Abstract
Background
Mouse models of atopic march suggest that systemic, skin-derived Thymic Stromal Lymphopoietin (TSLP) mediates progression from eczema to asthma.
Objective
We investigated whether circulating TSLP is associated with eczema, allergic sensitization, or recurrent wheezing in young children.
Methods
A prospective analysis of the relationship between plasma levels of TSLP to allergic sensitization and recurrent wheezing was conducted in the birth cohort from the Urban Environment and Childhood Asthma (URECA) study. Plasma TSLP levels were measured at 1, 2, and 3 years of age and analyzed for correlation with clinical parameters in each of the three years. Only those children with consecutive samples for all three years were included in this analysis.
Results
We detected TSLP in 33% of 236 children for whom plasma samples were available for all three years. Overall, a consistently significant association was not found between TSLP and eczema or allergic sensitization. With regard to recurrent wheezing, children with detectable TSLP at one year of age were significantly less likely to experience recurrent wheezing by 3 years compared with those children without detectable TSLP, but this was only seen in children without aeroallergen sensitization at 3 years. (p<0.01).
Conclusions and Clinical Relevance
Contrary to our expectations circulating TSLP was not significantly associated with eczema, allergen sensitization, or recurrent wheezing during the first three years of life. Early presence of circulating TSLP was significantly associated with reduced incidence of recurrent wheeze in those children not sensitized to aeroallergen. These findings suggest a possible underlying distinction between pathogenesis of developing atopic vs. non-atopic recurrent wheeze.
Keywords: TSLP, atopy, birth cohort, recurrent wheeze, preschool wheeze, URECA
Introduction
Studies in mice have identified the cytokine Thymic Stromal Lymphopoietin (TSLP) to be one of the most sensitive markers for defects in epidermal differentiation 1-4 and an important factor in the pathogenesis of both atopic dermatitis (AD) and asthma 5-11. TSLP promotes CD4+ T-cell polarization into T helper type 2 (Th2) cells associated with both atopic dermatitis and asthma9. Genome wide association studies in humans have identified a few genetic polymorphisms at the TSLP locus that were associated with altered asthma symptoms and response to medication12-14. Moreover, the link between SPINK5 and Netherton Syndrome, another atopic disease15, 16, may be due to activation of TSLP3.
Recent studies demonstrate that TSLP expressed by barrier-defective epidermis is released into the systemic circulation1, 17, in a stark contrast to overexpression of TSLP in asthmatic lung, where no serum TSLP is detected10. The discovery that disruption in the skin differentiation program1 or application of Vitamin D analogs18, 19 led to robust elevation of TSLP in circulation permitted the unequivocal demonstration that elevated TSLP levels in the skin were both required and sufficient to cause a marked increase in allergen-induced airway inflammation and hyperreactivity17, 20. This is characteristic of human AD patients developing asthma later in life. These findings raise the hypothesis that a cytokine (presumably TSLP) produced by the skin acts as a messenger to prime lung responses to allergen. If so, TSLP would be an excellent therapeutic target to reduce the incidence of asthma in children with a history of AD.
To examine whether circulating TSLP was present in humans and assess if it was contributing to the development of childhood allergic diseases, we measured plasma TSLP concentrations in an inner-city birth cohort enrolled in the Urban Environment and Childhood Asthma (URECA) study. We correlated yearly plasma TSLP levels to the development of multiple clinical parameters, including clinical symptoms of eczema, allergen sensitization, and recurrent wheezing through the first three years of life thereby testing our hypothesis for linkage between circulating TSLP and development of an allergic disposition and recurrent wheezing, antecedent markers for asthma.
