Abstract
Pisotriquetral (PT) osteoarthritis (OA) and enthesopathy of the flexor carpi ulnaris (FCU) are pathologies of the hypothenar eminence which both often remain undiagnosed, but can cause ulnar wrist pain. This study determined the prevalence of these pathologies in an older donor population. Twenty wrists were obtained from 10 cadavers with an age ranging from 65 to 94 years. Radiographs were taken of all wrists with the hand in pisotriquetral view and were assessed for osteoarthritic changes of the PT joint and signs of enthesopathy of the FCU. Ten wrists were grossly dissected and the other ten wrists were sagitally sectioned at a thickness of 10 μm. The wrists were analyzed for type and grade of osteoarthritis and signs of enthesopathy. On radiology, 2 out of 20 wrists showed no signs of osteoarthritis, 5 wrists showed severe changes. One wrist showed signs of enthesopathy. On macroscopy, 9 out of 10 wrists showed osteoartritic changes; 5 of these were severely osteoarthritic. On microscopy, all wrists showed some degree of osteoarthritis of which five showed severe changes. Signs of enthesopathy were seen in seven wrists. Pisotriquetral osteoarthritis has a high prevalence in the older donor population and may therefore be a cause of ulnar sided wrist pain. It should therefore always be considered in the differential diagnosis of ulnar sided wrist pain. By performing clinical examination with these pathologies in mind, diagnosis could be a lot faster. Furthermore, based on our results, radiographs seem to be not accurate in diagnosing osteoarthritis of the PT joint and enthesopathy of the FCU.
Keywords: Ulnar-sided wrist pain, Enthesopathy, Flexor carpi ulnaris, Pisiform, Pisotriquetral joint, Triquetrum, Osteoarthritis
Introduction
Osteoarthritis (OA) of the pisotriquetral (PT) joint and enthesopathy of the flexor carpi ulnaris (FCU) tendon are pathologies of the hypothenar eminence, which are often diagnosed late for several reasons: First, patients experience a vague pain at the palmar and ulnar side of the wrist often unrelated to trauma. Second, standard radiology is of little value for the diagnosis, and third these two pathologies are still quite unknown and often not included in the differential diagnosis [1].
OA is a chronic degenerative disease of the cartilage that affects the synovial joints [1]. OA of the PT joint can be a normal stage in aging, but can, specifically in this joint, also occur after a fracture, as a consequence of chronic repetitive trauma as occurring in several sports, or occur in rheumatoid arthritis. PT OA can also be aggravated after carpal tunnel release [2–5].
Stability of the pisiform is achieved by the attachment of several structures such as the FCU tendon, which continues as the pisometacarpal and the pisohamate ligament, the extensor and flexor retinaculum. The PT-joint is surrounded by a loose but strong joint capsule, which allows great mobility [6–8].
Enthesopathy is an inflammatory reaction at the level of an enthesis, which is the site of attachment of a tendon, ligament or joint capsule to the skeleton [9]. It is also referred to as tendinosis or tendinitis, specifically at the attachment site to the bone. Patients with enthesopathy of the FCU tendon often complain of activity related pain localized at the distal site of this tendon. Physical examination reveals tenderness approximately 3 cm proximal to the insertion of the FCU tendon on the pisiform in all patients as well as pain localized to the FCU tendon with resisted flexion and ulnar deviation of the wrist [10].
Diagnosing PT OA or enthesopathy of the FCU tendon is mainly based on clinical examination. Standard radiographs in semisupinated oblique view may reveal evidence of PT OA or evidence of enthesopathy of the FCU tendon like tendon calcifications near the pisiform bone. As clinical findings are usually unequivocal, ultrasound (US), computed tomography (CT) scanning and magnetic resonance (MR) imaging are rarely indicated [11]. US and MR images may show degeneration of the terminal portion of the FCU tendon and sometimes paratenon inflammation. As there is no synovial membrane around the FCU, no tenosynovitis is observed [12].
PT OA and enthesopathy can both be treated conservatively with splints, rest, and steroid injections. If conservative treatment fails, pisiformectomy or arthroscopic resection arthroplasty of the pisotriquetral joint can be performed [7, 13, 14].
Our hypothesis is that pathology of the PT joint has a high prevalence in the older population and therefore PT OA and enthesopathy of the FCU always belong in the differential diagnosis of ulnar wrist pain. The prevalence might currently be underestimated because of the common delay in diagnosis. The aim of this study was to determine the prevalence of OA of the PT joint and of enthesopathy of the FCU in a random donor population.
Methods
Twenty wrists, fixed with 3 % formaldehyde, were obtained from ten cadavers from the Anatomy Department of the University Medical Centre Utrecht. The population consisted of nine women and one man with an age ranging from 65 to 94 years. The mean age was 84 years. Medical histories of the patients were not available.
