Abstract
The standard of care for treatment of Barrett’s esophagus (BE) with early esophageal neoplasia, including high-grade dysplasia (HGD) and intramucosal adenocarcinoma (IMC), has undergone a revolution over the past several years. With the introduction and popularization of endoscopic ablative technologies, along with the refinement of endoscopic mucosal resection (EMR) techniques, the majority of cases of early neoplasia in the setting of BE now are managed by endoscopic approaches. As a result, many patients who previously would have been referred for esophagectomy now may be spared from this major surgical procedure with its inherent morbidity, potential for mortality, and negative impact on long-term gastrointestinal function. The esophageal surgeon must be knowledgeable about the indications for such endoscopic therapies, as well as their limitations and potential pitfalls, so as to apply them in the appropriate clinical scenarios.
Keywords: Endoscopic surgical procedure, Barrett’s esophagus (BE), esophagectomy
Introduction
Esophageal cancer is a highly lethal disease of which relatively few are cured. Data from the American Cancer Society predict an overall 5-year survival of only 17% for patients diagnosed with esophageal carcinoma in 2014 (1). Similar to other gastrointestinal malignancies, cancer of the esophagus is usually asymptomatic in its early stages, a fact that explains the common presentation of patients with the manifestations of advanced, incurable disease. The link between gastroesophageal reflux disease (GERD), Barrett’s esophagus (BE), and esophageal adenocarcinoma (EAC) has been well established (2). Fortunately, patients found to have BE with early esophageal neoplasia, such as high-grade dysplasia (HGD) [synonymous with high-grade intraepithelial neoplasia (HGIN) or carcinoma in situ (CIS)] or intramucosal adenocarcinoma (IMC), can be treated with the expectation of cure; this fact should not be lost in the pessimism surrounding the treatment of more advanced EAC. In addition, the frequency of detection of esophageal neoplasia at an early, curable stage appears to be increasing, an observation that may be explained by a number of factors (Table 1). Given the anticipated long-term survival for patients with early esophageal malignancy, quality of life considerations become important in deciding upon a management strategy that avoids morbidity while still assuring eradication of disease.
Table 1. Factors leading to the increased detection of esophageal adenocarcinoma at an early stage.
| • The liberal use of flexible upper endoscopy to investigate foregut symptoms |
| • The recognition of the potential for gastroesophageal reflux disease to cause BE, a malignant precursor, and esophageal adenocarcinoma |
| • Structured screening and surveillance programs for BE to detect early neoplasia prior to the onset of sentinel signs or symptoms |
| • The establishment of formal biopsy protocols for the assessment of dysplasia or occult invasive cancer in the setting of known BE |
| • Advancements in endoscopic imaging technologies (e.g., narrow-band imaging, confocal laser endomicroscopy) and vital staining dyes that have facilitated the detection of subtle esophageal mucosal abnormalities harboring early neoplasia |
BE, Barrett’s esophagus.
Contemporaneous to the increasing detection of early esophageal neoplasia has been the introduction of technologies that have improved the ability to eliminate such disease by endoscopic approaches. As a result, a revolution in the standard of care for the treatment of early neoplasia in the setting of BE has occurred. While only a few years ago, the recommended therapy for cases of BE with HGD or IMC was esophagectomy, assuming a medically suitable patient and the availability of an expert surgical team, the treatment paradigm has shifted such that most cases now are treated by endoscopic approaches. Guidelines recently proposed by the American Gastroenterological Association recommend endoscopic resection (ER) and ablation as the procedures of choice for BE with HGD in the majority of patients (3). Due to the widespread adoption of these effective and low-risk endoscopic therapies, many individuals who previously would have been referred for surgery are now spared from esophageal resection, with its high rate of morbidity, potential for mortality, and negative impact on long-term alimentary function.
Esophagectomy for early esophageal neoplasia
For decades, esophagectomy was the standard of care for BE with HGD or IMC. As a result, the indications, surgical techniques, perioperative outcomes, cure rates and long-term quality of life relative to esophageal resection and reconstruction have been extensively studied and elucidated. With the recent, rapid changes in treatment recommendations for early esophageal neoplasia, the physician must be mindful of this surgical experience so as to have a basis against which endoscopic alternatives should be compared. In the course of therapy for early neoplasia, the treating physician runs the risk of being over aggressive, recommending esophagectomy when less invasive endoscopic therapies would have been appropriate. Alternatively, the physician may risk being under aggressive, continuing on a course of endoscopic treatment when it should have been abandoned, leading to the development of incurable locoregionally advanced cancer or systemic metastases from what started as a readily curable disease process.
The rationale for esophageal resection in cases of BE with HGD has been based on two factors: (I) occult invasive carcinoma has been found in a significant proportion of esophagectomy specimens, averaging approximately 37% in multiple large surgical series, when surgery has been undertaken for the preoperative diagnosis of HGD (4); and (II) invasive cancer may arise within dysplastic BE over the short to medium term if the esophagus is left in situ. Esophagectomy, therefore, is both curative and prophylactic relative to the treatment of invasive disease. Of course, the ability to eliminate pathologic mucosa by surgical extirpation must be weighed against the invasiveness of the procedure and its implications with regards to perioperative morbidity, mortality, recovery time, and long-term impact on quality of life. Thus, esophagectomy in this circumstance may rightly be considered “radical prophylaxis” for a microscopic disease process (5).
