Abstract
Because hyperbaric oxygen treatment mobilizes bone marrow derived-stem/progenitor cells by a free radical mediated mechanism, we hypothesized that there may be differences in mobilization efficiency based on exposure to different oxygen partial pressures. Blood from twenty consecutive patients was obtained before and after the 1st, 10th and 20th treatment at two clinical centers using protocols involving exposures to oxygen at either 2.0 or 2.5 atmospheres absolute (ATA). Post-treatment values of CD34+, CD45-dim leukocytes were always 2-fold greater than the pre-treatment values for both protocols. Values for those treated at 2.5 ATA were significantly greater than the 2.0 ATA treatment group by factors of 1.9 to 3-fold after the 10th and before and after the 20th treatments. Intracellular content of hypoxia inducible factors -1,-2, and -3, thioredoxin-1 and poly-ADP-ribose polymerase assessed in permeabilized CD34+ cells with fluorophore-conjugated antibodies were twice as high in all post- versus pre-treatment samples with no significant differences between 2.0 and 2.5 ATA protocols. We conclude that putative progenitor cell mobilization is higher with 2.5 versus 2.0 ATA treatments, and all newly mobilized cells exhibit higher concentrations of an array of regulatory proteins.
Keywords: Vasculogenic stem cells; hyperoxia; nitric oxide synthase; CD34; hypoxia inducible factors (HIF-1,2,3a); thioredoxin-1; poly-ADP-ribose polymerase
1. Introduction
Stem/progenitor cells (SPCs) capable of multipotent differentiation can be mobilized from bone marrow and adipose tissue, enter the blood stream and migrate to peripheral sites where they may facilitate recovery from injuries1–3. SPCs mobilization occurs after wounding, physical exertion and in response to a variety of chemical agents4–10. Exposure to hyperbaric oxygen (HBO2) appears to be a reliable way to mobilize SPCs in humans and also has been shown in rodents and horses11–15. Animal studies indicate that one mechanism is based on activation of nitric oxide synthase type 3 (NOS-3) in bone marrow stromal cells with subsequent liberation of stem cell factor 11, 16. Separate from mobilization, HBO2 improves engraftment and differentiation of several progenitor cell types in organs such as spleen, bone marrow, brain, peripheral nerve, pancreas, cartilage and heart 17–23. One area of interest with circulating SPCs is identification of the sub-set having propensity to form vascular endothelium, so-called endothelial progenitor cells (EPCs) 24. Quantification of mobilized EPCs is based on flow cytometric detection of cell surface proteins and phenotypic manifestations of laboratory-grown clones 24, 25. Cells mobilized by HBO2 exhibit many of these surface markers and when cultured, some clones show lectin binding consistent with an endothelial phenotype 11, 12. Animal studies have documented that HBO2-mobilized SPCs form blood vessels in vivo and hasten wound healing 14, 16, 26.
HBO2-mobilized SPCs have greater content of hypoxia inducible factors (HIFs) and thioredoxin-1 (Trx), which in the murine model confers improved neovascularization 12, 14, 27. Subsequent to HBO2 treatments of refractory wounds and diabetic patients, the number of wound margin SPCs is increased and local HIFs and Trx appear to be within these localized SPCs 12, 13. This suggests that SPCs play a role in supplying factors required for wound healing. Hence, evaluating intracellular proteins may have greater importance to assess SPCs function versus ex vivo manipulations. Assessment of intracellular regulatory proteins of cells selected based on surface markers precludes studying ex vivo cell growth because of need to permeabilize the cell membranes.
HBO2 treatment involves breathing 100 percent O2 at 2 to 3 atmospheres absolute (ATA) pressure for 1.5 to 2 hours once or twice daily. HBO2 has been shown to improve refractory diabetic wounds and delayed radiation injuries in randomized trials and use is supported by independent evidence-based reviews 28–34. Several studies have failed to identify clinical efficacy 35, 36. Notably, these studies involved exposures to 2.0 ATA or use of face masks with questionable seals thus reducing the fraction of inspired O2; whereas several prospective randomized trials documenting therapeutic benefit utilized pressures of 2.4 or 2.5 ATA in pure O2-filled chambers or using head-covering hoods 34, 37. Whether clinical results may differ because of treatment protocols is unclear. The goal of this investigation was to evaluate whether mobilization of cells with surface markers considered consistent with SPCs (CD34+ and CD45-dim) and content of intracellular regulatory proteins differed between two commonly used HBO2 protocols 38.
