Abstract
Regression of established tumors can be induced by adoptive immunotherapy (AIT) with tumor draining lymph node (DLN) lymphocytes activated with bryostatin and ionomycin (B/I). We hypothesized that B/I-activated T cells cultured in IL-7 + IL-15 might proliferate and survive in culture better than cells cultured in IL-2, and that these cells would have equal or greater anti-tumor activity in vivo. Tumor antigen-sensitized DLN lymphocytes from either wild-type or T cell receptor transgenic mice were harvested, activated with B/I, and expanded in culture with either IL-2, IL-7 + IL-15 or a regimen of alternating cytokines. Cell yields, proliferation, apoptosis, phenotypes, and in vitro responses to tumor antigen were compared for cells grown in different cytokines. These T cells were also tested for anti-tumor activity against melanoma lung metastases established by prior i.v. injection of B16 melanoma cells. IL-7 + IL-15 or alternating cytokines resulted in much faster and prolonged proliferation and much less apopotosis of B/I-activated T cells than culturing the same cells in IL-2. This resulted in approximately tenfold greater yields of viable cells. Culture in IL-7 + IL-15 yielded higher proportions of CD8+ T cells and a higher proportion of cells with a central memory phenotype. Despite this, T cells grown in IL-7 + IL-15 had higher IFN-γ release responses to tumor antigen than cells grown in IL-2. Adoptive transfer of B/I-activated T cells grown in IL-7 + IL-15 or the alternating regimen had equal or greater efficacy on a “per-cell” basis against melanoma metastases. Activation of tumor antigen-sensitized T cells with B/I and culture in IL-7 + IL-15 is a promising modification of standard regimens for production of T cells for use in adoptive immunotherapy of cancer.
Keywords: Adoptive immunotherapy, Melanoma, IL-7, IL-15, IL-2, T lymphocytes
Introduction
The immune system, and particularly CD8+ T lymphocytes, can potentially be harvested from tumor-bearing hosts and used to eliminate cancers, as demonstrated by studies of numerous animal models and a few clinical trials [6, 9–12, 32, 34, 51]. Monoclonal antibodies, cytokines, and pharmacological methods have been used successfully in mouse models to activate lymphocytes isolated from tumors or tumor draining lymph nodes (DLN), which can then be adoptively transferred to tumor bearing hosts and cause regression of established tumors [6, 9–11, 20, 35, 36, 39, 52]. Adoptive immunotherapy (AIT), or the adoptive transfer of antigen-sensitized T cells activated and/or expanded in vitro continues to receive attention [1, 13, 22, 35, 44–46, 50].
The success of AIT relies on manipulation of a host’s lymphocytes to produce large numbers of tumor-specific cells that are optimized in effector functions. AIT circumvents the in vivo constraints that influence the magnitude and avidity of T cell responses against tumor, allowing the generation and activation of lymphocytes away from the suppressive tumor environment. In addition, it allows for the treatment of the host before reintroduction of the selected cells, thereby providing the optimal environment for anti-tumor responses.
We have shown that in vitro treatment of tumor antigen-sensitized draining lymph node (DLN) cells with bryostatin and ionomycin (B/I) selectively activates sensitized T lymphocytes that can induce regression of several different murine tumors, either primary or metastatic, and can confer long-term resistance to re-challenge [13, 44–46]. Bryostatin 1 is a macrocyclic lactone derived from Bugula neritina, a marine invertebrate. Bryostatin activates protein kinase C and ionomycin increases intracellular calcium [4, 7, 24, 38]. Together, these mimic signaling through the CD3/TcR complex and lead to activation and proliferation of T cells [4]. In the 4T1 mammary carcinoma model, we have previously shown that B/I selectively activates CD62L− or sensitized T cells and that the anti-tumor activity resides in the CD62L− fraction of lymphocytes obtained from donor lymph nodes; only the CD62L− subset proliferates after B/I activation and has anti-tumor activity [8].
Although it has been routine in our lab and others to use the T cell growth factor interleukin-2 (IL-2) to stimulate the proliferation of anti-tumor T cells in culture, IL-2 expansion may lead to the induction of regulatory T cells (T regs) and cause activation-induced cell death in T cells [14, 21, 37, 40, 48, 49, 53]. Other cytokines that stimulate T cell growth, via a gamma chain shared with the IL-2 receptor complex, may have a number of important advantages. In particular, IL-7 and IL-15 support the proliferation and survival of T cells more effectively than IL-2. Unlike IL-2, IL-7 and IL-15 do not cause activation-induced cell death, nor are they critical for the maintenance of T regs [14, 37, 48, 49]. Moreover, these alternate gamma chain cytokines have been found to support preferential differentiation of CD8+ T cells toward a central memory (Tcm) phenotype, which some have suggested may be more effective at inducing tumor regression than the effector cells that are more likely to develop when lymphocytes are grown in IL-2 [26, 27]. Indeed, IL-15 has been shown to be as effective or even somewhat more effective than IL-2 for the production and support of T cells used for AIT against melanoma and other tumors [2, 5, 26]. For these reasons, we hypothesized that growth of B/I-activated T cells in alternate gamma chain cytokines might stimulate greater expansion of T cells capable of mediating tumor regression and of establishing T cell memory in the adoptive host than similarly activated T cells grown in IL-2. In this report, we show that the combination of IL-7 and IL-15 yields many times the number of T cells activated with B/I compared to our standard optimal dose of IL-2, and that IL-7/15-cultured cells are as effective or more effective than similar cells grown in IL-2 at mediating tumor regression in vivo, without the addition of exogenous cytokines or vaccines.