The morbidity and mortality associated with esophagectomy fortunately have improved over recent decades, a trend that is likely to continue. While several well-publicized, population-based studies have reported perioperative mortality of 9% or more following esophagectomy (6-8), these data reflect outcomes ten years or older when surgery was performed for all stages of esophageal cancer in non-specialty centers. Accordingly, such results are not appropriate for comparisons to endoscopic therapies, most studies of which were more recently undertaken for early disease in specialty units.
More relevant for comparison are reports specific to esophagectomy for early esophageal neoplasia. A literature review from 2007 detailed the experience with esophagectomy for HGD over the 20-year period from 1987-2007 and found an overall perioperative mortality of 0.94% (4), roughly one-tenth the mortality rate quoted above for all cases of esophagectomy for cancer. In addition, when operating for early disease with a low potential for lymph node metastasis and high expectation for cure, the surgeon should consider operative approaches, such as transhiatal esophagectomy (THE) (9), minimally invasive esophagectomy (MIE) (10) or vagal-sparing esophagectomy (VSE) (11), that avoid some of the morbidity and negative impact on long-term alimentary function associated with more aggressive procedures.
Endoscopic therapies for early esophageal neoplasia
Endoscopic mucosal resection (EMR)
Inoue, Endo and other surgeons in Japan initially described EMR for curative treatment of superficial squamous cell carcinomas of the esophagus (12). The term “EMR” is a misnomer in that the excision typically occurs at the interface between the submucosa and muscularis propria. As a result, the specimen contains both mucosa and submucosa. The term ER is more appropriate, but has not gained widespread acceptance.
Based on the findings from series of patients undergoing esophagectomy with regional lymphadenectomy, early squamous cell carcinomas were determined to be at low risk of distant intramural spread or metastasis to regional lymph nodes; such tumors were considered amenable, therefore, to cure by endoscopic approaches. The selection criteria for undergoing EMR in Japan include tumors ≤30 mm in diameter, infiltration no deeper than the lamina propria, superficial tumor spread ≤ one-half the esophageal circumference, and the absence of lymphatic or venous invasion (12).
Physicians in Europe and the United States (U.S.) adopted EMR for excisional biopsy of small mucosal irregularities or discrete nodules in the setting of BE, as well as for potentially curative treatment of small foci of HGD or IMC. Two main applications exist for EMR: (I) provision of a wide and deep biopsy, particularly of small, discrete, mucosal nodules, for diagnosis and staging of metaplasia/neoplasia and to guide subsequent tailored therapies; and (II) excision with curative intent (with or without subsequent mucosal ablative therapy of surrounding non-nodular metaplasia/dysplasia) for neoplasia deemed to be at low-risk for metastasis to regional lymph nodes or systemic sites. Treatment adjuncts in such situations include radiofrequency (RF) ablation, cryotherapy, argon plasma coagulation (APC), multipolar electrocautery (MPEC) or photodynamic therapy (PDT).
In contrast to ablation, EMR possesses the obvious advantage of providing a generous specimen for histologic assessment, including a determination of the presence of invasive carcinoma, the depth of invasion, the degree of differentiation of the tumor, the presence of lymphovascular invasion, and the status of disease at the lateral and deep resection margins. EMR improves the staging of early esophageal neoplasia compared to standard biopsy techniques and imaging modalities such as endoscopic ultrasonography (EUS). While EUS commonly is utilized to assess the depth of tumor invasion in cases of EAC, particularly for bulky T3 or T4 lesions, it is quite inaccurate at determining the depth of invasion of superficial epithelial or mucosal neoplasms, where important differences are measured in microns and involve landmarks not ultrasonographically discernible.
A feature of EAC is its propensity to spread to regional lymph nodes, the likelihood of which is dependent upon the depth of tumor penetration. Several series from the surgical literature have evaluated outcomes after esophagectomy with regional lymphadenectomy for EAC and have correlated the incidence of nodal metastasis with the depth of tumor invasion. Neoplasia limited to the epithelium (HGD/HGIN/CIS) has no potential for nodal metastasis. Invasive tumors penetrating the basement membrane to involve the lamina propria or muscularis mucosa (IMC; T1a) appear to have a limited potential for nodal disease, in the range of 2-5% (13-18). For EAC penetrating just slightly deeper through the muscularis mucosa to involve the submucosa (submucosal carcinoma; T1b), the incidence of nodal metastasis appears to increase significantly to approximately 25%. The muscularis mucosa, therefore, appears a critical barrier to nodal spread. Tumors involving the muscularis propria or beyond (T2-4) have an even higher probability of nodal involvement, in the range of 50-80%.
The general consensus is that endoscopic therapies are appropriate with curative intent only when the neoplastic process appears to be limited to the epithelium or mucosa and the potential for lymph node metastasis or systemic spread is low. In select circumstances, however, such as the patient at high risk for undergoing esophagectomy, EMR may be considered the best therapeutic option even for submucosal tumors, accepting the modest risk of occult nodal disease.