2. Methods
2.1 Patient management protocols
All procedures were approved by Institutional Review Boards and patients signed informed consent. A consecutive series of patients was approached who had been referred for HBO2 treatment because of complications from radiotherapy for cancer. On the basis of current standard of care, they were to receive at least 20 HBO2 therapy sessions. Patient characteristics are shown in Table 1. Venous blood was collected prior to and after the 1st, 10th and 20th HBO2 treatment into Cyto-Chex BCT test tubes (Streck, Inc., Omaha, NE) that contain a proprietary preservative. Samples from the same day of treatment (pre- and post-HBO2) were analyzed concurrently within 3 days of collection.
Table 1. Patient characteristics.
Details for the Penn and Syracuse treatment centers show age/gender, cancer location, radiation dose (cGy), other health issues, tobacco and ethanol use. Penn-based patients were 62.1 ± 2.4 (mean ± SE) years old, 5 were female; Syracuse-based patients were 62.0 ± 2.5 years old (NS), 5 were female. Radiation dosage was known in 14 Penn-based patients (6409 ± 133 cGy) and 12 Syracuse-based patients (6635 ± 345, NS).
| Penn# | Age/Gender | Cancer | Radiat. | Other | Medications | Tobacco | Ethanol |
|---|---|---|---|---|---|---|---|
| 1 | 43M | Tongue | 6000 | HTN, HIV, COPD. Epilepsy, Asthma | Fosamprenavir, ritonavir, trimethoprim-sulfamethoxazole | None | None |
| 2 | 67M | Tongue | 6600 | HTN, DM-2, Adrenal CA | Amlodipine, terazosin, senna, MVI | None | Occasional |
| 3 | 68M | Prostate | Brachy, ND | Depression | Morphine, oxycodone, docusate sodium, omeprazole, citalipram, cyclobenzaprine HCl, gabapentin | Quit >6 weeks | None |
| 4 | 58F | Sinus | 5400 | HTN, DVT, Cataracts, Glaucoma | Amlodipine, warfarin, alendronate, latanoprost gtts, ciprodex gtts | None | None |
| 5 | 39F | Cervix | ND | Depression | Paclitaxel, methadone, gabapentin, bupropion HCl fluoxetine, pentosan polysulfate, alprazolam, clonazepam | None | Occasional |
| 6 | 55M | Neck | 6530 | HTN, CAD, CVA, Cholesterol, | Ibuprofen, ranitidine, MVI | None | None |
| 7 | 63M | Larynx | ND | COPD, Hypothyroid | Gabapentin, levothyroxine | Quit >3 yrs | Occasional |
| 8 | 68M | Tongue | ND | Lymphoma, Hypothyroid | Ramapril, levothyroxine | None | Occasional |
| 9 | 57M | Tonsil | 6300 | None | Pregabalin, oxycodone, lansoprazole, MVI, glycopyrrolate | Quit >3 yrs | Occasional |
| 10 | 67M | Tongue | 7000 | GERD | Esomepra zole | None | None |
| 11 | 48M | Tonsil | 6300 | Asthma | Albuterol, gabapentin, glycopyrrolate, oxycodone | Quit >5 yrs | None |
| 12 | 65F | Tongue | 5580 | Hypothyroid, Cataracts | Levothyroxine | None | Occasional |
| 13 | 76M | Prostate | ND | HTN, Cholesterol | Metoprolol, amlodipine, prevastatin | Quit >25 yrs | Occasional |
| 14 | 79M | Tongue | 6820 | HTN, DM-2, COPD | Metoprolol, losartan, HCTZ, irbesartan, chlorpheniramine-hydrocodone syrup | Quit >15 yrs | Occasional |
| 15 | 68F | Tongue | 7000 | HTN, GERD, Cholesterol, CAD | nebivolol, clopidogrel, rosuvastatin, metoclopramide | Quit >3 yrs | Occasional |
| 16 | 56M | Tongue | ND | Cholesterol, CAD | Pentoxifylline, rosuvastatin, oxycodone | None | Occasional |
| 17 | 78M | Prostate | Brachy, ND | HTN, Crohn’s | Atenolol | None | None |
| 18 | 56M | Neck | 6600 | HTN, Migraine, Cholesterol, Gout | Ezetimibe, pitavastatin, rizatriptan, allopurinal | Quit >15 yrs | Occasional |