Methods
Study Population
This study examines a subset, based on plasma availability, of the total 609 children who were enrolled in the Urban Environment and Childhood Asthma (URECA) study, an observational prospective study initiated in 2004 to determine the antecedents of asthma in children born into central urban areas where at least 20% of the population had incomes below the poverty line. Details of the design of the URECA study have been previously published21. Our subset of 378 children includes 118 children from Baltimore, 94 from Boston, 54 from New York, and 112 from St. Louis who had at least one plasma sample available at ages 1, 2, or 3 years (236 children had plasma samples at all 3 years). The majority of the children had at least one parent with a history of allergy or asthma (92%, n = 346); a small comparison group of children (8%, n = 32) were included having no family history of either condition. Mothers were recruited during pregnancy, and newborns born at a gestational age of ≥ 34 weeks were enrolled upon collection of a suitable umbilical cord blood specimen.
Respiratory Symptom Monitoring
Telephone interviews were administered every three months to collect data regarding the child's wheeze, cough, and allergic symptoms per parent report over the telephone. Recurrent wheezing was the primary outcome for the first three years of the URECA study and is defined as at least two episodes of wheezing in the first three years of life with at least one episode during the third year.
Clinic visits
The child's blood was drawn at each annual clinic visit for measurement of allergen-specific IgE levels. The specific IgE panel at age 1 included foods (egg, milk, peanut) and German cockroach only. At ages 2 and 3, the panel was expanded to include Dermatophagoides farinae, Dermatophagoides pteronyssinus, dog epithelium, cat dander/epithelium, mouse urine protein, and Alternaria alternata. Prick skin testing (Multi-Test II, Lincoln Diagnostics, Decatur, IL) was performed near age 3, in the majority of the children at age 33 months, for the following 14 indoor and outdoor allergens (Greer Laboratories): Alternaria tenuis, American and German cockroach mix, Aspergillus mix, cat hair, Dermatophagoides farinae, Dermatophagoides pteronyssinus, dog epithelia, German cockroach, mouse epithelia, Penicillium notatum, ragweed mix, rat epithelia, Timothy grass, and tree pollen (white oak at St. Louis and Baltimore sites; birch mix at Boston and New York sites). For the current analysis, aeroallergen sensitization at age 3 was defined as the presence of one or more positive (≥ 0.35 kU/L) sIgE responses to an aeroallergen or one or more positive skin tests (wheal ≥ 3 mm larger than the saline control). For age 2, aeroallergen sensitization was defined only using sIgE measurements since no skin test data were available at this time. Food sensitization at ages 1, 2, and 3 was defined as the presence of one or more positive (≥ 0.35 kU/L) sIgE responses to egg, milk, or peanut. Diagnosis of eczema was determined each year, using the EASI scoring system and was defined as an EASI score greater than or equal to 122.
TSLP measurement
Plasma TSLP levels were measured in available plasma samples from birth (cord blood), and ages 1, 2, and 3 using LEGEND MAX Human TSLP Enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (BioLegend Inc., San Diego, CA). The lower limit of detection of the assay was 10 pg/ml. Five Parameter Logistic (5-PL) Non-Linear Regression Curve-Fitting Model (SoftMax Pro 5 software, Molecular Devices Corporation, Sunnyvale, CA) was used to calculate TSLP concentrations based on standard controls. As a pilot study, we first confirmed that TSLP protein was detectable in human plasma using ELISA (BioLegend™), that it remained stable through several freeze-thaw cycles (data not shown). Using a small number of URECA samples, we detected TSLP in samples collected after birth but not in any of the cord blood samples (data not shown). Therefore, the subsequent analyses include only TSLP measurements at 1, 2, and 3 years after birth. TSLP levels in each sample were measured in duplicate; the two independent measurements differed by less than 10% across the entire population (data not shown). Because samples had been previously frozen and thawed, we confirmed that the samples with non-detectable TSLP were not degraded by comparing the distribution of sCD14 between children with detectable and non-detectable TSLP (Supplemental Figure 1).