Endoscopic mucosal resection techniques
A number of EMR techniques have been described, all sharing the basic strategy of endoscopic localization of a specific mucosal nodule or irregularity for excision using a snare cautery device. Differences in technique relate to the use of submucosal injection of saline (with or without dilute epinephrine) to lift the target lesion from the underlying muscle layer, and the manner in which the lesion subsequently is prepared for snare application.
The simplest variant of EMR is snare resection alone without elevation or submucosal injection. This technique is best applied to polypoid lesions of the esophageal mucosa, in that flat lesions cannot be snared without some form of mucosal elevation, though is infrequently applicable. A common resection method has been the use of submucosal injection of saline with dilute epinephrine (10-20 mL injectate, 1:100,000 solution) to separate the mucosa from the underlying muscularis propria. The target lesion can then be aspirated into a specially designed cap (Olympus EMR-001, Olympus America, Center Valley, Pennsylvania) attached to the end of a standard flexible adult endoscope (“cap-assisted” EMR). The cap is manufactured with an inner groove that allows seating of a standard electrocautery snare. Once the mucosa is within the cap, the snare can be tightened around the base of the lesion and cautery applied, amputating the specimen in the submucosal plane. Prior to application of cautery, the lesion should be gently tugged to give the endoscopist a sense of mobility from the muscularis propria and to prevent inadvertent full-thickness injury to the esophageal wall. The resected specimen typically remains within the cap and can be extracted as the endoscope is withdrawn.
A similar technique, and perhaps the one most commonly employed, utilizes a variceal banding device with supplied cap system to facilitate excision of the target lesion (Figure 1A-C). Various single-use multiband systems (Duette Multiband Mucosectomy System, Cook Medical, Bloomington, Indiana or Bard Six-Shooter, Bard Interventional Products, Billerica, Massachusetts) are commercially available. The procedure involves suction of the nodule or lesion into the cap (without prior submucosal injection) and application of a rubber variceal band to the base of the elevated mucosa (Figure 2) creating a pseudopolyp (“suck and ligate” EMR). The lesion is excised either above or below the band using snare electrocautery (Figure 3A,B). The specimen can then be retrieved using any of a variety of devices such as a net or polypectomy grasper (Figure 4).
Figure 1.

“Suck and ligate” endoscopic mucosal resection technique. (A) Endoscopic image of a small esophageal mucosal nodule in a long segment of Barrett’s esophagus. The nodule is situated at the 8 o’clock position; (B) Variceal banding device attached to the tip of a flexible endoscope; (C) The targeted mucosal lesion is sucked into the cap to facilitate application of a variceal band.
Figure 2.
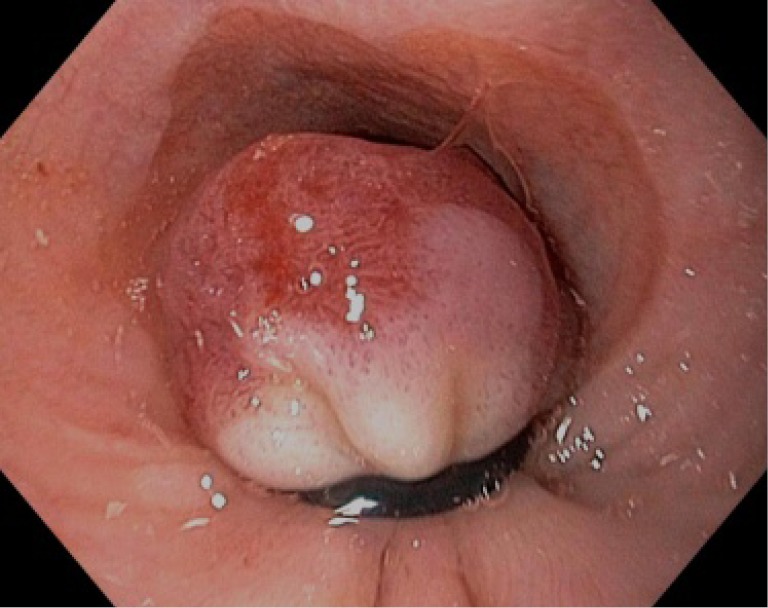
Variceal band applied to the esophageal mucosa with resultant pseudopolyp.
Figure 3.
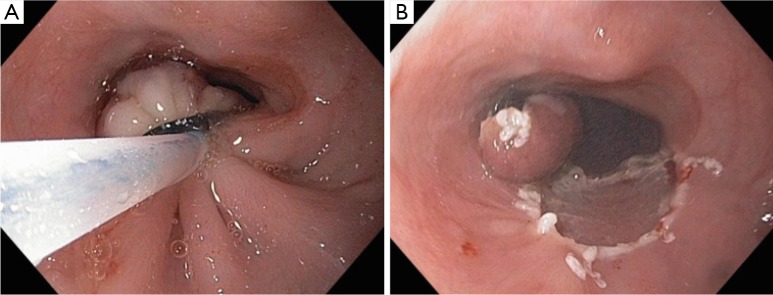
Snare excision of pseudopolyp. (A) Electrocautery snare placed just deep to the variceal band for amputation of the pseudopolyp; (B) Resultant specimen and mucosal/submucosal defect.
Figure 4.
