence analyses [4, 5]. The single-pass transmembrane protein NOTCH2 is a receptor in an evolutionary highly conserved cell–cell signaling pathway that relays signals between cells during development and in adult tissues. Binding of the single-pass membrane ligand of the Delta/Jagged family of proteins, presented by a neighboring cell, leads to activation of the NOTCH2 receptor. Through several enzymatic steps, NOTCH2 is cleaved and the Notch intracellular domain (NICD) released. NICD subsequently translocates to the nucleus where it leads to displacement of transcriptional repressors, thereby activating downstream target genes. NICD contains several domains, which include two nuclear localization sequences, a transactivation domain, and a conserved proline/glutamic acid/serine/threonine-rich motif (PEST).
Here, we describe phenotypic and genetic findings in two families with autosomal dominant HCS and four sporadic cases affected by this disease.
Methods
Patients
Patients were treated at Massachusetts General Hospital or at the NIH Clinical Center. Informed consent was obtained in accordance with protocols approved by the institutional review boards of the Massachusetts General Hospital, Boston, MA, USA and the National Institutes of Health, Bethesda, MD, USA. Clinical data were collected from medical records. Previously obtained radiographs and other available imaging studies were reviewed.
Bone histology
A piece of maxillary bone from patient HCS05 was obtained as surgical waste from a reconstructive craniofacial surgery to correct dental malocclusion and micrognathia. The tissue was fixed with 4 % paraformaldehyde, decalcified, embedded in paraffin, and processed for histology using standard techniques.
DNA extraction
Genomic DNA from peripheral blood leukocytes was extracted using proteinase K digestion followed by phenol–chloroform extraction and isopropanol precipitation as described [6].
Exome sequencing
Libraries were constructed from DNA from all four sporadic cases, and from one affected member in families HCS01 and HCS02, respectively. Exome sequencing was performed using the Agilent SureSelect Human All Exon Kit v2 [7] followed by massively parallel sequencing using Illumina HiSeq Sequencer. Sequence data were processed as described [8] using the PICARD data processing pipeline and the Firehose Pipeline of the Broad Institute, Cambridge, MA, USA. Variant calling was analyzed using GATK (Broad Institute) and snpEff [9].
Sanger resequencing
Intronic primers were used to amplify exon 34 of the gene-encoding NOTCH2 (primers available on request). The products were sequenced with Applied Biosystems Taq DyeDeoxy Terminator cycle sequencing kits and analyzed with the ABI 3730XL DNA Analyzer. Nucleotide sequences were analyzed and compared to UCSC refseq sequence (ID: NM_024408) using the software Sequencher (Gene Codes Corporation, Ann Arbor, MI, USA).
Construction of plasmid DNA encoding a NOTCH2 mutant
The plasmid hN2pcAMP encoding full-length human NOTCH2 was a gift from Dr. Spyros Artavanis-Tsakonas (Harvard Medical School, Boston, MA, USA). A BglII-BstEII fragment was PCR-amplified (right primer 5′-CACCGTGTTGACTTCTGCTC-3′, left primer 5′-GCAGAGTGGGGTGATGAACT-3′) and cloned into the TA vector using the TOPO TA Cloning Kit (Invitrogen, K4575). The NOTCH2 mutation c.6383delG was then introduced into this fragment by site-directed mutagenesis using QuikChange (Stratagene, La Jolla, CA, USA). The presence of the nucleotide change and the absence of additional mutations were confirmed by nucleotide sequence analysis. The BglII-BstEII fragment containing the mutation was swapped with the wild-type fragment in plasmid hN2pcAMP and the resulting vector named hN2_6383delG/pcAMP.
Immunohistochemistry
Using FuGENE 6 (Roche Applied Science, Indianapolis, IN, USA), HEK293 cells were transiently transfected with hN2pcAMP encoding wild-type NOTCH2 or with hN2_6383delG/pcAMP containing the mutant NOTCH2. Immunohistochemistry was performed as described [10] using an anti-NOTCH2 antibody (Sigma, SAB4502020, 1:200) as primary antibody and Alexa Fluor 488-labeled goat anti-rabbit IgG 1:1,000 (Invitrogen catalog #A11008) as secondary antibody. All slides were mounted with DAPI-containing mounting medium (Vector Laboratories, H-1200) and analyzed.