Radiology
Radiographs were taken of each wrist in semisupinated oblique view (30° supination) [15]. The radiographs were judged independently by three observers; a radiologist, a handsurgeon and a plastic surgeon. After assessment of the PT joint surface, joint space and presence of osteophytes, the radiographs were classified as normal, mild OA or OA. The radiographs were also assessed for calcifications in the FCU tendon and classified as ‘signs of enthesopathy’ or healthy.
Macroscopy
Ten wrists from ten different cadavers, five left and five right wrists, were dissected. The structures attaching to the pisiform were identified and the PT joint was opened and inspected. To visualize cartilage defects of the joint surfaces black Indian ink was applied [16] (Fig. 1). The severity of OA in the specimens was graded on a scale used by Bentley and Hill [17] for DIP joint OA, and classified by pattern on a scale designed by Yamaguchi et al. [18] by two of the authors (Table 1).
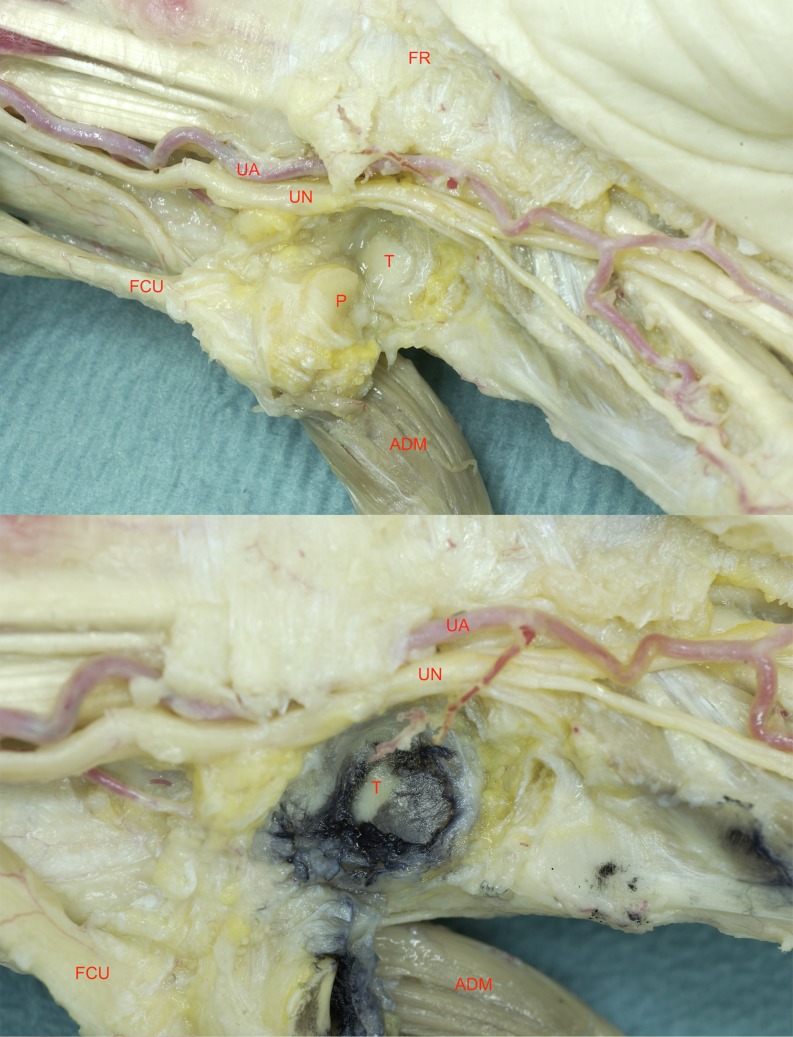
Fig. 1.
Anatomic dissection of the wrist (11–36) FR flexor retinaculum, UN ulnar nerve, UA ulnar artery, T triquetrum, P pisiform, FCU flexor carpi ulnaris tendon, ADM abductor digiti minimi muscle (a). After applicance of Indian ink to the PT-joint, damaged cartilage appears black, as this ink does not adhere to subchrondral bone (b)
Table 1.