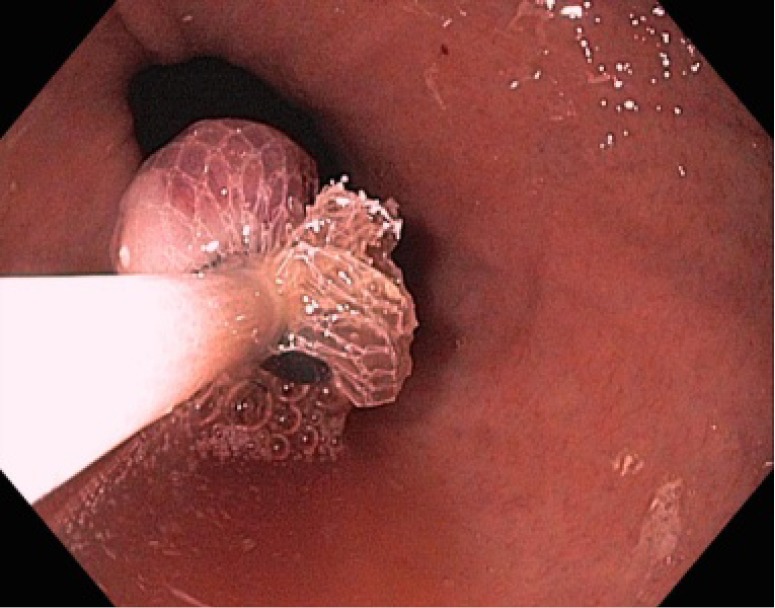
The specimen being retrieved with an endoscopic net.
An important principle underlying this technique is that the elasticity of the rubber band is not sufficient to hold the muscularis propria within it; excision of the pseudopolyp can proceed with confidence that only mucosa and submucosa are being removed and that a full-thickness perforation should not result (Figure 5). Advantages of this technique compared to cap-assisted resection are that submucosal injection is not necessary and that the snare does not need to be seated in the cap, a process that may be time-consuming and difficult to master. Disadvantages of the banding technique are the need to reintroduce the endoscope to position the snare, as well as the requirement of an additional instrument to retrieve the excised specimen. A prospective, randomized trial demonstrated equivalency between “cap-assisted” EMR and “suck and ligate” EMR in terms of the maximum diameter of the resected specimen, the resection area and complication rates (19).
Figure 5.
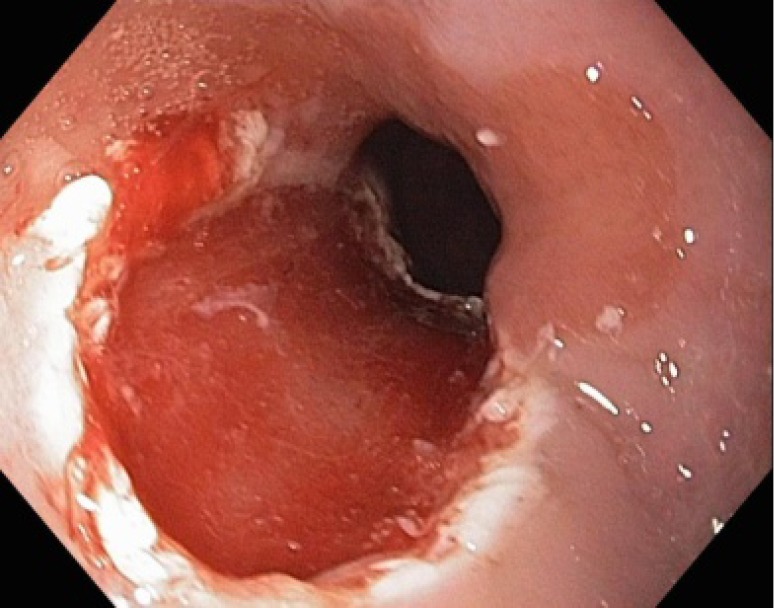
Defect in mucosa and submucosa created by endoscopic resection. The resection plane is typically at the interface between the submucosa and muscularis propria.
Ell et al. from Germany reported in 2007 on their initial experience with EMR for early EAC. Their cohort consisted of 100 patients selected from 667 referred with suspected intraepithelial neoplasia (20). Their criteria for an endoscopic treatment approach are listed in Table 2. The majority of tumors (69%) occurred in the setting of short-segment BE. EMR was combined with either APC for short-segment BE or PDT for long-segment BE in 49 patients. Complete local remission was noted in 99 out of the 100 patients by a mean of 1.9 months and a maximum of three resections. Metachronous or recurrent disease occurred in 11% of patients during a mean follow-up period of 36.7 months, though repeat treatment with EMR was successful in all cases. The calculated 5-year survival was 98%, with no cancer-related deaths during the surveillance period.
Table 2. Eligibility criteria for endoscopic mucosal resection in the setting of Barrett’s esophagus.
| Tumor characteristics suggesting a low-risk of lymphatic or systemic spread: |
| • Lesion diameter ≤20 mm |
| • Macroscopically polypoid or flat nodule without ulceration |
| • Well-differentiated or moderately differentiated adenocarcinoma |
| • Tumor limited to the mucosa on the basis of staging procedures (e.g., endoscopic ultrasonography) and proven on histologic examination of the resected specimen |
| • No invasion of lymphatics or veins on histologic examination of the resected specimen |
| No evidence of lymph node involvement or systemic metastasis on staging evaluation |
[Adapted from: Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3-10.].