| 19 | 70M | Tongue | 7000 | HTN, Hypothyroid, | Lisinopril, celecoxib, tramadol, trazodone, gabapentin, levothyroxine, MVI | None | Occasional |
| 20 | 60F | Tonsil | 6300 | None | Pentoxyfylline, oxycodone | None | Occasional |
| Syracuse# | Age/Gender | Cancer | Radiat. | Other | Medications | Tobacco | Ethanol |
|---|---|---|---|---|---|---|---|
| 1 | 50M | Tonsil | 6996 | ITP | Prednisone, oxycodone, xanax, fentanyl, sertraline | Chew | None |
| 2 | 73F | Tonsil | 7000 | COPD, Esoph Ca | Albuterol, fluticasone, esomeprazole, mometasone | Quit >5 yrs | None |
| 3 | 53M | Mouth | 7300 | HTN | Morphine, oxycodone, colace, omeprazole, citalipram, cyclobenzaprine, gabapentin | Quit >6 weeks | None |
| 4 | 72M | Prostate | 6600 | Cholesterol | Simvastatin | Quit >5 yrs | None |
| 5 | 55M | Mouth | ND | AFib, Aortic valve | Coumadin, carvedilol, amlodipine, fluticasone, albuterol, acetaminophen-hydrocodone | None | None |
| 6 | 61M | Mouth | ND | HTN, Esoph strictures | Bisacodyl, lisinopril, omeprazole, ranitidine, sulfamethoxazole, trazadone | Quit >8 weeks | Occas. |
| 7 | 60M | Mouth | 6600 | None | Ibuprofen, ranitidine, MVI | None | None |
| 8 | 53M | Palate | 7000 | Sinusitis | Pregabalin | Quit >3 yrs | Occas. |
| 9 | 57M | Larynx | ND | Laryngect. | Gabapentin, cyclobenzaprine, oxycodone, nortriptyline, HCTZ | Quit >3 yrs | None |
| 10 | 68M | Tongue | 6400 | Lung CA, COPD | Alendronate, travatan opthalmic sol, albuterol inh, levothyroxine, fluticasone inh, ferrous sulfate | Quit >5 yrs | None |
| 11 | 76F | Breast | ND | Aortic valve | Bumetanide, atorvastatin omeprazole, ASA, levothyroxine, KCl, fluticasone, naproxen, acetaminophen-hydrocodone, pseudoephedrine, Vitamin D, MVI | None | None |
| 12 | 79M | Prostate | ND | HTN, Colon CA | Coumadin, amlodipine, atorvastatin, HCTZ, bicalutamide | Quit >5 yrs | None |
| 13 | 57F | Larynx | 6996 | HTN | Lisinopril, methimazole, carvedilol | 1 PPD | None |
| 14 | 51M | Neck | ND | HTN, Cholesterol, Hypothyroid | Levothyroxine, bisoprolol, pantoprazole, pravachol, MVI | None | Occas. |
| 15 | 46M | Mouth | ND | None | Acetaminophen | 1 PPD | None |
| 16 | 69M | Prostate | 7740 | HTN, Cholesterol, Asthma | Albuterol inh, fluticasone inh, tiotropium inh, amlodipine, rosuvastatin, acetaminophen, fluticasone | None | None |
| 17 | 82F | Mouth | 3000 | HTN, CAD, Dialysis | Levothyroxine, isosorbide, vitamin b complex, donepezil, metoprolol, oxycodone, mesalamine, citalopram, nortriptyline, omeprazole, diphenoxylate, folate, MVI | None | None |
| 18 | 69F | Tongue | ND | HTN. Cholesterol, Asthma | Albuterol inh, amlodipine, atorvastatin | None | None |
| 19 | 46M | Palate | 6996 | Epilepsy | Lamictal, omeprazole, triamcinolone | None | None |
| 20 | 62M | Tongue | 6996 | HTN | HCTZ | Quit >5 yrs | None |
Abbreviations used are as follows: ND, not determined (not available from chart or the referring physicians); Brachy, brachytherapy; HTN, hypertension; HIV, human immunodeficiency virus infection; COPD, chronic obstructive pulmonary disease; DM-2, type 2 diabetes mellitus; Ca, cancer; DVT, deep venous thrombosis of a leg; CAD, coronary artery disease; CVA, cerebrovascular accident; GERD, gastro-esophageal reflux disease; Cholesterol, hypercholesterolemia; Esoph, esophageal; Afib, atrial fibrillation; Larengect., laryngectomy; aortic valve, history of aortic valve replacement, HCTZ, hydrochlorothiazide; MVI, multivitamin pill.