Methods
Mice
Virus-free C57Bl/6 mice (Charles River Laboratories, Cambridge, MA) were used between 8 and 12 weeks of age, caged in groups of six or fewer, and provided food and water ad libitum. Nude athymic BALB/c mice (National Cancer Institute, Bethesda, MD) were used to produce hybridoma ascites. T cell receptor (TcR) transgenic pmel-1 mice, with TcR specific for the peptide KVPRNQDWL derived from gp100 bound to class I of H-2b, were produced from breeding pairs obtained from Jackson Laboratories (Bar Harbor, Maine). OT-1 TcR transgenic mice with TcR specific for SIINFEKL peptide of ovalbumin were also produced from breeding pairs from Jackson Laboratories. All guidelines of the Virginia Commonwealth University Institutional Animal Care and Use Committee, which conform to the American Association for Accreditation of Laboratory Animal Care and the US Department of Agriculture recommendations for the care and humane experimental use of animals, were followed.
Tumor cell lines and hybridomas
B16-GMCSF and B16-F10 melanoma tumor cell lines were kindly provided by Dr. Richard Dutton (Trudeau Institute, Saranac Lake, NY) and by Dr. Rodney Prell (Cell Genesys, Inc., South San Francisco, CA), respectively. Melanoma cells were cultured in complete RPMI 1640 with 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 0.075% sodium bicarbonate, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM Hepes buffer, and 5 × 10−5 M 2-mercaptoethanol (Sigma, St Louis, MO). Tumor cells were harvested for inoculation of mice with 0.05% trypsin–EDTA (Invitrogen), washed twice with 1× PBS and resuspended in 1× PBS. All cells were incubated in 250 ml T-flasks (PGC, Gaithersburg, MD) at 37°C in humidified air with 5% CO2.
Draining lymph node sensitization
Wild-type C57Bl/6 or pmel-1 mice were inoculated with 1 × 106 B16-GMCSF cells. Ten days after footpad vaccination, popliteal tumor draining lymph nodes (tDLN) were harvested under sterile conditions.
Lymphocyte activation and in vitro expansion
DLN’s or spleens were harvested and dispersed into single cell suspensions in complete RPMI media at 1 × 106 cells/ml. Splenic mononuclear cells were enriched by Ficoll density gradient centrifugation and erythrocytes were removed by suspension in ammonium chloride. The cells were then activated by incubation with 5 nM bryostatin 1 (provided by the National Cancer Institute, Bethesda, MD) and 1 μM ionomycin (Calbiochem, San Diego, CA) (B/I), and 80 U/ml of rIL-2 (Chiron, Emeryville, CA) at 37°C for 18 h. Cells were washed three times with warm complete RPMI and resuspended at 1–2 × 106 cells/ml with 40 U/ml of rIL-2. The cells were allowed to proliferate in culture for an additional 7–14 days and were split every 2–3 days in order to maintain 1–2 × 106 cells/ml concentration. For the IL-7/15 groups, cells were activated with B/I in the presence of IL-7 + IL-15, each at 10 ng/ml, and were then washed and maintained in IL-7/15 throughout the culture period. For the alternating cytokine regimen, DLN cells were pulsed with B/I in the presence of IL-2, then washed and initially cultured in IL-7/15 overnight, washed and resuspended in IL-2 at 40 IU/ml. After 24 h incubation in IL-2, cell cultures were split, resuspended in IL-7 + IL-15 and maintained in IL-7/15 throughout the remaining time in culture.
Adoptive immunotherapy
For treatment of B16 melanoma lung metastases, mice were inoculated with 250,000 B16 melanoma cells i.v. 1 day prior to AIT, mice were pretreated with cyclophosphamide (CYP, Mead Johnson, Princeton, NJ), 100 mg/kg i.p. AIT consisted of i.v. infusion of lymphocytes from cultures. No systemic cytokines were administered. In some experiments, naïve mice not yet inoculated with tumor cells received adoptively transferred cells from culture and then were challenged with tumor cells 3–4 weeks later, without any CYP or exogenous cytokines.
Flow cytometry
Cells isolated from tDLN and expanded as described above were stained with a panel of antibodies and analyzed by dual color flow cytometry for surface marker expression on an ELITE Beckman Coulter flow cytometer. Fluorescently labeled Abs directed against the following markers were obtained from Pharmingen (San Diego, CA): Pan-DX5(DX5), CD4 (GK1.5), CD8 (53–6.7), CD44 (IM7), CD62L (MEL-14), and CD69 (H1.2F3). Appropriate isotype controls were used in all cases.
Assessment of lymphocyte apoptosis
Apoptosis was determined using the standard Annexin V-FITC Apoptosis Detection kit (BD Biosciences Pharmingen, San Diego, CA). B/I-activated and expanded DLN cells were washed twice with 1× PBS and resuspended in 1× binding buffer at a 200,000 cells/100 μl and double stained for Annexin V and propidium iodide (PI). Unstained cells were used as negative controls and staining with Annexin V alone or PI alone was used as single color positive controls. 20,000 cells per sample were analyzed. Annexin V+/PI− cells were considered early apoptotic cells, Annexin V+/PI+ cells were considered late apoptotic cells, and double negative cells were considered viable.
Enumeration of melanoma lung metastases
In all AIT experiments mice were euthanized by CO2 inhalation at times shown (about 3 weeks after tumor cell infusions) and lungs were removed, fixed in 10% formaldehyde, and black lung nodules were counted under a dissecting microscope, as previously described [30].
Cytokine release assays
Interferon-γ (IFN-γ) release in supernatant fluids from tumor sensitized, fresh or B/I-activated and expanded lymphocytes in response to stimulation with irradiated B16 melanoma cells or peptide-pulsed spleen cells for 24 h was assayed using ELISA assays from Pharmingen (San Diego, CA).
Isolation of RNA and RT-PCR
Total RNA was isolated from cells using Trizol (InVitrogen, Carlsbad, CA). Samples were treated with RQ DNase (Promega, Madison, WI) according to the manufacturer’s protocol. About 4 μg of RNA was used in the RT-PCR reaction, using BioScript Rnase H Minus RT (Bioline, Randolph, MA) according to the manufacturers protocol and oligo dT primer. Amplification of actin was used as an internal control. Granzyme B RNA was detected according to the procedures of Lian et al. [29], FasL RNA according to the procedures of Hallaas et al. [19], and Foxp3 as described by Fontenot [15]. RT-PCR products were separated on a 0.8% agarose gel and visualized with ethidium bromide.