Used grading system for scoring OA on macroscopy and microscopy
| Macroscopy Grade (0–6) |
Macroscopy Type (1–5) |
Microscopy Score = Grade × stage (0–24) |
|
|---|---|---|---|
| 0 = Grossly normal. 1 = Cartilage damage 2 = lesions extended to the subchondral bone, <50 % of the joint surface. 3 = >50 % exposed bone |
1 = Central 2 = Peripheral 3 = Fan-shaped 4 = Mixture of types 5 = Total |
Grade (0–6) 0 = Surface/cartilage intact 1 = Surface intact 2 = Surface discontinuity 3 = Vertical fissures 4 = Erosion 5 = Denudation 6 = Deformation |
Stage (0–5) 0 = NO OA activity 1 = <10 % affected 2 = 1–25 % 3 = 25–50 % 4 = >50 % affected |
Histology
The remaining wrists (n = 10) were sectioned at a thickness of 10 μm. The specimens were decalcified with a composite of 1 M sodium formiat and 35 % formic acid. After decalcification they were dehydrated in a series of alcohol and embedded in paraffin. At three different levels with an interval of 30 μm sections were cut in the sagittal plane. They were mounted on glass slides and stained according to the following methods. Hematoxylin and eosin (HE) stain was standardly applied to all sections. Safranin-O was applied to visualize the cartilage layer. Azan stain was applied to visualize collagen fibers. Elastic van Gieson (EVG) was applied to demonstrate elastic and collagen fibers [19]. Evaluation was performed by three of the authors. The specimens were scored according to the OARSI criteria for features of OA [20] (Table 1). The three different levels (ulnar to radial) were scored separately. The sections were also screened for enthesopathy signs: fibroblast hyperplasia, vascular hyperplasia, disorganised collagen. Each of these three signs was scored with one point. Where 0 points would imply no signs of enthesopathy, 3 points was graded as signs of enthesopathy. Abnormalities in the appearance of Sharpey’s fibers, the site of attachment where collagen fibers penetrate into the bone, were also examined.
Results
Radiology
On X-ray, five out of twenty wrists were considered as ‘OA’ by all three observers (Fig. 2). Two out of twenty wrists were considered as ‘no OA’ by all three observers. In the remaining 13 wrists there was a large interobserver variability. They were judged differently between the three observers and therefore not applicable. One wrist showed signs of enthesopathy; calcifications in the tendon at the site where it approaches the pisiform bone [11].
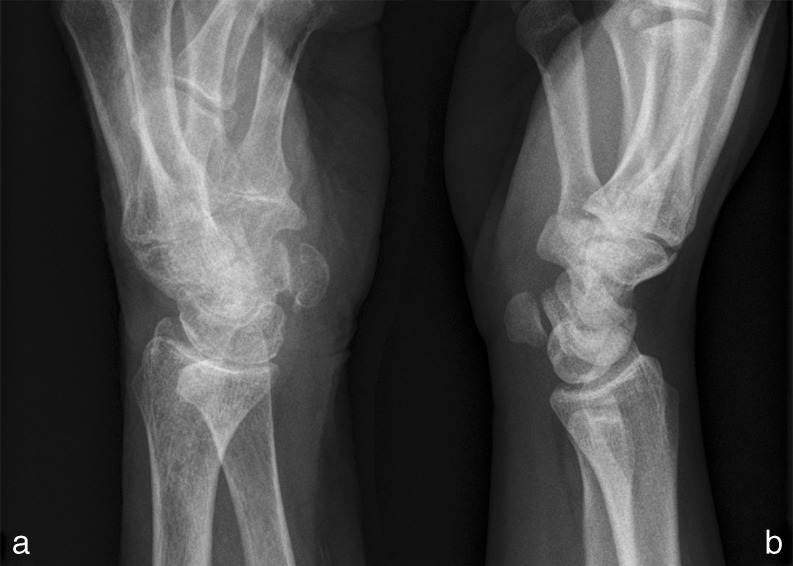
Fig. 2.
Radiograph of a wrist with OA of the PT-joint (a, 11-30 left) next to a radiograph of a wrist without OA (b) in semisupinated oblique view
Macroscopy
At macroscopic evaluation nine out of ten wrists showed some form of joint damage. Only one wrist appeared normal. In the pisiform, the most common grade of damage [17] was grade 1 which occurred in six out of ten wrists. In two wrists there was grade 2 damage and in one wrist there was grade 3 damage.
The triquetrum mainly showed grade 1 and 2 damage. Only one wrist showed no damage. Four wrists showed grade 1, four wrists showed grade 2 and one wrist showed grade 3 damage. Furthermore, most joint surfaces of the PT joint showed grade 1: damage to the articular cartilage, but no exposure of subchondral bone.
In the pisiform, the most common type [18] was central damage, which occurred in four wrists. Two wrists showed a mixed type of damage (grade 4), and the other four wrists showed different types.
In the triquetrum, the most common type was peripheral damage, which occurred in four wrists. Three wrists showed a mixed type, two wrists showed central damage. We did not find any macroscopic signs of enthesopathy in any of the ten wrists.