The standard Penn-based practice for delivering O2 involved placement of a balloon-cushioned face mask that is normally used for continuous positive airway pressure respiratory therapy. Treatments were conducted at 2.0 ATA for 2 h daily, 6 days/week. Intermittently the fractional inspired O2 content in the mask was verified to be 100%. Syracuse-based treatments were conducted in an acrylic chamber pressurized with pure O2 so that no special mask was required to assure 100% O2 delivery. Treatments were at 2.5 ATA for 90 minutes daily, 6 days/week.
2.2 Flow cytometry
CD34+ and CD45-dim cells and relative concentrations of intracellular proteins were evaluated with a 10-color FACSCanto (Becton Dickinson, San Jose, CA) using standard acquisition software following published techniques 12, 14, 27. Briefly, nucleated cells were segregated from debris by DRAQ5 DNA staining and gates were based on true-negative controls according to fluorescence-minus-one analysis. Anti-actin fluorescence confirmed uniform cell permeabilization for intracellular protein analysis. Fluorescence/cell was determined and used to compare pre- versus post-HBO2 cell populations.
2.3 Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies were purchased from the following sources: From BD Pharmingen, San Jose, CA. R-phycoerythrin (PE)-conjugated mouse anti-human CD34 (Clone 581, a class III CD34 epitope; catalogue number 555822), fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD45, catalogue number 5558710 and allophycocyanin (APC)-conjugated mouse anti-human poly-ADP ribose polymerase (PARP) catalogue number 558710; from R & D Systems, Minneapolis, MN, APC-conjugated anti-human hypoxia inducible factor (HIF)-1, catalogue number IC1935P; from Novus Biologicals, Littleton, CO, PE-conjugated anti-human HIF -2 (catalogue number NB100-122, FITC-conjugated anti-human HIF-3 (catalogue number NB100-2529) and anti-human Trx catalogue number EPR 6111 with secondary from Invitrogen, Grand Island, NY catalogue number T-2769.
2.4 Statistical analysis
Statistical analysis of stem cell numbers and quantitative changes in wound protein markers were carried out by repeated measures analysis of variance followed by the Tukey test for multiple comparisons (SigmaStat, Jandel Scientific, San Rafael, CA). Statistical significance was taken as p<0.05. Data sets were found to be normally distributed so results are displayed as mean ± SE, n=20 for all groups. Pre- and post-treatment comparisons were made within each type (2.0 ATA and 2.5 ATA) and between the 2.0 and 2.5 ATA treatments for each number (1st, 10th and 20th) by two-tailed t-test.