Statistical analysis
TSLP levels were highly skewed and were therefore analyzed using a dichotomous variable for detectable vs. non-detectable TSLP. Logistic regression was used to test for associations between detectable TSLP and the 4 clinical endpoints; recurrent wheeze, eczema, food sensitization and aeroallergen sensitization. Since the number of subjects with a given outcome tended to be small in some cases (i.e. eczema), exact logistic regression was also performed to confirm findings. Interaction by atopic status was examined for models of recurrent wheeze. As these models showed some significant interactions, stratified results are presented. Due to the exploratory nature of these analyses and the relatively small sample, no attempt was made to control for multiple comparisons. Our a priori hypothesis is that eczema should produce higher TSLP levels in skin, that this will result in higher TSLP levels in plasma, and that higher TSLP levels should, in turn, result in increased allergic sensitization and wheeze. All models were adjusted for child's gender and site, as well as mother's asthmatic history, which showed either significant associations or trends with several of the clinical outcomes and TSLP at years 1 and 3. Potential confounding effects from prenatal and early life exposure to tobacco smoke was investigated, but did not alter the results; the odds ratios are not adjusted for this in the tables. All statistical analyses were carried out using R 2.8.1 and SAS 9.2.
Results
Plasma TSLP is detected in a subset of URECA participants during the first three years of life
The general characteristics of the study population overall and stratified by detectable vs. non-detectable TSLP at age 3 are presented in Table 1. Three-fourths of the population is African-American, and 17% is Hispanic. Most of the families (71%) have a household income < $15,000 per year, and most of the mothers (78%) have a high school education or less. More asthma was reported among the mothers of children with detectable TSLP at age 3 vs. those without (57% vs. 44%, p=0.05), otherwise there were no significant differences by the presence of detectable TSLP. There were no differences between those included in this analysis and the remaining URECA cohort except with respect to site (data not shown). This has been controlled for in all analyses.
Table 1.
Demographic characteristics of children with TSLP measured at age 3 years, overall and stratified by detectable vs. non-detectable TSLP.
| Overall (n=259) | Detectable TSLP (n=73) | Non-detectable TSLP (n=186) | P-value | |
|---|---|---|---|---|
| Site | ||||
| Baltimore | 27% (70) | 30% (22) | 26% (48) | 0.26 |
| Boston | 25% (65) | 32% (23) | 23% (42) | |
| New York | 18% (47) | 15% (11) | 19% (36) | |
| St. Louis | 30% (77) | 23% (17) | 32% (60) | |
| Age of mother at child's birth | 24.4 ± 6.1 | 24.5 ± 6.2 | 24.3 ± 6.1 | 0.76 |
| Mother smokes | 22% (56) | 28% (20) | 19% (36) | 0.14 |
| Mother's education | 0.74 | |||
| Less than high school | 40% (103) | 40% (29) | 40% (74) | |
| High school | 38% (97) | 40% (29) | 37% (68) | |
| More than high school | 22% (58) | 19% (14) | 24% (44) | |
| Mother married | 11% (29) | 7% (5) | 13% (24) | 0.17 |
| Income < $15,000 | 71% (183) | 68% (50) | 72% (133) | 0.63 |
| Mother has ever had asthma | 47% (122) | 57% (41) | 44% (81) | 0.05 |
| Mother has ever had eczema | 30% (78) | 26% (19) | 32% (59) | 0.38 |
| Mother is atopic | 70% (178) | 74% (53) | 68% (125) | 0.38 |
| Child's race | 0.66 | |||
| Black | 74% (191) | 77% (56) | 73% (135) | |
| Hispanic | 17% (45) | 18% (13) | 17% (32) | |
| White/other | 9% (23) | 5% (4) | 10% (19) | |
| Child is male | 49% (128) | 42% (31) | 52% (97) | 0.16 |
| Child has ever been breastfed | 56% (144) | 60% (43) | 55% (101) | 0.48 |
Note: Similar findings were seen for those with TSLP measured at age 1 and 2.