Microscopy
At microscopic evaluation, all ten wrists showed some degree of OA at one of the three section levels (Table 2). One wrist (10–105) showed the most severe grade of OA at all three levels, in both pisiform and triquetrum (Fig. 3). Three wrists showed deformation of the subchrondral bone in the most radial level of the sections. The other six wrists showed variable damage to the cartilage layer (Fig. 4). The least affected wrist (10–115) showed grade 3 defects. In all affected wrists, both the pisiform and triquetum were affected (Fig. 5). Seven wrists showed signs of enthesopathy. There was a disorganisation of collagen fibers at the palmar side of the enthesis in seven wrists. Three wrists showed changes at the enthesis of the FCU tendon with all signs of enthesopathy (Fig. 6). Four wrists showed slight signs of enthesopathy. Three wrists showed no signs of enthesopathy. No signs of acute inflammation were visible. No specific changes in Sharpey’s fibers were seen. The interobserver variability on macroscopy and microscopy was neglectible. The results are represented in Table 2.
Table 2.
Compared radiological, histological and macroscopic results of osteoarthritic and degenerative changes in 20 wrists of a cadaveric population
| Cadaver (number) | Age (years) | Gender (M/F) | Radiology | Macroscopy | Microscopy | |
|---|---|---|---|---|---|---|
| OA OARSI score |
Enthesopathy | |||||
| 10-33 Left | 88 | F | N/A | P Type 5 Grade 3 T Type 5 Grade 2 |
– | |
| 10-33 Right | Osteoarthritis | – | P 16/12/24 T 8/16/24 |
3 Slight Enthesopathy |
||
| 10-116 Left | 94 | F | Osteoarthritis | P Type 4 Grade 2 T Type 2 Grade 2 |
– | |
| 10-116 Right | Osteoarthritis | – | P 16/6/24 T 12/8/24 |
3 Enthesopathy |
||
| 11-41 Right | 65 | F | N/A | P Type 2 Grade 1 T Type 4 Grade 2 |
||