Statistical analysis
In vivo experiments included at least six mice per group and were repeated at least twice. Differences in pulmonary metastases were assessed by analysis of variance (Wilcoxon-rank sum test) using JMPIN software (SAS Institute Inc., Cary, NC). Results are presented as the means ± standard error (SE) in each treatment group. In vitro assays were repeated at least twice. A P < 0.05 was used throughout to determine significant differences.
Results
Comparative analysis of T cell expansion and in vitro anti-tumor responses using different gamma chain cytokine regimens
Wild-type C57Bl/6 mice were inoculated with B16-GMCSF in the footpad, and popliteal tDLN were harvested and activated with B/I and expanded in either IL-2, IL-7/15 or an alternating regimen (IL-7/15 for 24 h, followed by IL-2 for 24 h, and then IL-7/15 for the remainder of the culture). The alternating regimen (IL-7/15–IL-2–IL-7/15) was based on observations with human PBL and the FVB mouse model of breast carcinoma, indicating that after 1–2 days in IL-7/15, expression of IL-7 receptors dropped dramatically, and this was rapidly restored by 24 h in IL-2 (data not shown). As indicated in Fig. 1a, B16-sensitized lymph node cells from wild-type C57Bl/6 mice cultured in IL-7/15 or in alternating cytokines both grew at a much faster rate and for more prolonged periods (2 vs 1 week) than cells cultured in IL-2 alone. To make the relative effects of alternate cytokine regimens more easily interpretable, TcR transgenic mice on a C57Bl/6 background were studied. B16-sensitized DLN cells from pmel-1 TcR transgenic mice (Fig. 1b) and splenic lymphocytes from pmel-1 mice sensitized with gp100 peptide (Fig 1c) grew more rapidly in IL-7/15 than in IL-2. We did not always see significantly higher yields of cells with alternating cytokines versus IL-7/15 (as shown in Fig. 1a), especially for pmel-1 cells. However, regardless of which cytokines were included in the B/I activation step, subsequent culture in IL-7/15 or alternating cytokines consistently resulted in much greater expansion of viable cells than in IL-2 in more than ten separate experiments, and was consistently 5- to 12-fold greater than in IL-2 alone for DLN cells. As shown in Fig. 1d, IL-7/15 or the alternating cytokine regimen induced greater proliferative activity, as assessed by tritiated thymidine uptake. Similar results were seen on days 3 and 6 of culture.
Fig. 1.

IL-7/15 induces stronger proliferation of antigen-sensitized, B/I-activated lymphocytes than IL-2. a Wild-type C57Bl/6 mice were injected with 1 × 106 B16-GMCSF cells in the footpad. Ten days later ipsilateral popliteal DLNs were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively, or after being pulsed with B/I + IL-2, were cultured in alternating cytokines for times shown. Fold-expansion is based on viable cell counts on each day, compared to the number plated immediately after pulsing with B/I. b Pmel-1 mice were injected with 1 × 106 B16-GMCSF cells in the footpad. Ten days later ipsilateral popliteal DLNs were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively, or after being pulsed with B/I + IL-2, were cultured in alternating cytokines for times shown. Fold-expansion is based on viable cell counts on each day, compared to the number plated immediately after pulsing with B/I. c Pmel-1 mice were injected with irradiated C57Bl/6 splenocytes pulsed with gp100 peptide i.v., weekly × 2. Ten days later splenic mononuclear cells were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in IL-2 (40 U/ml), IL-7/15 (10 ng/ml each), respectively for times shown. Fold-expansion is based on viable cell counts on each day, compared to the number plated immediately after pulsing with B/I. d Pmel-1 mice were injected with 1 × 106 B16-GMCSF cells in the footpad. Ten days later ipsilateral popliteal DLNs were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each), then cultured in microtiter well plates (at either 12,000 or 25,000 cells per well) in IL-2 (40 U/ml), IL-7/15 (10 ng/ml each) or alternating cytokines. On day 6, wells were pulsed with tritiated thymidine and harvested on glass fiber filters 24 h later and counted in a beta scintillation counter. Similar results were obtained on day 3
In order to determine the mechanism(s) of the more rapid and prolonged growth of T cells in IL-7/15, we examined cells cultured in IL-2 or IL-7/15 for early and late apoptosis at various time points. As shown in Fig. 2, the levels of early and late apoptotic cells increased progressively when cells were cultured in IL-2 and were much higher on days 6 and 8 than for cells cultured in IL-7/15. In repeat experiments, nearly 50% of cells cultured in IL-2 were apoptotic on day 6, versus only 16% of cells cultured in IL-7/15.
Fig. 2.
Decreased apoptosis and higher proportion of viable cells when B/I-activated lymphocytes are cultured in IL-7/15 or alternating cytokines compared to IL-2. B16-GMCSF sensitized DLNs from pmel-1 mice were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each), then expanded in IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively, or after being pulsed with B/I + IL-2, were cultured in alternating cytokines for times shown. Samples were taken on days 3, 6 and 8 of expansion and stained for Annexin V-FITC and propidium iodide (PI). Fluorescence of at least 20,000 cells per sample was analyzed by flow cytometry. Percent of cells that are viable are in the left lower quadrants; early and late apoptotic cells are in the right lower and right upper quadrants, respectively. The differences in apoptosis between IL-2 and IL-7/15 cultures are representative of three independent experiments
OT-1 mice received i.v. infusion of SIINFEKL-pulsed irradiated C57Bl/6 splenocytes. Spleen cells from these immunized mice were harvested, activated with B/I and then cultured with different cytokines and cytokine combinations for 7 days. Again, expansion was much greater in IL-7/15 than in IL-2 (five- to ten-fold greater in different experiments). RT-PCR analysis of T cells from each culture are shown in Fig. 3, and show that cells cultured in IL-7 + IL-15 expressed much lower levels of Foxp3 than the same cells grown in IL-2. There was a modest reduction in perforin expression in cells cultured in IL-7/15, but there was no difference in Granzyme B expression.