Results
Clinical characteristics
Family HCS01 has three affected members and the mode of inheritance is consistent with an autosomal dominant pattern (Fig. 1d). All affected individuals have typical disease characteristics (Table 1), including acroosteolysis (Fig. 1b). The female index case HCS01/12 suffered numerous fragility fractures; at age 44, her lumbar spine T-score was −5.0, but she remained fully functional and worked as a nurse. She died at age 46 of unknown causes. Her son HCS01/21 is severely disabled; his past medical history is significant for basilar invagination leading to intermittent respiratory arrests. He required surgical removal of the odontoid and posterior occiput to C5 arthrodesis. Because of his severe scoliosis, he suffers from restrictive lung disease and requires continuous noninvasive mechanical ventilation. He experienced multiple fractures; his forearm T-score is −8.0. His brother HCS01/22 has much milder manifestations of HCS. His PA spine T-score is −3.1 and he has also suffered multiple fractures, but remains fully functional and participates in several sports.
Fig. 1.
Clinical characteristics of patients with HCS and pedigree of HCS01. a Radiograph of the hand of the affected individual HCS03. Acroosteolysis with vanishing bones of the distal phalanges is pronounced at the second and third finger (arrows). b Hand photograph of affected mother and the two affected sons of family HCS01. Note the shortened fingers, especially the terminal phalanges with bulbous fingertips. c Craniofacial bone in HCS. Low-power view of a piece of maxilla removed at the time of craniofacial surgery from patient HCS05 revealed large areas of lamellar bone (lb), as is typical of maxilla, interspersed with small islands of woven (wb), which is atypical. Illumination with polarized light confirmed the orientation of collagen fibers within the bone (far right panels). Although maxillary bone has few marrow spaces, none were seen in this specimen; instead, fibrous tissue (ft) surrounded the trabeculae of lamellar and woven bone. Large, empty vascular-like structures (asterisks) were also noted, and are not typically found in maxillary bone. Areas of woven bone were hypercellular (double asterisks), and Sharpey fiber-like structures were present (arrow). Preexisting microfractures (arrowhead) were also observed. d Pedigree of family HCS01. The mother, who is the index case (arrow) and both sons are affected (solid symbols)
Table 1.
Clinical characteristics of patients with HCS studied in this report
| Patient | HCS01/12 | HCS01/21 | HCS01/22 | HCS02/12 | HCS02/21 | HCS03 | HCS04 | HCS05 | HCS06 |
|---|---|---|---|---|---|---|---|---|---|
| Gender | F | M | M | F | F | M | M | M | M |
| Age | 46 | 27 | 22 | 62 | 36 | 51 | 42 | 14 | 24 |
| Craniofacial | x | x | x | x | x | x | x | x | |
| Dental | x | x | x | x | x | x | x | x | |
| Acroosteolysis | x | x | x | x | x | x | x | x | x |
| Osteoporosis | x | x | x | x | x | x | x | x | x |
| Fractures | x | x | x | x | x | x | x | x | x |
| Short stature | x | x | x | x | x | x | x | ||
| Cystic kidneys | x |
Family HCS02 comprises an affected female (HCS02/12) and her affected daughter (HCS02/21). HCS02/12 suffered from cervical subluxation at C5–6 level with cord compression, which required surgical fusion. She experienced numerous fractures, which decreased in frequency when she reached her 30s. Her acroosteolysis did not become apparent until she reached her mid-20s and progressed slowly; she also has generalized joint laxity. The daughter (HCS02/21) has much milder manifestations of the disease.
HCS03, HCS04, and HCS05, and HCS06 are sporadic HCS cases, who presented with varying degrees of severity, yet share the typical characteristics of the disease (Table 1). The hand radiograph of HCS03 shows the typical acroosteolysis (Fig. 1a). In addition to the classic craniofacial and skeletal features, HCS05 was also found to have intestinal malrotation and mild hypospadias, both of which are uncommon but previously reported in HCS (3, 4). Patient HCS05 received bisphosphonates from age 6 to 12; the bone specimen (see below) was acquired at age 14 years. HCS06 has been described in detail elsewhere [11].