| 11-41 Left | N/A | – | P 12/15/24 T -/9/24 |
3 Slight Enthesopathy |
||
| 11-35 Right | 87 | F | N/A | P Type 2 Grade 1 T Type 4 Grade 1 |
||
| 11-35 Left | N/A | – | P 12/12/12 T 6/24/24 |
2 Slight Enthesopathy |
||
| 11-30 Right | 80 | F | Osteoarthritis | P Type 2 Grade 1 T Type 2 Grade 1 |
– | |
| 11-30 Left | Osteoarthritis | – | P -/10/16 T 16/24/- |
3 Slight Enthesopathy |
||
| 11-11 Right | 85 | F | N/A | P Type 0 Grade 0 T Type 0 Grade 0 |
– | |
| 11-11 Left | N/A | – | P 20/16/- T 16/16/- |
0 | ||
| 10-105 Left | 69 | M | N/A | P Type 5 Grade 3 T Type 5 Grade 3 |
– | |
| 10-105 Right | Enthesopathy | – | P 24/24/24 T 24/24/24 |
3 Enthesopathy |
||
| 10-115 Left | 85 | F | N/A | P Type 2 Grade 1 T Type 2 Grade 1 |
– | |
| 10-115 Right | N/A | – | P 12/9/- T 6/9/- |
1 | ||
| 11-36 Left | 90 | F | Healthy | P Type 2 Grade 1 T Type 4 Grade 1 |
– | |
| 11-36 Right | N/A | – | P 12/12/9 T 8/12/16 |
0 | ||
| 11-06 Right | 94 | F | N/A | P Type 5 Grade 1 T Type 5 Grade 2 |
– | |
| 11-06 Left | Healthy | – | P -/16/16 T 12/16/- |
2 Enthesopathy |
||
P pisiform, T triquetrum. The three values in the 6th column correspond with the levels of the sections, from ulnar to radial, respectively. In some joints, the section interval of 300 μm was too wide, which lead to no result in the radial section, or the triquetrum was not yet present in the ulnar section (−) M Male, F Female, N/A not applicable
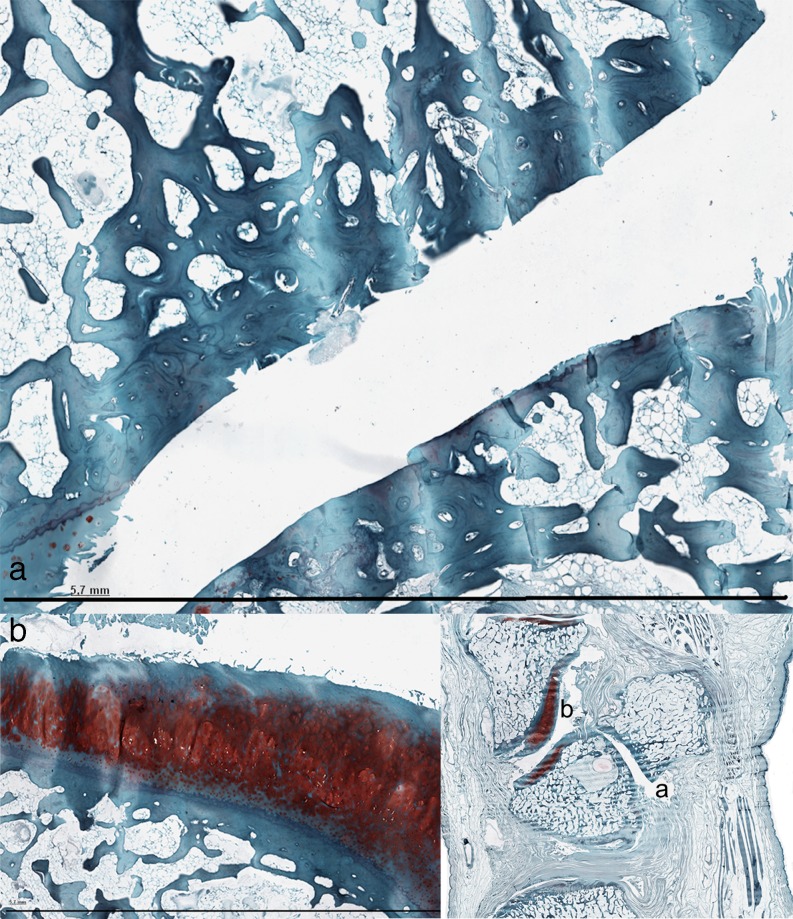
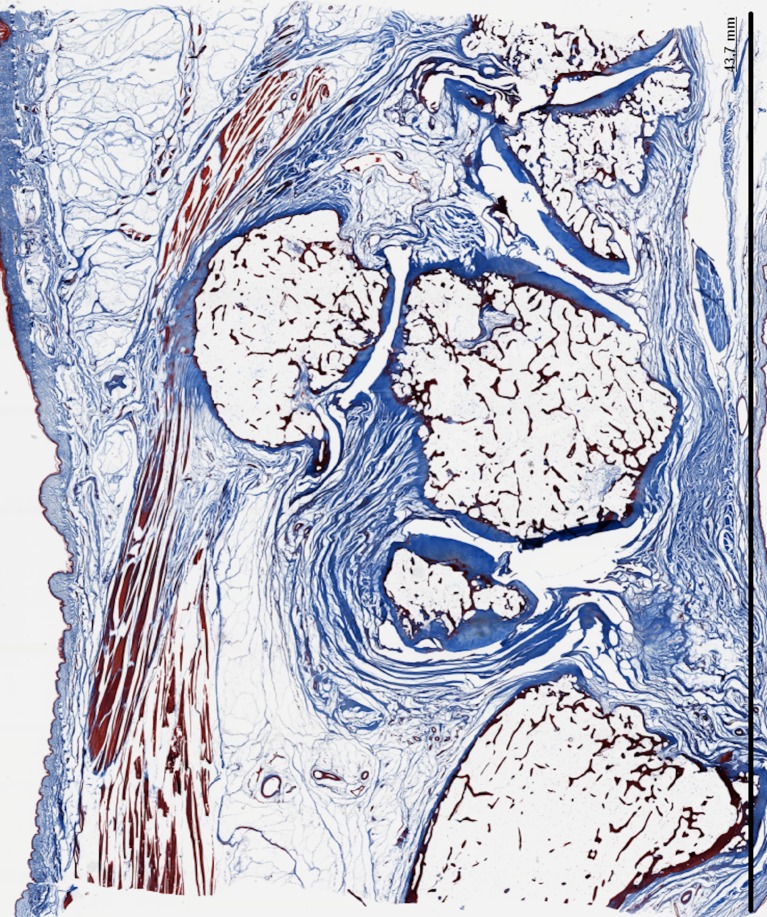
Fig. 3.
a Denudation and deformation of subchondral bone in 10-105 (Safranin-O stain) b Enlargement of the cartilage layer of the hamatum in the same specimen. The safranin-O stain shows only surface discontinuity of the cartilage layer, while the cartilage layer of the PT-joint is completely deformed
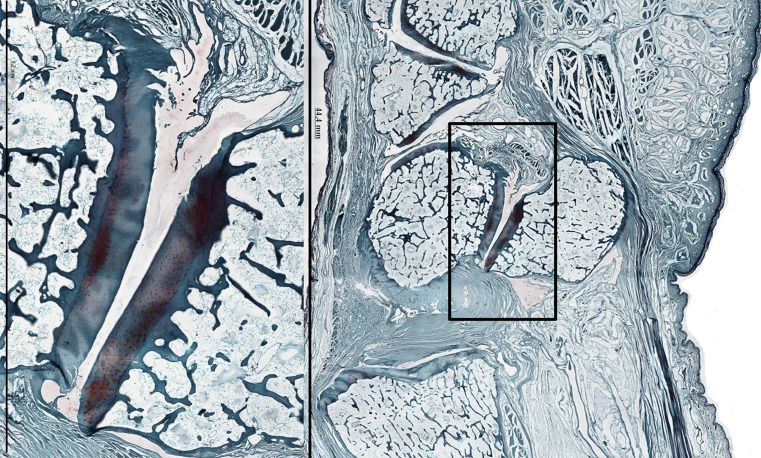
Fig. 4.