Fig. 3.
IL-7/15 induces less expression of Foxp3 than IL-2 in B/I-activated antigen-sensitized T cells. Naive or sensitized (from mice vaccinated with SIINFEKL-pulsed splenocytes) OT-1 T cells were activated with B/I in the presence of IL-2 for 18 h and then cultured in IL-2 (40 U/ml) or IL-7 + IL-15 (10 ng/ml each) for 7 days. On day 7, total RNA was isolated from each set using Trizol (Invitrogen) and then treated with RQ DNaze (Promega). About 4 ug of RNA was used for the reverse transcriptase, using BioScript RNase H Minus RT (BIOLINE) and oligo dT primers. The lowest panel shows amplification of β-actin as an internal control. Levels of expression of Foxp3, granzyme B, perforin, FasL were assayed. Products were migrated on 0.8% agarose gel and visualized by ethidium bromide. The RT-PCR results shown are representative of two separate experiments with different batches of cells from different animals, and the RT-PCR was repeated three times for each experiment, with similar results
The phenotypes of cells obtained from cultures in different cytokines and harvested on days 1, 3, and 7 after B/I activation are shown in Table 1. Early in these cultures, similar proportions of CD4+ and CD8+ cells were seen in both IL-2 and IL-7/15 cultures. However, IL-7/15 induced an increase in the proportion of CD8+ cells and a much higher proportion of cells expressing CD62L by day 7 of culture compared to IL-2. CD69 was also expressed by a higher proportion of cells cultured in IL-7/15. In three separate experiments, in which dual staining was performed, the proportion of CD44+CD62Lhigh cells was consistently higher for cells cultured in IL-7/15 (50–62%) than for cells cultured in IL-2 (20–42%) on days 6–8 of culture, as shown in Fig. 4.
Table 1.
IL-7/15 expands similar T cell subsets as IL-2 and maintains CD62L+CD8+ T cells better than IL-2
| Cytokine regimen | CD4 | CD8 | CD44 | CD62L | CD69 | |
|---|---|---|---|---|---|---|
| Pre-pulse | 7.30 | 34.6 | 58.7 | 72.5 | 13.1 | |
| Day 1 | IL-2 | 3.79 | 42.0 | 72.6 | 46.0 | 50.4 |
| Alternating | 4.96 | 35.1 | 74.5 | 52.1 | 60.0 | |
| IL-7/15 | 6.05 | 36.2 | 76.8 | 53.3 | 67.2 | |
| Day 3 | IL-2 | 9.93 | 75.6 | 86.2 | 32.7 | 14.1 |
| Alternating | 6.29 | 80.0 | 91.3 | 57.1 | 13.1 | |
| IL-7/15 | 6.94 | 87.3 | 95.2 | 69.7 | 43.8 | |
| Day 7 | IL-2 | 27.0 | 52.8 | 91 | 33.8 | 15.1 |
| Alternating | 6.06 | 94.3 | 87.7 | 71.7 | 43.4 | |
| IL-7/15 | 6.61 | 93.4 | 93.7 | 60.5 | 35.0 |
B16-GMCSF sensitized DLNs were harvested from pmel-1 mice and pulsed for 18 h at 1 × 106 DLN cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in either IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each)
Samples were taken on days 1, 3 and 7 of expansion and incubated with mAb against CD4, CD8, CD44, CD62L, and CD69. Fluorescence of 15,000 cells per sample was analyzed by flow cytometry
Numbers represent percentage of cells with the indicated phenotype
Fig. 4.
Culture of B/I-activated lymphocytes in IL-7/15 increases proportion of CD44+CD62L+ double positive cells compared to IL-2. B16-GMCSF sensitized DLNs were harvested from pmel-1 mice and pulsed for 18 h at 1 × 106 DLN cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in either IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively. Samples were taken on days 3, 6, and 8 of expansion and incubated with mAb against CD44 and CD62L. Fluorescence of 15,000 cells per sample was analyzed by flow cytometry. Numbers in upper right quadrants represent percentage of double positive cells for day 8 samples for each culture condition in this experiment. Results are representative of three separate experiments
Wild-type C57Bl/6 lymphocytes harvested from cultures grown in IL-7/15 on day 5 secreted higher levels of IFN-γ in response to B16 melanoma cells than those grown in IL-2 (Fig. 5a). Moreover, cells harvested after 14 days in culture with IL-7/15 secreted even higher levels of IFN-γ. Cells cultured in IL-2 did not survive that long and so could not be compared at this time point. DLN cells from pmel-1 mice vaccinated with B16-GMCSF cells and tested immediately after harvest secreted IFN-γ in response to gp100 peptide or B16 melanoma cells (Fig. 5b). When pmel-1 DLN cells were activated with B/I and then expanded in IL-2, IL-7/15 or alternating cytokines, the cells cultured with IL-2 initially secreted higher levels of IFN-γ than cells cultured in IL-7/15 (Not shown). However, starting on day 3 in culture, despite the higher levels of CD62L expression (indicating a central memory phenotype), B/I-activated IL-7/15-cultured cells or cells cultured in the alternating regimen demonstrated a progressive increase in IFN-γ response to antigen stimulation, while the response of cells grown in IL-2 gradually declined (results for day 7 in culture are shown in Fig. 5c).
Fig. 5.