3. Results
3.1 Circulating cells
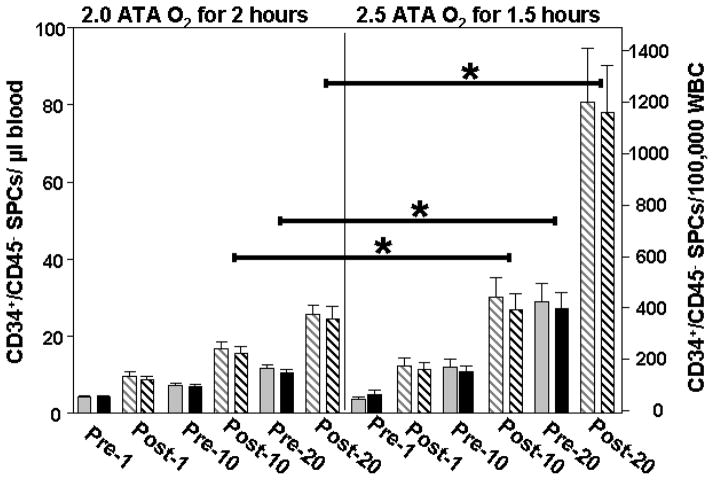
Circulating CD34+ and CD45-dim leukocytes increased in blood from 20 consecutive patients undergoing HBO2 therapy following a protocol of either 2.0 ATA or 2.5 ATA (Figure 1). There were no significant differences in age, gender or radiation dose between groups (Table 1). Following the 10th as well as before and after the 20th treatment cell counts were significantly higher with the 2.5 ATA versus the 2.0 ATA protocol. Findings were essentially the same whether normalized to volume of blood (left axis of Figure 1) or to total circulating leukocyte count (right axis) because total leukocyte counts for patients did not differ significantly over the course of the HBO2 treatments (data not shown).
Figure 1. Leukocyte mobilization by HBO2.
The number of circulating CD34+,CD45-dim cells in blood before and after the 1st, 10th and 20th treatment of 20 patients exposed to either at 2.0 or 2.5 ATA. Data were normalized to blood volume (grey boxes quantified on the left ordinate axis) or to total circulating leukocyte count (black boxes quantified on the right ordinate axis) and are mean ± SE, * indicates significant difference between 2.0 and 2.5 ATA groups (ANOVA). All post-HBO2 values are significantly different from pre-HBO2 values at each treatment time in both groups (t-test).
3.2 Intracellular protein concentrations
Significant elevations of intracellular regulatory proteins were found in permeabilized CD34+ cells after the 1st, 10th and 20th treatments with either protocol (Table 2). Because of variations in fluorescence intensity due to different lots of antibody and also flow cytometer laser intensities, only differences in cell fluorescence intensity for pre- and post-HBO2 samples analyzed on the same day were compared and not intensity across a 20 treatment course.
Table 2.
Intracellular protein content (fold-elevation post- versus prior to HBO2).
| Protein | Treatment # | 2.0 ATA Protocol | 2.5 ATA Protocol |
|---|---|---|---|
| HIF-1 | 1 | 2.35 ± 0.24 | 3.29 ± 0.55 |
| 10 | 2.65 ± 0.21 | 2.67 ± 0.22 | |
| 20 | 2.54 ± 0.38 | 2.77 ± 0.26 | |
| HIF-2 | 1 | 2.33 ± 0.24 | 2.68 ± 0.30 |
| 10 | 2.48 ± 0.15 | 2.54 ± 0.20 | |
| 20 | 2.54 ± 0.23 | 2.60 ± 0.21 | |
| HIF-3 | 1 | 2.27 ± 0.22 | 2.67 ± 0.31 |
| 10 | 2.38 ± 0.24 | 2.29 ± 0.15 | |
| 20 | 2.43 ± 0.26 | 2.27 ± 0.15 | |
| Trx | 1 | 2.34 ± 0.24 | 2.51 ± 0.26 |
| 10 | 2.36 ± 0.22 | 2.28 ± 0.13 | |
| 20 | 2.44 ± 0.24 | 2.50 ± 0.29 | |
| PARP | 1 | 2.36 ± 0.22 | 2.64 ± 0.26 |
| 10 | 2.39 ± 0.22 | 2.42 ± 0.19 | |
| 20 | 2.57 ± 0.27 | 2.47 ± 0.22 |
Data show mean ± SE fold-differences in fluorescence of post-versus pre-HBO2 permeabilized CD34+ cells using fluorophore-conjugated antibodies to proteins shown in column 1. All post-HBO2 values are significantly different from pre-HBO2 and there are no significant differences between the 2.0 and 2.5 ATA protocols.