In a follow-up report published in 2008, their patient cohort had increased to 349 patients with a mean follow-up of 63.6 months (21). The majority of patients underwent EMR, though only 20% were treated with some form of mucosal ablation. Perhaps due to this infrequent use of ablative therapies, the metachronous neoplasia rate increased to 21.5%. The complete response rate was 97%, however, and only 3.7% of patients required esophagectomy for failed endoscopic therapy. The five-year survival was 84% with no EAC-related deaths. Risk factors for recurrent disease included piecemeal resections, long-segment BE, lack of ablative therapy after EMR, and multifocal neoplasia. The data prove the efficacy and safety of EMR in a highly select subgroup of patients referred with IMC and treated at a high-volume specialty center.
Mucosal ablation
Several mucosal ablative technologies have been introduced in recent years for elimination of esophageal metaplasia or early neoplasia. The result of such therapies, however, is that the pathologic epithelium is destroyed, preventing subsequent histopathologic assessment and staging of the disease. The ideal mucosal ablation technology should fulfill a number of criteria (Table 3). As newer devices have been introduced into the marketplace, the fulfillment of these criteria has significantly improved, though none to date has proven perfect.
Table 3. Features of an ideal esophageal mucosal ablation technology.
| • Endoscopic |
| • Automated, quick and reliable |
| • Inexpensive |
| • Removes all Barrett’s esophagus in a single session |
| • Re-treatment possible |
| • Uniform treatment depth limited to mucosa |
| • No subsequent buried glands |
| • No complications |
| • Eliminates the need for surveillance |
PDT and APC were the two endoscopic ablative techniques most studied in years past. Both procedures, however, had significant shortcomings in that the depth of penetration was limited, variable and difficult to predict, complete elimination of pathologic mucosa was not guaranteed, buried BE under squamous epithelium was observed, and a significant complication rate from esophageal strictures, perforations and photosensitivity (with PDT) was reported. The point-and-shoot nature of APC also made it both unreliable and potentially dangerous. As a result of these limitations, the technologies were not widely adopted and endoscopic ablation with them did not supplant the role of esophagectomy for the vast majority of cases of BE with HGD or IMC.
With the recent introduction into clinical practice of RF ablation, and to a lesser extent cryotherapy, the landscape of endoscopic ablative therapies has undergone dramatic change. These modalities have proven quite effective while overcoming many of the limitations of their precursors. Endoscopic ablation of non-nodular (“smooth”) BE with RF, coupled with EMR of discrete mucosal lesions, has clearly been the most significant advance in the treatment of BE and associated early neoplasia over the past decade. The high rate of histologic complete response, along with an excellent safety profile, reasonable cost, and durability of treatment effect, make RF a nearly ideal ablation modality. Eradication of all BE appears to be associated with a lower rate of metachronous neoplasia than resection alone of focal dysplastic or invasive lesions.
Five RF ablation devices (the Barrx 360, Barrx Ultralong, Barrx 90, Barrx 60, and Barrx Channel catheter) are currently manufactured by Covidien Medical (Minneapolis, MN) (Figure 6). Each device consists of tightly approximated electrodes (250 µm spacing) that deliver high-frequency radio waves, generating heat. The established energy density and dosimetry have been shown to cause a reliable depth of tissue injury to the level of the muscularis mucosa, deep enough to destroy the target epithelium, yet not so deep as to injure the submucosa and cause subsequent esophageal stricture formation.
Figure 6.

The five radiofrequency ablation devices made by Covidien Medical (from left to right: Barrx 360, Barrx Ultralong, Barrx 90, Barrx 60 and Barrx Channel catheter).
The balloon-based Barrx 360 is typically used for ablation of circumferential BE. The ablation catheters, consisting of 3 cm of circumferential electrodes wrapped over a 4 cm balloon, come in a variety of diameters (18, 22, 25, 28 and 31 mm). A sizing balloon is initially passed through the esophagus at 1 cm increments to select an ablation catheter of an appropriate diameter. The ablation catheter is then advanced over a guidewire and positioned under endoscopic control at the upper limit of the target epithelium. Dosimetry studies have led to the use of an energy level of 10 Joules/cm2 for NDBE and 12 Joules/cm2 for cases of LGD/HGD (22,23). If the length of BE is greater than 3 cm, the ablation catheter is advanced, positioned to have slight overlap with the initial segment, and ablation is repeated. Once all targeted areas have been treated, the ablation catheter is withdrawn and the coagulum is debrided from the esophageal mucosa. A specially designed cap that fits on the tip of the endoscope is available to facilitate the debridement process. The ablation catheter is similarly cleaned then re-inserted. Ablation is repeated at each level such that two ablations are achieved across the entire segment of BE.