PT-joint in a Safranin-O stain of wrist 11-36 (right). Loss of matrix staining in the cartilaginous layer of the PT-joint, without deformation of the cartilage layer or denudation of subchondral bone
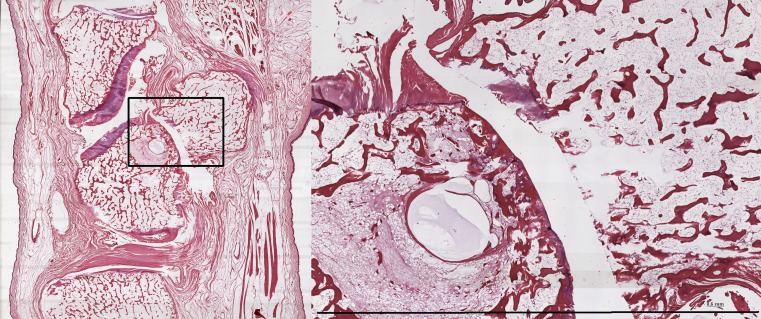
Fig. 5.
Overview of the enthesis and PT-joint of the Azan stain of wrist 11-30 (left)
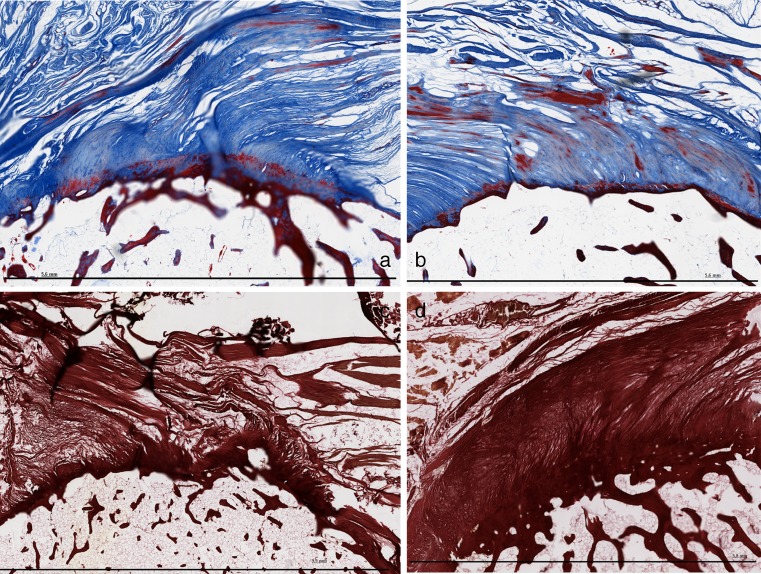
Fig. 6.
a Enthesis in the Azan stain of 11-30 b Enthesis in the Azan stain of 11-36 c Enthesis in EvG stain of 11.06 d Enthesis in EvG stain of 10-105
Discussion
The main results of the present study is that PT OA has a high prevalence in the older donor population. Nine out of ten PT-joints showed OA on macroscopy, and all ten PT-joints showed OA on microscopy. Radiographs are not sufficient as the only diagnostic tool in suspected pathology of the PT-joint and FCU-enthesis.
To the best of our knowledge this is the first study that compares radiology, macroscopy and microscopy of the PT joint. As the pairs, the two wrists of the same cadavers, were divided into two groups—one wrist went for dissection, and the other one for microscopic assessment—it was not possible to compare macroscopic and microscopic evaluations, and therefore we were not able to calculate the correlation between radiology, macroscopy and microscopy. However, Yamaguchi et al. [18] found that in 39 cadaver pairs, 29 pairs had degenerative changes of the PT joints in both hands.
Our results indicate that radiographs are easily misinterpreted. While radiographs should be taken to rule out other diagnoses, they are not sufficiently accurate in diagnosing pathology of the PT-joint and FCU-enthesis. Clinical findings are usually unequivocal, but if necessary, US or MR can be used in diagnosing FCU enthesopathy, and CT can be used to illustrate PT OA [11]. The large interobserver variability on radiology may also be partially explained by the difficulty of classifying radiographs of older specimens in which the bone densities are different than in somewhat younger subjects. Enthesopathy is an even more difficult diagnosis. Although changes related to enthesopathy can be imaged by conventional radiography, it fails to show enthesopathy in an early stage and only shows changes of patients with more advanced disease [21]. Also, enthesopathy can be either calcific or non-calcific. Calcific tendinitis of the FCU presents a characteristic radiographic appearance of amorphous calcification, caused by connective tissue degeneration near the pisiform [22]. However, non-calcific enthesopathy gives similar complaints but lacks the specific radiographic appearance.