4. Discussion
The results demonstrate that O2 partial pressure influences SPCs mobilization with repetitive treatments. Whether this is due to augmented NOS-3 activation requires additional study. SPCs mobilization in response to a variety of drugs is compromised by older age, prior radiotherapy and use of several types of chemotherapy (e.g. platinum compounds, alkylating agents, purine analogues and lenalidomide) 39. None of these agents were being administered to patients during our study. We have reported previously that SPCs mobilization in response to a single 2.0 ATA O2 exposure is the same between normal adults and those exposed to radiotherapy11. Obviously, all patients in this study received radiotherapy but there was no significant difference in radiation dosage or patient age between the 2.0 and 2.5 ATA treatment groups (Table 1).
There were no notable deviations in the pattern of SPCs mobilization among the patients despite taking a variety of medications listed in Table 1. Some of these medications are known to have positive effects on SPCs mobilization (e.g. short term statin use, paclitaxel, certain β-blockers such as nebivolol and carvedilol; while others have a negative impact on mobilization (e.g. bisphosphonates, long-term use of statins and trimethoprim/sulfamethoxazole) 40–46. None of the medications listed in Table 1 had been started in the time frame while patients were receiving HBO2 and all had been prescribed for over 2 months prior to patient enrollment. One patient in the 2.0 ATA group had HIV and one in the 2.5 ATA group had renal failure and was undergoing dialysis. HIV does not impede the efficacy of chemotherapeutic agent-mediated SPCs mobilization and renal failure may modify mobilization by some drugs but does not completely abrogate responses 47–50. Whether these disorders influence HBO2-mediated mobilization will require additional study. Clearly, there are differences in mobilization mechanisms between chemotherapeutic agents and HBO2. Contrary to many of the stem cell mobilization drugs HBO2 does not activate platelets or elevate leukocyte counts which can be thrombogenic 13, 51–53.
Intracellular regulatory protein contents were elevated in all post-HBO2 samples with no significant differences between protocols. Elevations are likely a characteristic of the bone marrow SPCs population primed for mobilization and a higher percentage is released with higher O2 dose. Lower protein levels in pre-HBO2 samples at the 10th and 20th treatments may reflect preferential perivascular sequestration of newly mobilized cells and/or protein degradation in cells remaining in the circulation for many hours. The difference in protein contents of newly mobilized SPCs has not been appreciated in mobilization studies involving chemotherapeutic agents. This is probably because responses to chemical agents proceed over a much longer time course.
A weakness of this investigation is that perhaps alternative or additional surface markers should be used to better characterize the mobilized cells. With regard to neovascularization potential, this is difficult to determine given the ongoing debate over EPCs characterization 38. Elevated intracellular proteins of HBO2 –mobilized cells suggest they may have improved propensity for growth/differentiation based on animal studies 14, 27. HIF-3 and PARP were probed because they provide evidence that cells were not merely circulating endothelial cells or cells undergoing apoptosis. PARP levels would be expected to be quite low in apoptotic cells 54. EPCs can be distinguished from mature CECs by determining ‘clonogenic’ proliferative capacity, but not by flow cytometric evaluation of surface markers 38. Our approach for assessing intracellular markers after membrane permeabilization precludes ex vivo growth analysis, which is why we probed for HIF-3. In animals we have found HBO2 –mobilized SPCs that form new blood vessels and hence not CECs are well endowed with HIF-3, whereas HIF-3 normally is highly tissue restricted (to thymus, lung and a lesser extent in brain, heart and kidney) 14, 55. Therefore, we conclude that the cells mobilized by hyperoxia are SPCs and that treatment pressure influences mobilization efficiency. Functional consequences of this response require further study.
Highlights.
The number of circulating CD34+-CD45-dim leukocytes are doubled in humans within 2 hours of exposure to oxygen at 2.0 or 2.5 atmospheres absolute (ATA).
Repetitive exposures to 2.5 ATA leads to a further 1.9 to 3.0-fold elevation of CD34+-CD45-dim cells with up to 20 treatments versus 2.0 ATA treatments.
Newly mobilized CD34+-CD45-dim leukocytes exhibit higher concentrations of an array of regulatory proteins.