Bone histology
Examination of the maxillary bone from patient HCS05 (Fig. 1c) revealed areas of large lamellar trabecular bone commonly seen in normal maxillary bone, but interspersed with areas of woven bone, as revealed by polarized light microscopy. Areas of woven bone were also hypercellular, with many instances of empty lacunae, more so than what is seen in normal maxillary bone. There were no discernable osteoblasts or osteoclasts on the surface of the bone. On occasion, Sharpey fiber-like structures were seen, as well as microfractures across the trabeculae that are not processing artifacts. Marrow spaces in the maxilla are usually filled with adipocytes and small islands of hematopoiesis. Instead, this affected sample contained loose fibrous tissue throughout, with large, empty vascular-like structures that are also not seen in normal maxillary bone.
Genetic findings
Exome sequencing, which was performed while disease-causing mutations in NOTCH2 were reported [4, 5], produced 32 megabases per individual with an average coverage of 150×. More than 80 % of targeted exons had a coverage >20×. The number of missense, nonsense, indels, and splice mutations not found in dbSNP 131 is listed in Table 2. The only gene that revealed heterozygous mutations that were predicted to be deleterious (missense, nonsense, indels, and splice site mutations) in all six exomes was NOTCH2. These NOTCH2 mutations (Table 3), all located in exon 34, were confirmed by Sanger sequence analysis for all nine HCS patients. Five of the identified mutations were novel; the nonsense mutation c.7198C>T had been described previously [5].
Table 2.
Exome sequencing: number of deleterious variants found in patients with HCS
| Number of individuals | # Genes with novel missense mutations | # Genes with novel nonsense mutations | # Genes with novel indels | # Genes with novel splice site mutations |
|---|---|---|---|---|
| 1 | 1,539 | 58 | 461 | 35 |
| 2 | 192 | 4 | 156 | 7 |
| 3 | 49 | 3 | 103 | 4 |
| 4 | 22 | 1 | 86 | 2 |
| 5 | 14 | 1 | 64 | 1 |
| 6 | 10 | 0 | 30 | 0 |
Table 3.
Mutations identified in exon 34 of NOTCH2 in patients with HCS
| Individual | Mutation | Protein |
|---|---|---|
| HCS01/12/21/22 | c.6450delT | Val2151LeufsX4 |
| HCS02/12/21 | c.6383delG | Gly2128ValfsX8 |
| HCS03 | c.6586C>T | Gln2196X |
| HCS04 | c.6578-6588delTCCATGCCCAG | Val2193AlafsX7 |
| HCS05 | c.6662-6663delTG | Val2221GlufsX22 |
| HCS06 | c.7198C>T | Arg2400X |
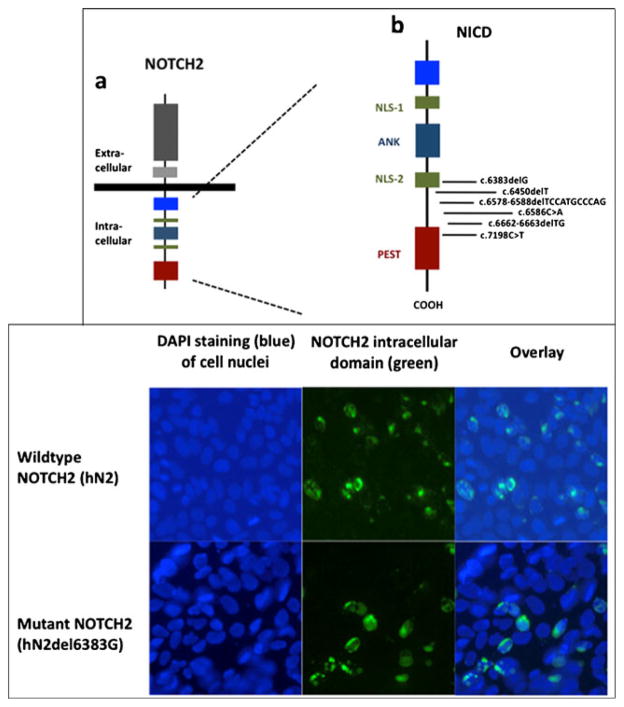
Of the six different NOTCH2 mutations, two were nonsense mutations; four were deletions comprising 1, 2, and 11 bp, respectively, which led to a shift in the open-reading frame with subsequent translation into unrelated amino acid sequence, followed by a termination codon (see Table 3). All six mutations resulted in deletion of the carboxyl-terminal PEST sequence. The mutation c.7198C>T is located directly within the PEST sequence, leading to Arg2400X (Fig. 2, upper panel). The mutation identified in HCS02, c.6383delG (leading to Gly2128GlufsX2), is located within the last nuclear localization signal.