IFN-γ release in response to tumor antigen after expansion in IL-2 or IL-7/15 or alternating cytokines. a Pmel-1 mice were injected with 1 × 106 B16-GMCSF cells in the footpad. Ten days later ipsilateral popliteal DLNs were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each), then expanded in IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively, or after being pulsed with B/I + IL-2, were cultured in alternating cytokines for times shown. Cells were harvested from cultures at times shown on x-axis. Cells were incubated with stimulants at a ratio of 10:1 in 24 well plates at a concentration of 2 × 106 lymphocytes/ml. Supernatants were collected 24 h after exposure to stimulants and assayed for IFN-γ using the murine IFN-γ ELISA kit. Baseline refers to the response of cells immediately after harvest of DLN. IL-2-cultured cells were tested at 5 days, but did not survive long enough to be tested at 14 days. b, c B16-GMCSF sensitized DLNs from pmel-1 mice were harvested and pulsed for 18 h at 1 × 106 cells/ml in B (5 nM)/I (1 μM) + IL-2 (80 U/ml) or B/I + IL-7/15 (10 ng/ml each) then expanded in IL-2 (40 U/ml) or IL-7/15 (10 ng/ml each), respectively, or after being pulsed with B/I + IL-2, were cultured in alternating cytokines for times shown. Samples were taken on days 0 (prior to B/I pulse, shown in b), 1, 3 (not shown), and 7 (shown in c) of expansion and stimulated with media alone (NIL), with irradiated splenocytes (IRR SPLEEN), or irradiated splenocytes pulsed with the specific peptide, gp100 (GP100) or with irradiated B16 melanoma cells. Cells were incubated with stimulants at a ratio of 10:1 in 24 well plates at a concentration of 2 × 106 lymphocytes/ml. Supernatants were collected 24 h after exposure to stimulants and assayed for IFN-γ using the murine IFN-γ ELISA kit. Data are shown as the mean of duplicates ± SD
Treatment of B16 melanoma lung metastases with T cells grown in IL-2 or IL-7/15
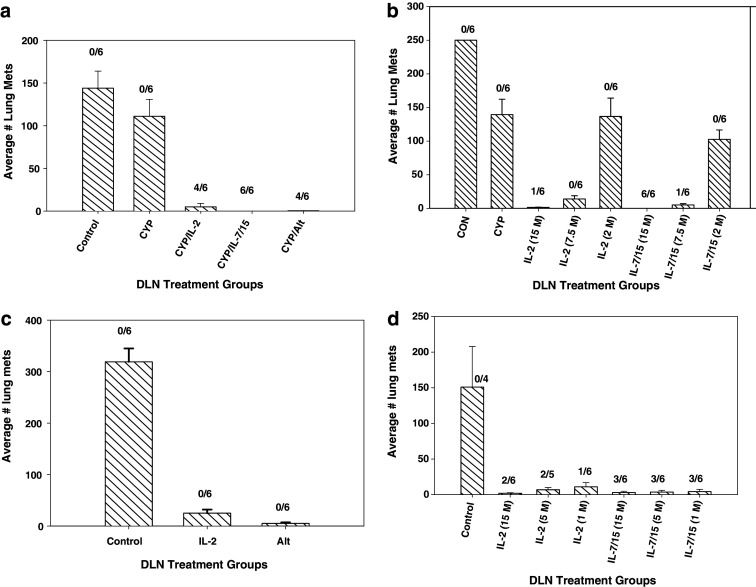
As shown in Fig. 6a, which is representative of results in four separate experiments, pmel-1 DLN cells sensitized in vivo, activated with B/I and grown in either IL-2, IL-7/15 or alternating cytokines markedly inhibited the growth of B16 melanoma lung metastases after adoptive transfer at a dose of 15 × 106 per mouse. However, cells grown in IL-7/15 seemed to be marginally more effective in every experiment, with complete cures of some if not all mice treated. In an attempt to determine more clearly the relative potency of T cells grown in these alternate regimens, a cell dose-response experiment was performed, as shown in Fig. 6b). At each of the two lower cell doses (2 and 7.5 × 106), there was a trend for IL-7/15 cultured cells to be more effective, but comparisons of the number of metastases to mice treated with IL-2 cultured cells did not reach statistical significance. However, at 15 × 106 cells per mouse, IL-7/15 cultured cells were significantly more effective than IL-2 cultured cells. More important than the actual numbers of metastatic lung nodules perhaps, all mice treated with IL-7/15 cells were tumor-free compared to only one of six mice treated with IL-2 cultured cells. In order to demonstrate the potential of these B/I-activated T cells to confer long-term memory and resistance to tumor growth, naïve mice received pmel-1 cells activated with B/I and grown in either IL-2 or IL-7/15, without any CYP or cytokines in vivo. Three weeks later, these mice and untreated controls were challenged i.v. with B16 melanoma cells. As shown in Fig. 6c, d, the adoptively transferred lymphocytes conferred substantial protection against lung metastases, but IL-7/15-cultured cells and cells grown in alternating cytokines were again somewhat more effective than cells grown in IL-2.
Fig. 6.