Acknowledgments
This work was supported by funds provided by NIH grant R01-DK094260 and the Office of Naval Research to SRT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clnical uses of blood stem cells. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- 2.Gil-Ortega M, Garidou L, Barreau C, et al. Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation. Stem Cells. 2013;31:1309–1320. doi: 10.1002/stem.1375. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Fiorina P, Pietramaggiori G, Scherer SS, et al. The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant. 2010;19:1369–1381. doi: 10.3727/096368910X514288. [DOI] [PubMed] [Google Scholar]
- 5.Fukaya E, Margolis DJ, Miller CJ, et al. Hyperbaric oxygen, vasculogenic stem cells and wound healing. Wound Rep Reg. 2013 in press. [Google Scholar]
- 6.Albanese P, Caruelle D, Frescaline G, et al. Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood: Structure/function insights. Exp Hematol. 2009;37:1072–1083. doi: 10.1016/j.exphem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehman J, Li J, Parvathaneni L, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. Journal of Clinical Investigation. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 11.Thom SR, Bhopale VM, Velazquez OC, et al. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 12.Thom SR, Milovanova TN, Yang M, et al. Vasculogenic stem cell mobilization and wound recruitment in diabetic patients: Increased cell number and intracellular protein content associated with hyperbaric oxygen therapy. Wound Rep Reg. 2011;19:149–161. doi: 10.1111/j.1524-475X.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma YH, Lei YH, Zhou M, et al. Effects of hyperbaric oxygen therapy in the management of chronic wounds and its correlation with CD34(+) endothelial progenitor cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2011;91:3214–3218. [PubMed] [Google Scholar]
- 14.Milovanova TN, Bhopale VM, Sorokina EM, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. Journal of Applied Physiology. 2009;106:711–728. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhar M, Neilsen N, Beatty K, et al. Equine peripheral blood-derived mesenchyman stem cells: isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment. Equine Vet J. 2012;44:600–605. doi: 10.1111/j.2042-3306.2011.00536.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24:2309–2318. doi: 10.1634/stemcells.2006-0010. [DOI] [PubMed] [Google Scholar]
- 17.Aljitawi OS, Xiao Y, Eskew JD, et al. Hyperbaric oxygen improves engraftment of ex vivo expanded and gene transduced human CD34+ cells in a murine model of umbilical cord blood transplantation. Blood Cell Mol Dis. 2013 Aug 14; doi: 10.1016/j.bcmd.2013.07.013. pii::S1079-9796(1013)00164-00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YS, Chio CC, Chang CP, et al. Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediators Inflamm. 2013;2013:512978. doi: 10.1155/2013/512978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherng JH, Chang SC, Chen SG, et al. The effect of hyperbaric oxygen and air on cartilage tissue engineering. Ann Plast Surg. 2012;69:650–655. doi: 10.1097/SAP.0b013e3182745f95. [DOI] [PubMed] [Google Scholar]
- 20.Khan M, Meduru S, Gogna R, et al. Oxygen cycling in conjunction with stem cell transplantation induces NOS3 expression leading to attenuation of fibrosis and improved cardiac function. Cardiovas Res. 2012;93:89–99. doi: 10.1093/cvr/cvr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Yang Y, Xu P, et al. The role of beta-catenin signaling pathway on proliferation of rats neural stem cells after hyperbaric oxygen therapy in vitro. Cell Mol Neurobiol. 2011;31:101–109. doi: 10.1007/s10571-010-9559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Yang Q, Wang S, et al. Hyperbaric oxygen therapy improves neurogenesis and brain blood supply in piriform cortex in rats with vascular dementia. Brain Inj. 2010;24:1350–1357. doi: 10.3109/02699052.2010.504525. [DOI] [PubMed] [Google Scholar]
- 23.Pan H, Chin C, Yang D, et al. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochem Res. 2009;34:1304–1316. doi: 10.1007/s11064-008-9910-7. [DOI] [PubMed] [Google Scholar]
- 24.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mund JA, Estes ML, Yoder MC, et al. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milovanova T, Bhopale VM, Sorokina EM, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia inducible factor-1. Mol Biol Cell. 2008;28:6248–6261. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett M, Feldmeier J, Hampson N, et al. The Cochrane Library. 1. 2008. Hyperbaric oxygen therapy for late radiation tisue injury (Cochrane review) [DOI] [PubMed] [Google Scholar]