Fig. 2.
Upper panel: a domain structure of NOTCH2 and b localization of the identified mutations within the NOTCH intracellular domain (NICD). NLS-1 proximal nuclear localization domain, NLS-2 distal nuclear localization domain, ANK ankyrin repeats, PEST pro-line/glutamic acid/serine/threonine-rich domain. NOTCH2 is a large single-pass type I transmembrane receptor protein, which is activated by binding to a ligand presented by a neighboring cell. Upon activation, the NCID is cleaved off and released, enters the nucleus, and derepresses transcription of target genes. PEST domain targets the molecule for proteasomal degradation. The mutations found in patients with HCS lead to truncation of the molecule at the very carboxyl-terminal end and loss of the PEST sequence. Lower panel: nuclear localization of wild-type and mutant NOTCH in transfected HEK293 cells. HEK 293 cells were transiently transfected with plasmid encoding wild-type NOTCH2 (upper row hN2) or with plasmid encoding NOTCH2 harboring the mutation identified in HCS02 (lower row hN2del6383G). Blue DAPI staining of cell nuclei. Immunohistochemistry using anti-NOTCH2 antibody and green Alexa Fluor 488. Overlay demonstrated nuclear localization of the mutant NOTCH2 that was not different from wild-type
We tested the hypothesis that mutation c.6383delG disrupts the nuclear localization signal. Immunohistochemical analysis of transfected HEK293 cells revealed that nuclear localization of the mutant was indistinguishable from that of wild-type NOTCH2 (Fig. 2, lower panel).
Discussion
In this study, we examined nine patients with Hajdu–Cheney syndrome and identified the genetic mutations responsible for clinical manifestations of the syndrome. All nine patients, five patients from two families and four additional sporadic cases, had the typical clinical findings of HCS, a rare disease characterized by focal osteolysis, severe osteoporosis, craniofacial characteristics, and numerous other abnormalities. Through exome sequencing of one affected family member from each of the two families and all sporadic cases, we identified deleterious mutations in exon 34 of NOTCH2 in all patients. Five of these mutations were novel; the mutation found in our patient HCS06 was identical to a mutation reported previously [5] (designated HCS-03 in the previous report).
Signaling through the plasma-spanning NOTCH receptors is a critical and highly conserved pathway determining cell fate decisions and important cell functions in various tissues [12]. Several developmental and multisystem diseases are caused by mutations in the NOTCH signaling system [13]. Binding of the single-pass ligand of the Delta or Jagged family expressed on a neighboring cell triggers the cleavage of the NICD, which translocates to the nucleus and activates transcriptional programs. Termination of the NOTCH signal is achieved by targeting NICD for proteasomal degradation.
All six mutations identified in our study were truncating mutations: two were nonsense mutations and four were frameshift mutations. Because these mutations were located in the last exon, the resulting mRNA is predicted to escape nonsense mediated decay [14]. They were all clustered between the last nuclear localization signal (NLS) and the PEST sequence of NOTCH2 (Fig. 2, upper panel), likely resulting in removal of the PEST sequence. These sequences rich in proline (P), glutamic acid (E), serine (S), and threonine (T) promote the rapid intracellular degradation of the protein containing them [15]. The absence of this destabilizing PEST motif is therefore predicted to lead to a longer half-life of the protein, hence a “gain-of-function.” This concept is supported by two experimental findings: The same mutation that was found in our patient HCS06, Arg2400X, was observed in 3 out of 109 patients with B cell lymphoma; in a Notch-sensitive luciferase reporter assay, luciferase activity was significantly increased in CHO cells carrying this mutation (named nsmN2) [16]. Secondly, mutations that remove the PEST sequence of the closely related NOTCH1 also lead to an increase in activity in vitro in luciferase assays. In addition, these investigators could demonstrate a longer half-life of the protein using metabolic labeling studies [17]. Our findings are fully consistent with three recent reports describing mutations in the last exon of NOTCH2 that are all predicted to lead to a loss of the PEST sequence and therefore a gain-of-function in patients with HSC [4, 5, 18].