The anatomic dissections showed that the most common sites for chondromalacia or exposed subchondral bone were radial and distal. This is in line with Yamaguchi et al. [18], who showed that degenerative changes in the PT joint occur most frequently in the distal, distal-radial, and radial aspect of the pisiform and triquetrum and in the distal-ulnar aspect of the triquetrum. This can partially be explained by the theory that during wrist extension the pisiform is pressed against the distal triquetrum while it translates distally. As ulnar deviation applies large forces to the PT joint as well, this causes peak shearing forces on the radial and distal aspects of the pisiform [6, 8, 18, 23].
It can be questioned whether OA is a normal stage in aging. Figure 7 reveals that there was more OA in the PT-joint than in adjacent joints. In this image, the hamatotriquetral and metacarpal joint were undamaged whilst the PT-joint was severely damaged. It is questionable whether OA in the PT-joint could be subscribed to more factors than only age-dependent cartilage degradation.
Fig. 7.
Denudation of subchondral bone in the PT-joint of specimen 10-105 (HE) with enlargement of the PT-joint in specimen 10-105 on the right
It was difficult obtaining information about enthesopathy from recent articles; there is not much published yet about the histological findings in patients with enthesopathy. In a study of McGonagle et al., it was mentioned that the gold standard for validation that entheseal tenderness represents enthesopathy (e.g. histologic confirmation) posed considerable problems because of the extreme difficulty in obtaining tissue [21]. Enthesopathy mainly occurs at the palmar side of the pisiform. We hypothesize that this is because our hands mainly rest on the pisiform possibly leading to tendinosis at the specific site of the enthesis.
The current study has a few limitations. The most important limitation of our study is the relatively small number of cadavers, which did not allow statistical evaluations. Another limitation is that prolonged storage in aqueous fixatives such as formaldehyde may lead to proteoglycan diffusion into the fixative and thereby loss of matrix staining [20] in the cartilaginous layer (Safranin-O stain). As a result some of our sections might have been false-positive. However, the most severe cases demonstrated denudated or deformed bone and the cartilage was completely lost excluding false-positive scoring.
Furthermore, as we randomly chose these cadavers nine where female and one was male. This might influence our results as demonstrated in a study of Beckers et al. [6] in which PT OA was found significantly more in females than in age-matched males. Finally we did not include reference material with younger subjects.
For the surgeon it is important to differentiate between enthesopathy and OA. According to Budoff et al. [10], the exact location of the pain may help. In case of pisiform pathology patients will point exactly to the pisiform bone whilst they point about 3 cm more proximally if the pain is elicited by FCU tendinopathy.
Pain is most severe in wrists with denudation of the bone since cartilage does not have a nerve supply. In our cadaveric population five wrists showed exposed subchrondral bone on macroscopy and six wrists showed either denudation or even bone deformation on microscopy. Unfortunately we do not know whether these persons suffered from wrist pain as we do not possess the medical histories.
The relevance of these presented results is that PT OA and enthesopathy of the FCU are pathologies which should not be forgotten in the differential diagnosis of ulnar sided wrist pain.
Conclusion
According to the present study, pisotriquetral osteoarthritis and enthesopathy of the flexor carpi ulnaris have a high prevalence in the older donor population and should therefore always be considered in the differential diagnosis of ulnar sided wrist pain. By performing clinical examination of the wrist with these pathologies in mind, diagnosis could be a lot faster. While radiographs should be taken to rule out other diagnoses, they are not sufficiently accurate in diagnosing osteoarthritis of the PT joint and enthesopathy of the FCU.
Acknowledgments
We wish to thank S. Plomp and F. van Zoomeren for their help with the macroscopic specimens. We also thank the people from the pathology department especially W. Hermsen for his help with the preparation of the histologic specimens and N. Stathonikos for his help with scanning the sections. Our gratitude goes to Dr. H.J. Prins and Dr. P. van Minnen for their valuable judgment.