For smaller tongues or islands of BE, the Barrx 90, Barrx Ultralong, Barrx 60, or Barrx Channel Catheter may be utilized. The Barrx 90 is 13 mm wide and 20 mm in length (Figure 7A,B) while the Barrx Ultralong is the same width but 40 mm in length. Each device is designed to fit over the tip of a standard flexible adult upper endoscope while the Barrx Channel catheter is designed to fit down the biopsy channel (Figure 8). An energy level of 12 J/cm2 (40 W/cm2) is appropriate with these devices for both non-dysplastic and dysplastic BE. The endoscope with attached device is inserted transorally, the target epithelium is ablated under direct endoscopic visualization, and ablation is repeated. The ablation catheter is repositioned to the next target zone, and the process carried out until all regions have been ablated twice. The coagulum is debrided using the tip of the device, the scope withdrawn, the ablation catheter cleaned, and the process repeated. Thus, four ablations are performed to each zone. Patients typically are brought back at 2-month intervals for repeat endoscopy and ablation until a complete response, as confirmed by endoscopic biopsies, has been achieved.
Figure 7.
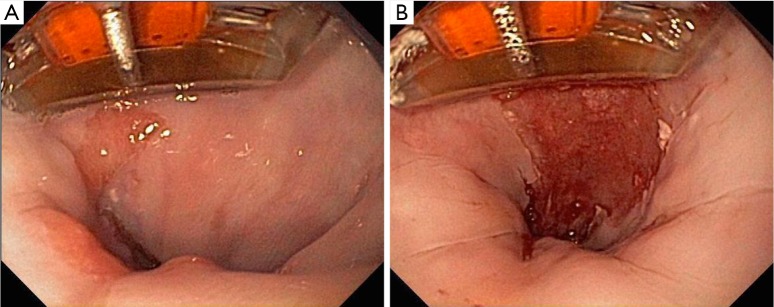
Focal ablation. (A) Barrx 90 attached to the tip of a flexible endoscope with targeted tongue of Barrett’s esophagus; (B) Coagulum that results after radiofrequency ablation (×2) of targeted mucosa. The coagulum is debrided with the tip of the device and the ablation is repeated (×2).
Figure 8.
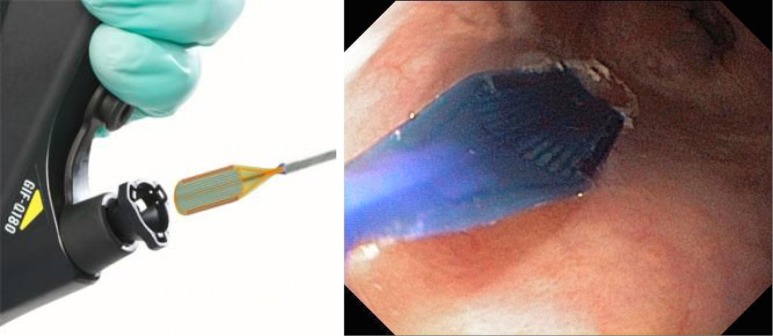
Barrx Channel catheter designed to pass through the biopsy channel of a flexible endoscope.
Multiple studies have demonstrated the safety and efficacy of RF ablation for both dysplastic and nondysplastic BE (NDBE). The most significant study to date relative to dysplastic BE was a multicenter, randomized (2:1), sham-controlled trial from 20 centers in the U.S. One hundred twenty-seven patients with BE and HGD or LGD underwent RF ablation or sham treatment (24). At a median follow-up of 12 months, 81.0% of treated patients achieved complete eradication (CE) of HGD (compared to 19.0% in the sham group), 90.5% had CE of LGD (compared to 22.7% in the sham group), and 77.4% had CE of IM (compared to 2.3% in the sham group) on intention-to-treat analysis. Patients who underwent RF ablation also had less disease progression (3.6% versus 16.3%, P=0.03) and developed fewer cancers (1.2% versus 9.3%, P=0.045) compared to the sham control group. The complication rate and side effect profile associated with treatment were quite low, with 6% developing an esophageal stricture after ablation.
In a subsequent report from this trial, the durability of response to RF ablation was assessed (25). At 2 years follow-up, CE of dysplasia was noted in 95% of patients, while CE of NDBE was found in 93%. By 3 years follow-up, the CE of dysplasia was 98%, while the CE of NDBE was 91%. The development of invasive EAC was found in 0.55% of patients per year, while an esophageal stricture occurred in 7.6%. Finally, in a recent meta-analysis assessing 18 studies of efficacy and 6 studies of durability, the CE of dysplasia was 91% and the CE of NDBE was 78% (26). Progression to EAC was found in 0.2% of patients, and the stricture rate was 5%.
The use of circumferential EMR for excising entire short or long segments of BE has also been evaluated. While complete circumferential excision is feasible, a high rate of subsequent esophageal stenosis has been found [up to 88% in a recent multicenter trial (27)], particularly if the excisions are performed in a single session. Based on these reports, ablation of residual BE appears preferable to stepwise circumferential resection.