Adoptive immunotherapy of B16 melanoma lung metastases with T cells activated with B/I and cultured in IL-2, IL-7/15 or alternating cytokines. a Recipient C57BL/6 mice were injected intravenously with 250,000 B16F10 melanoma cells and randomized to five treatment groups: untreated control (CON), cyclophosphamide (CYP) treated (100 mg/kg), and CYP + AIT with B/I-activated DLN cells from B16-GMCSF sensitized pmel-1 mice cultured in IL-2 or IL-7/15 or alternating cytokines at 15 × 106 cells/mouse. CYP was given 3 days after tumor inoculation and cultured lymphocytes were infused i.v. the following day. After 24 days, lungs were harvested and lung metastases were counted. The data shown are the mean number of lung metastasis ± SE of six mice per group. All treated groups were significantly different from CON and from CYP only group (Wilcoxon/Kruskal Wallis rank sum test, for all groups: χ 2 = 24.2, df = 4, P < 0.0001; CON vs CYP: P = 0.1705; CYP vs IL2: P = 0.0033; CYP vs IL7/15: P = 0.0021; CYP vs ALT: P = 0.0033; IL2 vs IL7/15: P = 0.1396; IL2 vs ALT: P = 0.8474; IL7/15 vs ALT: P = 0.1380. Numbers above each histogram are the number of mice with no detectable lung nodules out of six mice in each group. b Recipient C57BL/6 mice were injected intravenously with 250,000 B16F10 melanoma cells and randomized to eight treatment groups: untreated control (CON), cyclophosphamide (CYP) treated (100 mg/kg), and CYP + AIT with B/I-activated DLN cells from B16-sensitized pmel-1 mice cultured in IL-2 or IL-7/15 at doses of 15, 7.5, or 2 × 106 cells/mouse [cytokines used for expansion and cell doses (in parentheses) shown along the x-axis]. CYP was given 3 days after tumor inoculation and cultured lymphocytes were infused i.v. the following day. After 24 days, lungs were harvested and lung metastases were counted. The data shown are the mean number of lung metastasis ± SE of six mice per group. All treated groups were significantly different from CON; groups treated with AIT at T cell doses of 7.5 × 106 and 15 × 106 were significantly different from CYP only group and IL-7/15 group at 15 × 106 was significantly different from IL-2 group at same cell dose (Wilcoxon/Kruskal Wallis test—comparing all groups: χ 2 = 43.0, df = 7, P < 0.0001; CON vs. CYP: P = 0.0021; CYP vs IL-2 at 7.5 million cells: P = 0.0039; CYP vs IL-7/15 at 7.5 million cells: P = 0.0039; CYP vs IL-2 at 15 million cells: P = 0.0037; CYP vs IL-7/15 at 15 million cells: P = 0.0021; IL-2 vs IL-7/15 at 15 million: P = 0.0069; Other comparisons not significantly different). Numbers above each histogram are the number of mice with no detectable lung nodules out of six mice in each group. c Naïve C57BL/6 mice randomized to three treatment groups: untreated controls or infusion of B/I-activated DLN cells from B16-sensitized pmel-1 mice cultured in IL-2 or alternating cytokines 25 × 106 cells/mouse. Three weeks later, mice were injected intravenously with 250,000 B16F10 melanoma cells and after 24 days, lungs were harvested and lung metastases were counted. The data shown are the mean number of lung metastasis ± SE of six mice per group. Wilcoxon–Kruskal Wallis test—comparing all groups: χ 2 = 13.4, df = 2, P = 0.0012; CON vs ALT: P = 0.0039; CON vs IL-2: P = 0.0062; ALT vs IL-2: P = 0.0174. Similar results were obtained in a separate experiment using cells grown in IL-2, IL-7/15 or alternating cytokines. Numbers above each histogram are the number of mice with no detectable lung nodules out of six mice in each group. d Naïve C57BL/6 mice randomized to seven treatment groups: untreated controls or infusion of B/I-activated DLN cells from B16-sensitized pmel-1 mice cultured in IL-2 or IL-7 + IL-15, each at 15, 5, or 1 million cells/mouse [cytokines used for expansion and cell doses (in parentheses) shown along the x-axis]. Three weeks later, mice were injected intravenously with 250,000 B16F10 melanoma cells and after 24 days, lungs were harvested and lung metastases were counted. The data shown are the mean number of lung metastasis ± SE of six mice per group. Wilcoxon–Kruskal Wallis test—comparing all groups: χ 2 = 12.95, P = 0.044; CON vs each IL-2 group: P < 0.01; CON vs each IL-7/15 group, P < 0.01; all other comparisons NS. Numbers above each histogram are the number of mice with no detectable lung nodules out of six mice in each group
Discussion
These results demonstrate that culturing B/I-activated lymphocytes from tumor or tumor antigen-sensitized mice in IL-7 + IL-15 results in a much higher yield of viable cells with equal or greater activity against melanoma metastases in vivo than the same cells cultured in IL-2. This results from greater proliferation and greatly reduced apoptosis in culture. These results are consistent with those of Klebanoff et al. [26, 27], who showed that culturing antigen-sensitized T cells in IL-15 prior to AIT produced greater numbers of cells in vivo than cells cultured in IL-2. However, they did not comment on the relative growth of cells in vitro in the two different cytokines. In preliminary experiments using the 4T1 mammary carcinoma model (not shown here), we had found that culturing B/I-activated tumor antigen-sensitized lymphocytes in IL-7 or IL-15 alone produced expansion that was at most only 2- to 3-fold greater than with IL-2 alone (data not shown), but that culturing tumor-sensitized DLN cells in the two cytokines combined produced dramatically increased expansion (5- to 10-fold) of DLN cells in culture. This led to the experiments shown here using the B16 melanoma model. The finding that IL-7 combined with IL-15 was markedly more effective than IL-2 or either of the alternate cytokines alone is consistent with the suggestion made previously that, even though both IL-7 and IL-15 have roles in the maintenance and self-renewal of memory precursors, effector CD8+ T cells actually need both IL-7 and IL-15 for optimal proliferation and to become long-lived memory cells [23].
Carrio et al. [5] showed that IL-2 induced greater proliferation of CTL and higher levels of IFN-γ secretion than IL-7 or IL-15 alone. This difference resulted in part from the preferential selection of effector T cells by IL-2 and selection of memory T cells by IL-7 and IL-15. Our data contrast with the data presented by Carrio et al.; we found that IL-7 or IL-15, and especially the combination of both IL-7 and IL-15, expands T cells to a much greater extent than IL-2. The contradictory results may be caused by a difference in the protocol used to produce activated T cells. Carrio et al. initially activated naïve T cells in vitro with splenocytes pulsed with specific peptide plus IL-2, while our protocol employs pharmacological activation with bryostatin-1 and ionomycin of T cells previously sensitized in vivo. The strength of signaling through the TcR pathway plays a role in prolonging T cell survival both in vitro and in vivo by regulating the capacity of primed T cells to respond to homeostatic cytokines, to survive cytokine withdrawal, and to accumulate in vivo [18, 28]. It is possible that T cell activation with B/I produces stronger and more prolonged TcR pathway signaling, which would increase the capacity of sensitized T cells to respond to homeostatic cytokines, like IL-7 and IL-15. This may also explain why we did not see as marked a difference among the regimens in T cell phenotypes, since it has been suggested that absence of antigen may contribute to differentiation toward central memory cells in the presence of IL-15 [41]. Interestingly, we also did not observe a shift away from IFN-γ production which is supposed to be characteristic of the differentiation toward central memory cells and away from terminally differentiated effector cells [16, 23]. In fact, we found that B/I-activated T cells grown in IL-7/15 had increased IFN-γ responses compared to cells grown in IL-2. Moreover, we did not observe significant levels of IL-2 secretion in response to tumor cells or tumor antigen for any of the expansion groups (data not shown). Future studies will determine which T cells (e.g., CD62L+ or CD62L−) are the main source of IFN-γ in each expansion protocol, using flow cytometric analysis of surface markers and intracellular cytokine.