Table 2 shows the distribution of eczema, aeroallergen and food sensitivity and recurrent wheeze in the population in which TSLP was measured. TSLP was detected in 33% of samples measured at year one, 36% at year two, and 28% at year three. The highest level measured was 402 pg/ml. Percentages of non-detectable versus detectable TSLP were similar across years (Tables 3a and 3b, p = 0.25).
Table 2.
Year 3 outcomes among URECA children with TSLP measured at age 3 years (n=259)
| Clinical outcome | % (n) |
|---|---|
| Eczema | 9% (23) |
| Aeroallergen sensitivity (atopy) | 42% (110) |
| Food sensitivity | 38% (97) |
| Recurrent wheeze | 32% (83) |
| Modified asthma predictive index (MAPI25) | 14% (33) |
Table 3a and 3b.
Distribution of TSLP by year for entire sample (3a) and subset of the sample including only participants with TSLP measurements at all 3 years (3b).
| Year | N | < LOD (10 pg/ml) % (n) | ≥ LOD Mean ± std (pg/ml) |
|---|---|---|---|
| 1 | 352 | 67% (236) | 57.6 ± 68.2 |
| 2 | 275 | 64% (177) | 46.6 ± 35.3 |
| 3 | 259 | 72% (186) | 47.3 ± 50.9 |
| Year | N | < LOD (10 pg/ml) % (n) | ≥ LOD Mean ± std (pg/ml) |
|---|---|---|---|
| 1 | 236 | 67% (159) | 54.9 ± 64.6 |
| 2 | 236 | 64% (150) | 48.2 ± 35.6 |
| 3 | 236 | 71% (167) | 48.0 ± 52.2 |
Cochran-Mantel-Haenszel test for difference across years: 2.78, p=0.25
LOD, limit of detection
Eczema was weakly associated with plasma TSLP levels and plasma TSLP was not associated with allergic sensitization
Eczema, defined by the Eczema Area and Severity Index (EASI)22 (range, 0-72) was of mild severity among children with detectable TSLP, and not significantly different from the entire URECA cohort. Children with eczema (EASI score ≥ 1) in year 2 were twice as likely as those without eczema to have detectable TSLP levels at year 3 [adjusted OR: 2.12 (1.02, 4.42)]. However, no other significant associations were seen between presence of eczema and detectable TSLP in the concurrent or later years (Table 4). Likewise, neither aeroallergen sensitivity (Table 5) nor food sensitization (Table 6) showed any consistent association with the presence of detectable TSLP or eczema. One exception was a borderline association of detectable TSLP with food sensitization at year 2, which was significant in the adjusted model [adjusted OR: 1.71 (1.01, 2.90)].
Table 4.
Association between eczema and the presence of detectable TSLP at the concurrent or subsequent year.
| Eczema | N | Detectable TSLP % (n) | Odds Ratio (95% CI) | Adjusted Odds Ratio1 (95% CI) |
|---|---|---|---|---|
| Year 1 | Year 1 | |||
| Yes | 90 | 30.0% (27) | 0.83 (0.50, 1.40) | 0.77 (0.44, 1.35) |
| No | 262 | 34.0% (89) | ||
| Year 1 | Year 2 | |||
| Yes | 65 | 29.2% (19) | 0.68 (0.37, 1.24) | 0.54 (0.28, 1.06) |
| No | 187 | 38.0% (71) | ||
| Year 1 | Year 3 | |||
| Yes | 61 | 27.9% (17) | 0.92 (0.49, 1.76) | 0.68 (0.33, 1.38) |
| No | 183 | 29.5% (54) | ||
| Year 2 | Year 2 | |||
| Yes | 44 | 36.4% (16) | 1.04 (0.53, 2.03) | 0.92 (0.46, 1.83) |
| No | 231 | 35.5% (82) | ||
| Year 2 | Year 3 | |||
| Yes | 39 | 43.6% (17) | 2.28 (1.12, 4.61) | 2.12 (1.02, 4.42) |
| No | 209 | 25.4% (53) | ||
| Year 3 | Year 3 | |||
| Yes | 23 | 26.1% (6) | 0.88 (0.33, 2.33) | 1.01 (0.37, 2.74) |
| No | 234 | 28.6% (67) |
Adjusted for site, gender of the child, and mother's asthmatic status
Table 5.