References
- 1.Strackee SD, Bos KE. Pathologie van het os pisiforme en piso-triquetrale gewricht—een diagnose die frequent laat wordt gesteld. Ned Tijdschr Traum. 1997;6:150–157. [Google Scholar]
- 2.Stahl S, Calif E. Latent pisotriquetral arthrosis unmasked following carpal tunnel release. Orthopedics. 2010;33(9):673. doi: 10.3928/01477447-20100722-06. [DOI] [PubMed] [Google Scholar]
- 3.Helal B. Chronic overuse injuries of the piso-triquetral joint in racquet game players. Br J Sports Med. 1978;12(4):195–198. doi: 10.1136/bjsm.12.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayan GM. Pisiform ligament complex syndrome and pisotriquetral arthrosis. Hand Clin. 2005;21(4):507–517. doi: 10.1016/j.hcl.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Guo X, Fan Y, Li ZM. Effects of dividing the transverse carpal ligament on the mechanical behavior of the carpal bones under axial compressive load: a finite element study. Med Eng Phys. 2009;31(2):188–194. doi: 10.1016/j.medengphy.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Beckers A, Koebke J. Mechanical strain at the pisotriquetral joint. Clin Anat. 1998;11(5):320–326. doi: 10.1002/(SICI)1098-2353(1998)11:5<320::AID-CA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Collins ED, Gharbaoui I. Imaging and anatomic study of the pisiform bone/ulnar nerve relationship-evaluation of the preferred surgical approach for the excision of the pisiform bone. Tech Hand Up Extrem Surg. 2010;14(3):150–154. doi: 10.1097/BTH.0b013e3181ccb7c3. [DOI] [PubMed] [Google Scholar]
- 8.Moojen TM, Snel JG, Ritt MJ, Venema HW, den Heeten GJ, Bos KE. Pisiform kinematics in vivo. J Hand Surg [Am] 2001;26(5):901–907. doi: 10.1053/jhsu.2001.26199. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports. 2009;19(4):520–527. doi: 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 10.Budoff JE, Kraushaar BS, Ayala G. Flexor carpi ulnaris tendinopathy. J Hand Surg [Am] 2005;30(1):125–129. doi: 10.1016/j.jhsa.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Blum AG, Zabel JP, Kohlmann R, Batch T, Barbara K, Zhu X, Dautel G, Dap F. Pathologic conditions of the hypothenar eminence: evaluation with multidetector CT and MR imaging. Radiographics. 2006;26(4):1021–1044. doi: 10.1148/rg.264055114. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe A, Souza F, Vezeridis PS, et al. Ulnar-sided wrist pain. II. Clinical imaging and treatment. Skelet Radiol. 2010;39:837–857. doi: 10.1007/s00256-009-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss KE, Rodner CM. Osteoarthritis of the wrist. J Hand Surg. 2007;32A:725–746. doi: 10.1016/j.jhsa.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Belliappa PP, Burke FD. Excision of the pisiform in piso-triquetral osteoarthritis. J Hand Surg (Br) 1992;17(2):133–136. doi: 10.1016/0266-7681(92)90072-A. [DOI] [PubMed] [Google Scholar]
- 15.Gardner-Thorpe D, Giddins GE. A reliable technique for radiographic imaging of the pisotriquetral joint. J Hand Surg (Br) 1999;24(2):252. doi: 10.1054/jhsb.1998.0187. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113–S116. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Bentley BS, Hill RV. Assessing macroscopic and microscopic indicators of osteoarthritis in the distal interphalangeal joints: a cadaveric study. Clin Anat. 2007;20(7):799–807. doi: 10.1002/ca.20511. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi S, Viegas SF, Patterson RM. Anatomic study of the pisotriquetral joint: ligament anatomy and cartilagenous change. J Hand Surg [Am] 1998;23(4):600–606. doi: 10.1016/S0363-5023(98)80044-0. [DOI] [PubMed] [Google Scholar]
- 19.Popko M, Bleys RL, De Groot JW, et al. Histological structure of the nasal cartilages and their perichondrial envelope. Rhinology. 2007;45(2):148–152. [PubMed] [Google Scholar]
- 20.Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. OsteoArthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.McGonagle D. Diagnosis and treatment of enthesitis. Rheum Dis Clin N Am. 2003;29(3):549–560. doi: 10.1016/S0889-857X(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 22.Gandee RW, Harrison RB, Dee PM. Peritendinitis calcarea of flexor carpi ulnaris. AJR Am J Roentgenol. 1979;133(6):1139–1141. doi: 10.2214/ajr.133.6.1139. [DOI] [PubMed] [Google Scholar]
- 23.Viegas SF, Yamaguchi S, Boyd NL, Patterson RM. The dorsal ligaments of the wrist: anatomy, mechanical properties, and function. J Hand Surg [Am] 1999;24(3):456–468. doi: 10.1053/jhsu.1999.0456. [DOI] [PubMed] [Google Scholar]
- 24.Agha RA, Webb B. A cadaveric investigation into the links between macroscopic and microscopic osteoarthritic changes at the hip. Clin Anat. 2006;19:115–124. doi: 10.1002/ca.20197. [DOI] [PubMed] [Google Scholar]