Melchionda et al. [33] showed that IL-7 and IL-15 were comparable to or better than IL-2 at expanding Ag sensitized CD8+ T cells when given as an adjuvant in vivo along with immunization. There was, however, no additive or synergistic effect when IL-7 and IL-15 were combined. In our in vitro B/I activation model, we have shown that the combination of IL-7 and IL-15 has an additive effect on expansion, increasing T cell numbers 5- to 12-fold over IL-2. We found that this difference in expansion resulted partly from the protective roles of IL-7 and IL-15 against apoptosis [3, 25, 42, 43]. By day 6 of expansion, 40–50% of IL-2 expanded T cells were apoptotic. In contrast, only 15–18% of IL-7/15 expanded T cells were apoptotic. The greater rate of apoptosis in IL-2 expanded cells may be the result of IL-2 mediated activation-induced cell death [40, 47]. To strengthen this argument, future experiments should look for differences in expression levels of FasL and antiapoptotic molecules like Bcl-2 and Bcl-XL.
Although it has been shown that T cells grown in IL-15 persisted longer after adoptive transfer and provided better protection against tumor challenge than cells grown in IL-2, IL-15-expanded T cells were actually less effective at inducing regression of established tumors in a model using OVA-transfected EL-4 [5, 41]. However, Klebanoff et al. [26, 27] found that IL-15-cultured pmel-1 cells were more effective at inducing regression of established subcutaneous B16 melanomas than T cells cultured in IL-2. This was related to a shift towards a central memory phenotype, greater proliferation of IL-15 cultured cells in vivo and increased trafficking to lymphoid tissues. We did observe a moderate shift toward a greater number of CD62L+ cells when B/I-activated T cells were cultured in IL-7/15 versus IL-2, and the IL-7/15 cells were somewhat more effective at inducing tumor regression than IL-2 cultured cells, but this difference was not as dramatic as the earlier results of Klebanoff et al. This could relate to a number of differences, including the use of antigen-sensitized versus naïve T cells, the use of B/I to re-activate the cells in vitro, treating pulmonary metastases rather than subcutaneous tumors, or the combined use of IL-7 and IL-15 instead of IL-15 alone. It seems likely that the ability of T cells to traffic to lymphoid organs, which is favored by the use of IL-15 and the Tcm phenotype, may not be as important for treatment of pulmonary metastases as it would be for subcutaneous solid tumors or for response to a vaccine. In addition, the successful regression of B16 melanoma tumors in the studies reported by Klebanoff was achieved by a complex regimen combining AIT with sublethal irradiation, vaccination with a recombinant virus encoding the target antigen and administration of exogenous cytokines. It has also been suggested that cells grown for short periods of time may be more effective than cells cultured for several weeks, as the latter leads to more terminally differentiated cells [17]. In all of the AIT experiments shown here, we harvested cells from culture after 6–7 days, although IL-7/15 cultured cells were capable of growing much longer without needing an additional activation with B/I or antigen.
Preliminary studies have been reported with human PBL from melanoma patients vaccinated with a peptide tumor antigen to examine the relative merits of using alternate cytokines to expand T cells for AIT [31]. These studies did not show any consistent major differences in the yield or phenotypes of cells grown in IL-7, IL-15 or combinations compared to IL-2. This may not necessarily mean that results similar to what we are reporting here would not be achieved with human T cells, since this may depend on the source of cells, the prior sensitization and the method of in vitro activation. In addition, the concentration of IL-2 used in the reported human experiments was 300 versus 40–80 IU/ml (which we have found to be the optimal concentration for expansion of B/I-activated cells) in our experiments. This concentration also maintains antigen specificity more reliably than higher doses. We also used lower concentrations of IL-7 and IL-15 than were used in the human studies. Although we did not find that IL-7/15-cultured cells were markedly more effective than cells grown in IL-2 on a per cell basis, they were always as effective or slightly more effective in vivo. The major advantage of using the alternate or alternating cytokines appears to be the increased yield of viable cells, which may be of critical importance for clinical use, especially when the number of starting antigen-sensitized T cells is limited. This would likely be the case with vaccine-draining lymph nodes, an approach we plan to apply clinically. The comparatively simple regimen described here, with a modest dose of CYP followed by infusion of T cells cultured for short periods of time may be particularly applicable to clinical use. Future experiments will examine more detailed comparisons of the functions, phenotypes and fates after adoptive transfer of cells grown in IL-7/15 versus those grown in IL-2.