Association between presence of detectable TSLP and aeroallergen sensitivity (atopy) at the concurrent or subsequent year.
| Detectable TSLP | N | Aeroallergen Sensitivity % (n) | Odds Ratio (95% CI) | Adjusted Odds Ratio1 (95% CI) |
|---|---|---|---|---|
| Year 1 | Year 2 | |||
| Yes | 80 | 17.5% (14) | 1.31 (0.64, 2.69) | 1.22 (0.57, 2.62) |
| No | 172 | 14.0% (24) | ||
| Year 1 | Year 3 | |||
| Yes | 81 | 46.9% (38) | 1.33 (0.78, 2.28) | 1.29 (0.74, 2.24) |
| No | 163 | 39.9% (65) | ||
| Year 2 | Year 2 | |||
| Yes | 98 | 13.3% (13) | 0.78 (0.39, 1.58) | 0.85 (0.41, 1.77) |
| No | 177 | 16.4% (29) | ||
| Year 2 | Year 3 | |||
| Yes | 90 | 41.1% (37) | 0.95 (0.56, 1.60) | 1.03 (0.60, 1.77) |
| No | 158 | 42.4% (67) | ||
| Year 3 | Year 3 | |||
| Yes | 73 | 45.2% (33) | 1.17 (0.68, 2.02) | 1.20 (0.68, 2.11) |
| No | 186 | 41.4% (77) |
Adjusted for site, gender of the child, and mother's asthmatic status
Table 6.
Association between presence of detectable TSLP and food sensitivity at the concurrent or subsequent year.
| Detectable TSLP | N | Food Sensitivity % (n) | Odds Ratio (95% CI) | Adjusted Odds Ratio1 (95% CI) |
|---|---|---|---|---|
| Year 1 | Year 1 | |||
| Yes | 116 | 31.9% (37) | 1.16 (0.72, 1.88) | 1.12 (0.68, 1.84) |
| No | 233 | 28.8% (67) | ||
| Year 1 | Year 2 | |||
| Yes | 80 | 35.0% (28) | 1.12 (0.64, 1.95) | 1.18 (0.66, 2.09) |
| No | 172 | 32.6% (56) | ||
| Year 1 | Year 3 | |||
| Yes | 81 | 43.2% (35) | 1.43 (0.83, 2.46) | 1.42 (0.80, 2.52) |
| No | 161 | 34.8% (56) | ||
| Year 2 | Year 2 | |||
| Yes | 98 | 40.8% (40) | 1.66 (0.99, 2.78) | 1.71 (1.01, 2.90) |
| No | 177 | 29.4% (52) | ||
| Year 2 | Year 3 | |||
| Yes | 90 | 43.3% (39) | 1.40 (0.83, 2.39) | 1.42 (0.82, 2.48) |
| No | 156 | 35.3% (55) | ||
| Year 3 | Year 3 | |||
| Yes | 73 | 41.1% (30) | 1.22 (0.70, 2.12) | 1.17 (0.65, 2.09) |
| No | 184 | 36.4% (67) |
Adjusted for site, gender of the child, and mother's asthmatic status
Detectable plasma TSLP at an early age correlates with fewer episodes of recurrent wheezing among non-atopic children
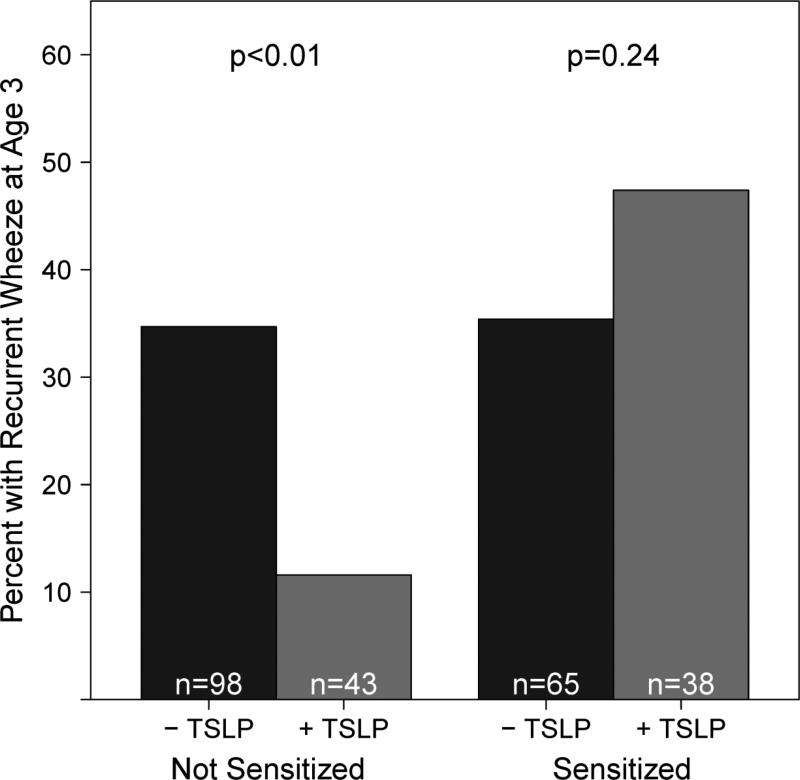