- 29.Clarke R, Tenorio C, Hussey J, et al. Hyperbaric oxygen treatment of chronic radiation proctitis: A randomized and controlled doouble blind crossover trial with long-term follow-up. Int J Rad Oncol Biol Phys. 2008;72:134–143. doi: 10.1016/j.ijrobp.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;4:CD004123. doi: 10.1002/14651858.CD004123.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. Physical Med and Rehabilitation. 2009;1:471–489. doi: 10.1016/j.pmrj.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Fife CE, Buyukcakir C, Otto G, et al. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15:322–331. doi: 10.1111/j.1524-475X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 33.Duzgun AP, Satir AZ, Ozozan O, et al. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot & Ankle Surg. 2008;47:515–519. doi: 10.1053/j.jfas.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Londahl M, Katzman P, Nilsson A, et al. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diab Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893–4900. doi: 10.1200/JCO.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation. Diab Care. 2013;36:1961–1963. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. JADA. 1985;111:49–54. doi: 10.14219/jada.archive.1985.0074. [DOI] [PubMed] [Google Scholar]
- 38.Pober JS. Just the FACS or stalking the elusive circulating endothelial progenitor cell. Arterioscler Thromb Vasc Biol. 2012;32:837–838. doi: 10.1161/ATVBAHA.112.246280. [DOI] [PubMed] [Google Scholar]
- 39.Jantunen E, Kvalheim G. Mobilization strategies in hard-to-mobilize patients with lymphoid malignancies. Eur J Haematol. 2010;85:463–471. doi: 10.1111/j.1600-0609.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 40.Hristov M, Fach C, Becker C, et al. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192:413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Sorrentino SA, Doerries C, Manes C, et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol. 2011;57:601–611. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Besler C, Doerries C, Giannotti G, et al. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071–1082. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Pang L, Lei W, et al. Inhibition of Sca-1 positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. 2010;7:571–580. doi: 10.1016/j.stem.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs M, Scheid C, Schulz A, et al. Trimethoprim/sulfamethoxazole prophylaxis impairs function of mobilised autologous peripheral blood stem cells. Bone Marrow Trans. 2000;26:815–816. doi: 10.1038/sj.bmt.1702586. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Yang YJ, Yang T, et al. Statins and stem cell modulation. Ageing Res Rev. 2013;12:1–7. doi: 10.1016/j.arr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez AP, De Arriba F, Rivera J, et al. Successful mobilization of hematopoietic peripheral blood progenitor cells with paclitaxel-based chemotherapy as initial or salvage regimen in patients with hematologic malignancies. Haematologica. 2008;93:1436–1437. doi: 10.3324/haematol.13056. [DOI] [PubMed] [Google Scholar]
- 47.Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;144:822–829. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 48.Hill QA, Pearce R, Cook G. Unsuccessful stem cell remobilization for autologous transplantation is predicted by renal impairment and a stem cell yiled < 0.5 million cells/kg at first mobilization. Bone Marrow Trans. 2012;47:1372–1373. doi: 10.1038/bmt.2012.20. [DOI] [PubMed] [Google Scholar]
- 49.Re A, Cattaneo C, Skert C, et al. Stem cell mobilization in HIV seropositive patients with lymphoma. Haematologica. 2013;98:1762–1768. doi: 10.3324/haematol.2013.089052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabarre J, Azar N, Autran B, et al. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated lymphoma. The Lancet. 2000;355:1071–1072. doi: 10.1016/S0140-6736(00)02041-9. [DOI] [PubMed] [Google Scholar]
- 51.Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 52.Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thom SR. Platelet function in humans is not altered by hyperbaric oxygen therapy. Undersea and Hyperbaric Med. 2006;33:81–83. [PubMed] [Google Scholar]
- 54.Gajdusek C, Onoda K, London S, et al. Early molecular changes in irradiated aortic endothelium. J Cell Physiol. 2001;188:8–23. doi: 10.1002/jcp.1091. [DOI] [PubMed] [Google Scholar]
- 55.Gu YZ, Moran SM, Hogenesch JB, et al. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expression. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]