References
- 1.Alexander JP, Kudoh S, Melsop KA, Hamilton TA, Edinger MG, Tubbs RR, Sica D, Tuason L, Klein E, Bukowski RM, Finke JH. T-cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- 2.Bathe OF, yot-Herman N, Malek TR. IL-2 during in vitro priming promotes subsequent engraftment and successful adoptive tumor immunotherapy by persistent memory phenotypic CD8(+) T cells. J Immunol. 2001;167:4511–4517. doi: 10.4049/jimmunol.167.8.4511. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 6.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–890. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143:1283–1289. [PubMed] [Google Scholar]
- 8.Chin CS, Miller CH, Graham L, Parviz M, Zacur S, Patel B, Duong A, Bear HD. Bryostatin 1/ionomycin (B/I) ex vivo stimulation preferentially activates L-selectinlow tumor-sensitized lymphocytes. Int Immunol. 2004;16:1283–1294. doi: 10.1093/intimm/dxh130. [DOI] [PubMed] [Google Scholar]
- 9.Cohen PA, Peng LM, Kjaergaard J, Plautz GE, Finke JH, Koski GK, Czerniecki BJ, Shu SY. T-cell adoptive therapy of tumors: mechanisms of improved therapeutic performance. Crit Rev Immunol. 2001;21:215–248. [PubMed] [Google Scholar]
- 10.Crossland KD, Lee VK, Chen W, Riddell SR, Greenberg PD, Cheever MA. T cells from tumor-immune mice nonspecifically expanded in vitro with anti-CD3 plus IL-2 retain specific function in vitro and can eradicate disseminated leukemia in vivo. J Immunol. 1991;146:4414–4420. [PubMed] [Google Scholar]
- 11.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Sci. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong TA, Mosman TR. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989;143:2887–2893. [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8(+) T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 19.Halaas O, Vik R, Espevik T. Induction of Fas ligand in murine bone marrow NK cells by bacterial polysaccharides. J Immunol. 1998;160:4330–4336. [PubMed] [Google Scholar]
- 20.Harada M, Okamoto T, Omoto K, Tamada K, Takenoyama M, Hirashima C, Ito O, Kimura G, Nomoto K. Specific immunotherapy with tumour-draining lymph node cells cultured with both anti-CD3 and anti-CD28 monoclonal antibodies. Immunol. 1996;87:447–453. doi: 10.1046/j.1365-2567.1996.487568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haux J, Johnsen AC, Steinkjer B, Egeberg K, Sundan A, Espevik T. The role of interleukin-2 in regulating the sensitivity of natural killer cells for Fas-mediated apoptosis. Cancer Immunol Immunother. 1999;48:139–146. doi: 10.1007/s002620050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 24.Kazanietz MG, Lewin NE, Gao F, Pettit GR, Blumberg PM. Binding of [26-3H] bryostatin 1 and analogs to calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1994;46:374–379. [PubMed] [Google Scholar]
- 25.Keller AM, Borst J. Control of peripheral T cell survival: a delicate division of labor between cytokines and costimulatory molecules. Hum Immunol. 2006;67:469–477. doi: 10.1016/j.humimm.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Lian RH, Maeda M, Lohwasser S, Delcommenne M, Nakano T, Vance RE, Raulet DH, Takei F. Orderly and nonstochastic acquisition of CD94/NKG2 receptors by developing NK cells derived from embryonic stem cells in vitro. J Immunol. 2002;168:4980–4987. doi: 10.4049/jimmunol.168.10.4980. [DOI] [PubMed] [Google Scholar]
- 30.Lipshy KA, Kostuchenko PJ, Hamad GG, Bland CE, Barrett SK, Bear HD. Sensitizing T-lymphocytes for adoptive immunotherapy by vaccination with wild-type or cytokine gene-transduced melanoma. Ann Surg Oncol. 1997;4:334–341. doi: 10.1007/BF02303584. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29:284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 32.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 33.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell MS, Darrah D, Yeung D, Halpern S, Wallace A, Voland J, Jones V, Kan-Mitchell J. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 35.Morse MA, Clay TM, Lyerly HK. Current status of adoptive immunotherapy of malignancies. Expert Opin Biol Ther. 2002;2:237–247. doi: 10.1517/14712598.2.3.237. [DOI] [PubMed] [Google Scholar]
- 36.Nijhuis EWP, Wiel-van Kemenade EVD, Figdor CG, Van Lier RAW. Activation and expansion of tumour-infiltrating lymphocytes by anti-CD3 and anti-CD28 monoclonal antibodies. Cancer Immunol Immunother. 1990;32:245–250. doi: 10.1007/BF01741708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettit GR, Herald SL, Doubek DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104:6846–6848. doi: 10.1021/ja00388a092. [DOI] [Google Scholar]
- 39.Plautz GE, Cohen PA, Shu S. Considerations on clinical use of T cell immunotherapy for cancer. Arch Immunol Ther Exp (Warsz) 2003;51:245–257. [PubMed] [Google Scholar]
- 40.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/S1074-7613(00)80566-X. [DOI] [PubMed] [Google Scholar]
- 41.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 42.Roychowdhury S, May KF, Jr, Tzou KS, Lin T, Bhatt D, Freud AG, Guimond M, Ferketich AK, Liu Y, Caligiuri MA. Failed adoptive immunotherapy with tumor-specific T cells: reversal with low-dose interleukin 15 but not low-dose interleukin 2. Cancer Res. 2004;64:8062–8067. doi: 10.1158/0008-5472.CAN-04-1860. [DOI] [PubMed] [Google Scholar]
- 43.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008;86:312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 44.Tuttle TM, Bethke KP, Inge TH, McCrady CW, Pettit GR, Bear HD. Bryostatin 1-activated T cells can traffic and mediate tumor regression. J Surg Res. 1992;52:543–548. doi: 10.1016/0022-4804(92)90126-K. [DOI] [PubMed] [Google Scholar]
- 45.Tuttle TM, Fleming MF, Hogg PS, Inge TH, Bear HD. Low-dose cyclophosphamide overcomes metastasis-induced immunosuppression. Ann Surg Oncol. 1994;1:53–58. doi: 10.1007/BF02303541. [DOI] [PubMed] [Google Scholar]
- 46.Tuttle TM, McCrady CW, Inge TH, Salour M, Bear HD. γ-Interferon plays a key role in T-cell-induced tumor regression. Cancer Res. 1993;53:833–839. [PubMed] [Google Scholar]
- 47.Van PL, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/S1074-7613(00)80103-X. [DOI] [PubMed] [Google Scholar]
- 48.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 49.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 50.Yee C. Adoptive T cell therapy—immune monitoring and MHC multimers. Clin Immunol. 2003;106:5–9. doi: 10.1016/S1521-6616(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 51.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshizawa H, Chang AE, Shu S. Specific adoptive immunotherapy mediated by tumor-draining lymph node cells sequentially activated with anti-CD3 and IL-2. J Immunol. 1991;147:729–737. [PubMed] [Google Scholar]
- 53.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25 + Foxp3 + regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]