Cryotherapy
Cryotherapy also has been utilized for endoscopic ablation of BE with or without dysplasia. The current technology (truFreeze® Spray Cryotherapy, CSA Medical, Lutherville, Maryland) consists of a 7 French catheter advanced via the biopsy channel of a flexible upper endoscope through which liquid nitrogen (–196 °C) is delivered at a low pressure (2-3 pounds per square inch) (Figure 9). The technique requires placement of a specialized decompressive tube into the esophagus and stomach to prevent perforation from barotrauma. Dosimetry is based upon the time the mucosa is exposed to the cryogen and is a matter of some debate. The target lesion is treated under direct visualization and may be smooth or nodular, increasing the applicability of the technology to cases not suitable for RF ablation. In addition, the treatment zone may be focal or diffuse. Another advantage of spray cryotherapy over RF ablation is that non-cellular connective tissue elements, such as collagen and fibrin, are relatively resistant to freezing, thus allowing selective necrosis of cellular elements while preserving the extracellular matrix.
Figure 9.

Spray cryotherapy delivering liquid nitrogen at a low pressure via a catheter advanced through the biopsy channel of a flexible endoscope.
The available data supporting the safety and efficacy of cryotherapy in the ablation of BE are much more limited than the experience reported for RF ablation. A small, multi-institutional case series reported on 23 patients undergoing cryotherapy for BE or cancer, 17 for the diagnosis of HGD (28). The safety profile was excellent, and the CR rates were 94% for HGD, 88% for all dysplasia, and 53% for NDBE, similar to the results reported by others following RF ablation.
Studies comparing esophagectomy and endoscopic therapies for early esophageal neoplasia
Three retrospectively reviewed case series have compared surgical and endoscopic treatment of BE with HGD or esophageal IMC. The first report, from the Mayo Clinic group published in 2009, compared outcomes in 178 patients with IMC treated between 1998 and 2007 (29). Endoscopic therapy was undertaken in 132 patients (74%) and 46 patients (26%) underwent an initial esophagectomy. Endoscopic therapy consisted of EMR alone in 75 (57%) and a combination of EMR with PDT in 57 (43%). At a mean follow-up of 43 months in the endoscopic cohort, 24 patients (18.2%) experienced persistent or recurrent cancer, 9 requiring esophagectomy, 1 undergoing chemoradiation, and 14 being treated with repeat EMR. The overall mortality during the follow-up interval was 17%. For the cohort undergoing an initial esophagectomy, the mean follow-up was 64 months and the overall mortality was 20%. The survival was thought to be comparable between the two groups.
The second report, from 2011, described the experience at the University of Southern California (30). Their cohort consisted of 101 patients with either HGD or IMC, 40 treated via endoscopy and 61 undergoing esophagectomy. The endoscopic treatment group underwent a total of 109 EMRs and 70 ablation sessions. The median number of endoscopic interventions per patient was three. The metachronous neoplasia rate was 20%, with three patients (7.5%) subsequently requiring esophagectomy for endoscopic treatment failure. Comparing endoscopic and surgical therapy, the former was associated with lower morbidity (0% versus 39%), though similar overall (94% in both groups) and disease-free survival at three years.
The third report, also from 2011, assessed outcomes at two high-volume specialty centers in Germany between 1996 and 2009 (31). Seventy-six patients who underwent EMR and APC in Wiesbaden were compared to 38 patients who underwent transthoracic esophagectomy with two-field lymphadenectomy for IMC at the University of Cologne. The groups were matched for age, gender, depth of invasion, and differentiation. Similar to the prior studies, endoscopic treatment was associated with equivalent cure rates compared to esophagectomy, but with lower morbidity and no mortality.
Conclusions
Esophageal cancer remains a highly lethal disease, a fact due mainly to the frequency with which patients present in an advanced stage. Early detection, therefore, is critical, underscoring the importance of the liberal use of endoscopy for assessment of foregut symptoms, screening of patients at high-risk for development of EAC, and surveillance of BE, a known malignant precursor.
The recommended treatment strategy for early esophageal neoplasia in the setting of BE has undergone a revolution in the span of just a few years. With the introduction, refinement, and popularization of EMR techniques and ablative technologies, the vast majority of cases of BE with HGD or IMC now can be treated successfully by endoscopic means. While esophagectomy for early neoplasia can be undertaken with a low mortality rate in appropriate candidates and by experienced centers, the morbidity of such a major surgical procedure remains considerable, as does the potential for negatively impacting long-term quality of life and gastrointestinal function. An endoscopic treatment approach, in carefully selected patients and by expert endoscopists, has been shown to provide cure rates equal to esophagectomy for early stage disease, but with lower morbidity, virtually no mortality, and fewer side effects.
A few comments are relevant, however, when considering a curative endoscopic strategy. The initial endoscopic assessment is critical for detection, mapping and staging of disease, including a meticulous visual inspection of the esophagus for suspicious nodules or subtle irregularities that might harbor a focus of invasive cancer, and perhaps utilizing advanced technologies such as narrow-band imaging, confocal laser endomicroscopy, or vital stains to highlight mucosal detail. Multiple biopsies should be taken of non-nodular BE, as per established protocols, and EMR should be used liberally for excision of suspicious focal lesions. Careful assessment of biopsy and EMR specimens by an experienced gastrointestinal pathologist is critical, as major treatment decisions may hinge on subtle interpretations of histopathologic findings. If such expertise is not available locally, the specimens should be sent to a recognized expert in the assessment of esophageal pathology for a second opinion. Compliance on the part of the patient and rigorous follow-up on the part of the treating physician both are essential, as multiple endoscopic sessions are required for the initial diagnosis, eventual treatment, and subsequent surveillance. As persistent or metachronous neoplasia is not infrequent, the patient must understand that they are agreeing to a prolonged process of evaluation and therapy spanning over years, not merely a single intervention. The patient must also be aware that endoscopic treatment might ultimately fail, leading to esophagectomy at some point in the future or even, rarely, the development of incurable malignancy.