The presence of detectable TSLP at any age was not associated with recurrent wheeze at age 3 (Table 7). Initially, we hypothesized that atopic children with detectable TSLP would be more likely to develop recurrent wheeze. We therefore stratified our children with recurrent wheezing at age three years by aeroallergen sensitivity status. In non-sensitized children (i.e., no detectable IgE or positive skin test to aeroallergens) detectable plasma TSLP at age one year was inversely related to recurrent wheezing at age three [adjusted OR 0.33, (0.11, 0.99), Table 7, Figure 1]. There were similar but non-significant trends noted for detectable TSLP in years 2 [adjusted OR 0.60 (0.25, 1.43)] and 3 [adjusted OR 0.50 (0.19, 1.34), Table 7]. In aeroallergen sensitized children, there were no significant relationships between detectable TSLP and recurrent wheeze.
Table 7.
Association between presence of detectable TSLP and recurrent wheeze at age 3, overall and stratified by aeroallergen sensitivity at age 3.
| Detectable TSLP | N | Recurrent Wheeze % (n) | Odds Ratio (95% CI) | Adjusted Odds Ratio1 (95% CI) |
|---|---|---|---|---|
| Total Population | ||||
| Year 1 | ||||
| Yes | 114 | 31.6% (36) | 0.92 (0.57, 1.48) | 0.99 (0.59, 1.67) |
| No | 227 | 33.5% (76) | ||
| Year 2 | ||||
| Yes | 90 | 31.1% (28) | 0.90 (0.52, 1.57) | 0.80 (0.44, 1.46) |
| No | 162 | 33.3% (54) | ||
| Year 3 | ||||
| Yes | 71 | 29.6% (21) | 0.81 (0.45, 1.48) | 0.79 (0.41, 1.51) |
| No | 173 | 34.1 % (59) | ||
| Not sensitized to Aeroallergens | ||||
| Year 1 | ||||
| Yes | 43 | 11.6% (5) | 0.25 (0.09, 0.69) | 0.33 (0.11, 0.99) |
| No | 98 | 34.7% (34) | ||
| Year 2 | ||||
| Yes | 52 | 23.1% (12) | 0.68 (0.31, 1.51) | 0.60 (0.25, 1.43) |
| No | 85 | 30.6% (26) | ||
| Year 3 | ||||
| Yes | 40 | 17.5% (7) | 0.46 (0.18, 1.14) | 0.50 (0.19, 1.34) |
| No | 101 | 31.7% (32) | ||
| Sensitized to Aeroallergens | ||||
| Year 1 | ||||
| Yes | 38 | 47.4% (18) | 1.64 (0.73, 3.71) | 1.52 (0.63, 3.68) |
| No | 65 | 35.4% (23) | ||
| Year 2 | ||||
| Yes | 34 | 41.2% (14) | 1.20 (0.51, 2.79) | 0.98 (0.36, 2.64) |
| No | 65 | 36.9% (24) | ||
| Year 3 | ||||
| Yes | 31 | 45.2% (14) | 1.37 (0.59, 3.22) | 1.08 (0.40, 2.94) |
| No | 72 | 37.5% (27) | ||
Adjusted for site, gender of the child, and mother's asthmatic status
Figure 1.