Two of the six mutations that we identified in our patient cohort are noteworthy: the nonsense mutation c.7198C>T in HCS06 was located directly within the PEST sequence. However, it still truncates the majority of the PEST motif (Fig. 2, upper panel), consistent with our proposed model. Mutation c.6383delG found in HCS02 is notable because it is not located distal to, but rather within the last NLS (Fig. 2, upper panel), possibly leading to a disruption of this NLS. The NICD of NOTCH2 possesses two putative nuclear localization signals. Since the mutation c.6383delG lies within the second (distal) NLS of NOTCH2 and possibly interrupting it, we tested the hypothesis that c.6383delG in NOTCH2 does not interfere with the nuclear localization. Our immunohistochemistry studies do confirm this hypothesis which is necessary (but not sufficient) for the “gain-of-function” explanation of disease. In line with our results are detailed studies of the function of the two very similar NLS motifs in closely related NOTCH1. They demonstrated that deletion of the proximal, but not distal NLS motif, leads to the inability of the NOTCH1 intracellular domain to enter the nucleus, a prerequisite for its proper function [19].
Also recently, serpentine fibula polycystic kidney syndrome has been shown to be caused by mutations in the last exon of NOTCH2, providing evidence that these two conditions are allelic variants and that the phenotypic variability of the disease can be significant [20].
Notch signaling is important in early development (somite formation) of the skeleton, as well as for the differentiation and function of osteoblasts and osteoclasts in postnatal life. The precise mechanism leading to the predominant bone phenotype in HCS, especially the significant osteoporosis and the acroosteolysis that is often apparent later in life, is unclear [21]. Notch signaling and the results of its perturbations are context and cell-stage specific. For example, in the mouse, activation of Notch under the control of the Col2.3 promoter leads to osteosclerosis and development of woven bone due to increased proliferation of immature osteoblast [22]. In contrast, activation of Notch in early osteoblasts under the control of the osterix or Col3.6 promoter leads to an initial decrease in osteoblast function and resulted in low bone volume [23].
Notch has inhibitory effects on osteoclastogenesis and is required for RANKL-mediated osteoclast differentiation [24]. Notch2, in collaboration with NF-kB, regulates the expression of NFATc1, a key transcription factor in osteoclastogenesis. Increased osteoclastogenesis through Notch2 activation is therefore a possible explanation for the bone loss in HCS [25].
The affected bone in patient HCS05 that was available for histological analysis was characterized by a mixture of large, lamellar trabeculae interspersed with islands of woven bone, both surrounded by a loose fibrous tissue rather than yellow marrow. The woven bone was hypercellular and many empty lacunae were noted. Not only were active osteoblasts absent, but also osteoclasts, suggesting a low bone turnover, in spite of the age of the patient. These findings could be related to the 6 years of treatment with bisphosphonate, although the patient had not been treated for 2 years prior to the time of surgery.
In conclusion, we identified heterozygous mutations in NOTCH2 exon 34 in each of the affected individuals of two families, and in four sporadic HCS patients; five of these mutations were novel. All six mutations are predicted to lead to a loss of the protein-destabilizing PEST sequence and therefore to a gain-of-function. Our findings confirm that heterozygous NOTCH2 mutations are the cause of HCS and expand the mutational spectrum of this disorder.
Acknowledgments
We like to thank all patients and family members who participated in this study. We thank the Broad Institute for generating high-quality sequence data supported by NHGRI funds (U54 HG003067, PI Eric Lander), Harald Jüppner for support and Spyros Artavanis-Tsakonas for the Notch2 cDNA plasmid. This research was supported, in part, by grants from the National Institute of Diabetes and Digestive and Kidney Disease (K08-DK081669-01 to M.M.) and by the Division of Intramural Research, NIDCR, a part of the Intramural Research Program of the NIH, DHHS.
Footnotes
Conflicts of interest None.