Esophagectomy will continue to play a role for a minority of cases of BE with HGD or IMC, such as for patients unwilling to stay the course of a prolonged endoscopic treatment regimen, tumor characteristics portending a significant risk of nodal metastasis, or cases difficult to manage by endoscopic means, and offers definitive therapy in a single intervention. Surgeons should offer resection options such as THE, MIE or VSE, with low perioperative morbidity and mortality while providing a good long-term quality of life, in order for esophageal resection to remain competitive as a treatment alternative.
The esophageal surgeon must be well-versed in the indications for endoscopic resective and ablative therapies so that they are appropriately applied. Before any treatment decisions are made, the patient should be evaluated and counseled by both an experienced endoscopist and an esophageal surgeon on the available management options, including the pros and cons of each. The best treatment decision for a given patient will depend upon patient factors, such as their desires, their comorbidities, the specifics of their disease, and the salvageability of their esophagus, physician expertise, and local or regional institutional resources.
While the science of endoscopic therapies has progressed a long way in recent years, much is still unknown. Long-term outcome data spanning a decade or more are lacking. Factors predicting failures of ablation, as well as ways to prevent recurrence of neoplasia or metaplasia, require further study. The frequency and duration of surveillance in patients having achieved a complete response to endoscopic treatment is still a topic of debate, as is the cost effectiveness of therapy for various indications including dysplasia and NDBE. Improved methods for detecting submucosal invasion and, more importantly, lymphatic spread would be ideal, as would the identification of biologic or genetic markers predicting a high risk of occult carcinoma or progression to invasive malignancy.
Endoscopic therapies for early esophageal neoplasia are the new standard of care. In few areas of thoracic surgery has a treatment paradigm changed so dramatically and so rapidly with such promising results.
Acknowledgements
The author has been a paid consultant to Covidien, Inc. on projects unrelated to this manuscript.
Disclosure: The author declares no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29 [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31 [DOI] [PubMed] [Google Scholar]
- 3.American Gastroenterological Association , Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91 [DOI] [PubMed] [Google Scholar]
- 4.Williams VA, Watson TJ, Herbella FA, et al. Esophagectomy for high grade dysplasia is safe, curative, and results in good alimentary outcome. J Gastrointest Surg 2007;11:1589-97 [DOI] [PubMed] [Google Scholar]
- 5.Barr H.Ablative mucosectomy is the procedure of choice to prevent Barrett’s cancer. Gut 2003;52:14-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222 [DOI] [PubMed] [Google Scholar]
- 7.Connors RC, Reuben BC, Neumayer LA, et al. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg 2007;205:735-40 [DOI] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37 [DOI] [PubMed] [Google Scholar]
- 9.Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72; discussion 372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando HC, Luketich JD, Buenaventura PO, et al. Outcomes of minimally invasive esophagectomy (MIE) for high-grade dysplasia of the esophagus. Eur J Cardiothorac Surg 2002;22:1-6 [DOI] [PubMed] [Google Scholar]
- 11.Peyre CG, DeMeester SR, Rizzetto C, et al. Vagal-sparing esophagectomy: the ideal operation for intramucosal adenocarcinoma and barrett with high-grade dysplasia. Ann Surg 2007;246:665-71; discussion 671-4 [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Endo M, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc 1992;6:264-5 [DOI] [PubMed] [Google Scholar]
- 13.Rice TW, Zuccaro G, Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998;65:787-92 [DOI] [PubMed] [Google Scholar]
- 14.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703-10 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85 [PubMed] [Google Scholar]
- 16.Altorki NK, Lee PC, Liss Y, et al. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg 2008;247:434-9 [DOI] [PubMed] [Google Scholar]
- 17.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27 [DOI] [PubMed] [Google Scholar]
- 18.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8 [DOI] [PubMed] [Google Scholar]
- 19.May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc 2003;58:167-75 [DOI] [PubMed] [Google Scholar]
- 20.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3-10 [DOI] [PubMed] [Google Scholar]
- 21.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200-6 [DOI] [PubMed] [Google Scholar]
- 22.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc 2007;65:185-95 [DOI] [PubMed] [Google Scholar]
- 23.Sharma VK, Kim HJ, Das A, et al. A prospective pilot trial of ablation of Barrett’s esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system). Endoscopy 2008;40:380-7 [DOI] [PubMed] [Google Scholar]
- 24.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277-88 [DOI] [PubMed] [Google Scholar]
- 25.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011;141:460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73 [DOI] [PubMed] [Google Scholar]
- 28.Greenwald BD, Dumot JA, Horwhat JD, et al. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus 2010;23:13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:39-47 [DOI] [PubMed] [Google Scholar]
- 31.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg 2011;254:67-72 [DOI] [PubMed] [Google Scholar]