The presence of detectable plasma TSLP (-TSLP indicates non-detectable levels, +TSLP indicates detectable levels) in year 1 is inversely associated with the occurrence of recurrent wheeze in year 3 among non-sensitized children only (not sensitive to any aeroallergens) at age 3. P-values indicate the association between recurrent wheeze at age 3 and detectable TSLP at age 3 for each group.
Data relating to presence of upper respiratory viral infection did not show a significant difference between those children with detectable or non-detectable plasma TSLP during the first three years of life.
Discussion
Independent studies have demonstrated that a skin-produced TSLP is both sufficient and required to predispose mice to airway hypersensitivity1, 17, 23, although one study has provided data suggesting that circulating TSLP is not necessary for developing the atopic march23. One human study has found serum TSLP in children to correlate with their eczema status but not with atopy24; however, this study excluded children with respiratory illnesses or those with undetectable TSLP levels from the analysis. To test the hypothesis that circulating TSLP (as a biomarker of epidermal TSLP production) linked atopic dermatitis with asthma in humans, we performed a prospective longitudinal analysis of the association between circulating TSLP and eczema, allergen sensitization, and recurrent wheezing in a birth cohort during the first three years of life. Although we did find association between mild eczema in year two and TSLP levels in year three, we did not see a robust association between eczema and TSLP. This may reflect the paucity of children with moderate to severe eczema in the URECA cohort, the short follow-up period prohibiting the detection of the long term associations, or the use of an eczema diagnosis that fits several different pathologies (e.g. atopic vs. non-atopic eczema), some of which are linked to TSLP production and others that are not.
Importantly, we detected no positive association of recurrent wheezing or aeroallergen sensitization with TSLP or with eczema. These findings are consistent with the null hypothesis (no linkage between persistent plasma TSLP levels, atopy and recurrent wheeze) in this population, but they cannot rule out the possibility that transient peaks of TSLP coinciding with allergen exposure play a role in the development of the atopic march. Testing this hypothesis will necessitate a different study design. Moreover, it is important to note that there was no significant association between eczema and recurrent wheezing in our cohort, (data not shown). Therefore, a positive correlation between plasma TSLP levels and subsequent asthma later in life may emerge as we follow these children through school age to identify associations that may not have developed by age 3 years.
An intriguing finding in this study is the protective effect of TSLP on recurrent wheezing in non-aeroallergen sensitized children. Children in this group with detectable plasma TSLP at one year of age were four fold less likely to report recurrent wheezing at 3 years of age than non-atopic children without detectable TSLP in their plasma. One possible explanation for our data would be if TSLP provided protection from respiratory infections. However, examination of the prevalence of respiratory infection found no difference between the groups. There was also no difference in season of birth (data not shown). We acknowledge that due to the number of comparisons made in this analysis it is also possible that this is a chance finding. However, considering that this is a high-risk population, any suggestion of reduced incidence of wheezing may be significant.