Contributor Information
W. Zhao, Endocrine Unit, Thier 10, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114, USA
E. Petit, Endocrine Unit, Thier 10, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114, USA
R. I. Gafni, CSDB, NIDCR, National Institutes of Health, Bethesda, MD, USA
M. T. Collins, CSDB, NIDCR, National Institutes of Health, Bethesda, MD, USA
P. G. Robey, CSDB, NIDCR, National Institutes of Health, Bethesda, MD, USA
M. Seton, Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
K. K. Miller, Neuroendocrine Unit, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
M. Mannstadt, Email: mmannstadt@partners.org, Endocrine Unit, Thier 10, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114, USA
References
- 1.Hajdu N, Kauntze R. Cranio-skeletal dysplasia. Br J Radiol. 1948;21:42–48. doi: 10.1259/0007-1285-21-241-42. [DOI] [PubMed] [Google Scholar]
- 2.Cheney WD. Acro-osteolysis. Am J Roentgenol Radium Ther Nucl Med. 1965;94:595–607. [PubMed] [Google Scholar]
- 3.Brennan AM, Pauli RM. Hajdu–Cheney syndrome: evolution of phenotype and clinical problems. Am J Med Genet. 2001;100:292–310. doi: 10.1002/1096-8628(20010515)100:4<292::aid-ajmg1308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Isidor B, Lindenbaum P, Pichon O, et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet. 2011;43:306–308. doi: 10.1038/ng.778. [DOI] [PubMed] [Google Scholar]
- 5.Simpson MA, Irving MD, Asilmaz E, et al. Mutations in NOTCH2 cause Hajdu–Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43:303–305. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- 6.Schipani E, Weinstein LS, Bergwitz C, et al. Pseudohypoparathyroidism type Ib is not caused by mutations in the coding exons of the human parathyroid hormone (PTH)/PTH-related peptide receptor gene. J Clin Endocrinol Metab. 1995;80:1611–1621. doi: 10.1210/jcem.80.5.7745008. [DOI] [PubMed] [Google Scholar]
- 7.Gnirke A, Melnikov A, Maguire J, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol. 2009;27:182–189. doi: 10.1038/nbt.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cingolani P. snpEff: variant effect prediction. 2012 http://snpeff.sourceforge.net.
- 10.Zhao W, Hirose T, Ishikawa M, Oshima Y, Hirai S, Ohno S, Taniguchi H. Neonatal pancreatic cells redifferentiate into both neural and pancreatic lineages. Biochem Biophys Res Commun. 2007;352:84–90. doi: 10.1016/j.bbrc.2006.10.179. [DOI] [PubMed] [Google Scholar]
- 11.Crifasi PA, Patterson MC, Bonde D, Michels VV. Severe Hajdu–Cheney syndrome with upper airway obstruction. Am J Med Genet. 1997;70:261–266. [PubMed] [Google Scholar]
- 12.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penton A, Leonard L, Spinner N. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23(4):450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 15.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Kumano K, Nakazaki K, et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci. 2009;100:920–926. doi: 10.1111/j.1349-7006.2009.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang MY, Xu ML, Histen G, Shestova O, Roy M, Nam Y, Blacklow SC, Sacks DB, Pear WS, Aster JC. Identification of a conserved negative regulatory sequence that influences the leukemogenic activity of NOTCH1. Mol Cell Biol. 2006;26:6261–6271. doi: 10.1128/MCB.02478-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majewski J, Schwartzentruber JA, Caqueret A, et al. Mutations in NOTCH2 in families with Hajdu–Cheney syndrome. Hum Mutat. 2011;32:1114–1117. doi: 10.1002/humu.21546. [DOI] [PubMed] [Google Scholar]
- 19.Jeffries S, Capobianco AJ. Neoplastic transformation by Notch requires nuclear localization. Mol Cell Biol. 2000;20:3928–3941. doi: 10.1128/mcb.20.11.3928-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray MJ, Kim CA, Bertola DR, Arantes PR, Stewart H, Simpson MA, Irving MD, Robertson SP. Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu–Cheney syndrome. Eur J Hum Genet. 2012;20:122–124. doi: 10.1038/ejhg.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunziata V, di Giovanni G, Ballanti P, Bonucci E. High turnover osteoporosis in acro-osteolysis (Hajdu–Cheney syndrome) J Endocrinol Invest. 1990;13:251–255. doi: 10.1007/BF03349553. [DOI] [PubMed] [Google Scholar]
- 22.Engin F, Yao Z, Yang T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154(2):623–634. doi: 10.1210/en.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–6412. doi: 10.1128/MCB.00299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldridge D, Shchelochkov O, Kelley B, Lee B. Signaling pathways in human skeletal dysplasias. Annu Rev Genomics Hum Genet. 2010;11:189–217. doi: 10.1146/annurev-genom-082908-150158. [DOI] [PubMed] [Google Scholar]