This study is, to our knowledge, the first prospective study in young children addressing the role of TSLP in association with development of allergen sensitization and recurrent wheeze. The recruitment criteria for the study provided a high likelihood for capturing individuals that will develop asthma later in life. It is a weakness, however, that our participants, chosen for parental history of allergic manifestations, did not manifest a high degree of eczema. We, therefore, cannot exclude a possible role of skin-derived TSLP in recurrent wheezing in children with more severe eczema. More years of follow-up will be needed to conclusively determine the rate of asthma among this study population.
Plasma TSLP is a surrogate for epithelial TSLP production. Instead of predisposing to allergies we find that the presence of TSLP without allergen sensitization reduced the incidence of recurrent wheeze in relation to other, unknown drivers of wheezing. Since we do not know what causes wheezing in children without evidence of sensitization, and because there was no difference in occurrence of viral respiratory illness between children with detectable and non-detectable plasma TSLP, we cannot offer an explanation or a mechanism for the seemingly protective effects of TSLP. Nevertheless, the findings in this report suggest such an additional predisposing factor exists and give pause to the efforts at addressing TSLP as a therapeutic target in prevention or treatment of childhood asthma requiring further investigation into the reasons for this outcome.
In summary, this work establishes that TSLP can be detected in the plasma of children as early as 1 year of age. We have not found a substantial positive correlation between plasma TSLP and overall eczema, allergic sensitization or recurrent wheezing in our cohort. On the contrary, our detailed analysis has uncovered the possibility that detectable circulating TSLP during the first three years of life may have a dampening effect rather than an enhancing effect upon recurrent wheeze during this early period of development. It remains to be seen whether this effect will hold through the school age years.
Supplementary Material
Supplemental Figure 1. The distribution of sCD14 was similar for children with detectable TSLP (solid line) and those without detectable TSLP (dashed line) in years, 1, 2 and 3. P-values from Kolmogorov-Smirnov two-sample tests were non-significant (year 1: p=0.99, year 2: p=0.96, year 3: p=0.83).
Acknowledgements
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, and HHSN272201000052I. Additional support was provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, and 1UL1RR024156; and from Lincoln Diagnostics (which provided Multi-Test®). Additional funding for this project was provided by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital.
References
- 1.Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumortier A, Durham A-D, Di Piazza M, Vauclair S, Koch U, Ferrand G, et al. Atopic Dermatitis-Like Disease and Associated Lethal Myeloproliferative Disorder Arise from Loss of Notch Signaling in the Murine Skin. PLoS ONE. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–8. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–80. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 8.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–7. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 12.Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–74. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 13.Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. TSLP Promoter Polymorphisms are Associated with Susceptibility to Bronchial Asthma. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunninghake GM, Soto-Quiros ME, Avila L, Kim HP, Lasky-Su J, Rafaels N, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowcock AM, Cookson WOCM. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Human Molecular Genetics. 2004;13:R43–R55. doi: 10.1093/hmg/ddh094. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Xia Y, Zhang W, Li J, Wang P, Li H, et al. A functional polymorphism in the SPINK5 gene is associated with asthma in a Chinese Han Population. BMC Med Genet. 2009;10:59. doi: 10.1186/1471-2350-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106:1536–41. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Hener P, Zhang Z, Ganti KP, Metzger D, Chambon P. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J Invest Dermatol. 2009;129:498–502. doi: 10.1038/jid.2008.232. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and lowcalcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. doi:10.1371/journal.pbio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 23.Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–60. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126:212–6. doi: 10.1016/j.jaci.2010.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The distribution of sCD14 was similar for children with detectable TSLP (solid line) and those without detectable TSLP (dashed line) in years, 1, 2 and 3. P-values from Kolmogorov-Smirnov two-sample tests were non-significant (year 1: p=0.99, year 2: p=0.96, year 3: p=0.83).