Abstract
Objective
To examine the additive effect of age on disability for adults with SCI.
Design
Prospective cohort study.
Setting
Spinal Cord Injury Model Systems.
Participants
The study sample individuals with SCI with a discharge motor-FIM score and at least one follow-up motor-FIM score who also provided measures of other covariates (N=1,660). Seventy-nine percent of the sample was male, 72% was white, 16% had paraplegia, incomplete; 33.0% had paraplegia, complete; 30% had tetraplegia, incomplete; and 21% had tetraplegia, complete. The participants’ median age at injury was 32 years (range, 6–88).
Main Outcome Measures
The primary study outcome was the motor subscale of the Functional Independence Measure (motor-FIM). We used a mixed models approach to examine the additive effect of age on disability for individuals with SCI.
Results
When controlling for motor-FIM at discharge from rehabilitation, level and severity of injury, age at injury, gender, and race, the Age × Time interaction was not significant (p=0.07). Age at SCI was significantly associated with motor-FIM (F1,238=22.49, p<0.0001). Two sensitivity analyses found significant interactions for both Age × Time (p=0.03; p=0.02) and Age × Time2 (p=0.01; p=0.006) models. Trajectory of motor-FIM scores are moderated slightly by age at the time of injury. The older participants were at time of injury, the greater curvature and more rapid decline was found in later years.
Conclusions
These findings indicate that age moderately influences disability for some individuals with SCI, and the older one is upon injury the greater the influence age has on disability. They serve as an important empirical foundation for the evaluation and development of interventions designed to augment accelerated aging experienced by individuals with SCI.
Keywords: Spinal Cord Injury, Aging, Disability
In recent history, life expectancy for individuals with spinal cord injuries has not improved. In fact, the gap between life expectancy for individuals with SCI and the general population is actually increasing.1–3 With this trend comes the possibility of an increased incidence and severity of health problems associated with aging in individuals with SCI. Older age in individuals with SCI is associated with a variety of health complications, including: bowel complications, diminished renal function, higher rates of ventilator use, increased rates of hospitalizations and skilled nursing home admissions, increase cardiovascular disease, increased healthcare costs, and increased prevalence of pressure sores.4–11 As a group, these findings suggest that adults with SCI are experiencing “accelerated aging”, due to the occurrence of health conditions more frequently and earlier in the lifespan.12
If individuals with SCI are experiencing accelerated aging, they may also experience accelerated decline in independence in activities of daily living (i.e., self-care, mobility, bowel and bladder functioning). Consistent with this hypothesis, published studies have reported associations between age and greater disability in activities of daily living, social integration, and quality of life in individuals following SCI.10,13–15 On the other hand, some longitudinal research indicates that time since injury is positively associated with satisfaction with life and the community integration, suggesting that some accommodation to the spinal cord injury can take place over time.16,17 Krause and colleagues conducted a long-term health and well-being analysis for individuals with SCI and found a complicated pattern related to mortality and employment and demographic predictors.18
Longitudinal studies offer advantages over cross-sectional studies, providing the opportunity to follow individuals with SCI and observe changes over time. Unfortunately, there is a paucity of longitudinal studies in individuals with SCI.12 As a result, there are significant knowledge gaps regarding the effects of aging in individuals with SCI. The primary purpose of this study is to address this gap by directly examining the additive effect of age on disability for adults with SCI, using a longitudinal design.
METHODS
Participants
The participants from this study came from those enrolled in the SCI Model Systems between 1988 and 2011 (U.S. Department of Education, National Institute on Disability and Rehabilitation Research). Participants who sustained a traumatic SCI with neurologic deficit were admitted to each model system within 1 year of injury, and completed inpatient rehabilitation. All participants provided informed consent for data collection, and research protocols were approved by each model system’s local institutional review board. Data collectors gathered information about demographics, injury and medical characteristics, independence in activities of daily living, and psychosocial well-being from the individuals with SCI. Data were captured at variable intervals; however, the majority of the data was captured during the initial hospitalization, at 1 year, and every additional 5 years post-SCI. Details about this database have been described elsewhere.19
In order to be included in the analyses in this study we required at least two time-points describing independence in activities of daily living. As a baseline score for independence in activities of daily living, we required a score at discharge from rehabilitation. At least one follow-up score was also required. Data for independence in activities of daily living were first entered in the database in 1988, and follow-up data were first entered in 1995. Thus, data in these analyses represent data collected between 1988 and 2011.
Measures
To describe the sample, we examined age at time of injury, sex, race (dichotomized into white or minority), years since injury onset, injury level and severity, and whether death or change in residential status occurred. Injury level and severity were categorized into six groups based on a combination of level of SCI and severity of SCI at discharge from rehabilitation. Individuals were grouped as tetraplegia (C1-C8), complete; tetraplegia, incomplete; tetraplegia, minimal deficit; paraplegia (T1-S5), complete; paraplegia, incomplete; or paraplegia, minimal deficit. Minimal deficit was defined as minimal neurologic damage, so the individuals had limited disability. We dichotomized residential status (private vs. non-private home location, which were nursing home, group home, or other non-private home locations) and tracked death, as is reported in the database, to assist in the explanation of loss to follow-up.
The primary study outcome variable was the Motor subscale of the Functional Independence Measure (motor-FIM).20 The motor subscale is comprised of items assessing self-care, mobility, and bowel and bladder functioning (13 items). Each item is rated on a 7-point scale describing the amount of assistance required to complete the activity. A score of ‘1’ represents complete dependence and a score of ‘7’ designates complete independence (possible scale score range, 13–91). The FIM subscales are adequately valid and reliable and the FIM remains the most widely used measure of functional independence in neurological rehabilitation.21
Statistical Analyses
All data were examined for normality prior to analyses and data were transformed as needed. We computed means (and standard deviations) of continuous and n(%) of categorical demographic variables to describe the sample. We employed a mixed models approach to examine the trajectories of motor-FIM over time.22 We anchored the analyses with the motor-FIM at discharge from rehabilitation. Follow-up FIM scores were data collected after that point. This approach allowed us to model the pattern between age and disability for the entire population (fixed effects) and curves specific to each individual (random effects). We allowed the intercept and the time terms (linear and quadratic) to be random (individual specific) and used spatial power to model the covariance structure. We considered mixed models using age at injury, motor-FIM at discharge from rehabilitation, level and severity of SCI at discharge, gender, race, time, time squared and the potential interactions among age and the time variables. The time terms were calculated from date of injury. We ran two sensitivity analyses: (1) dropping individuals who died or relocated to a non-private residence and to examine whether these variables influenced the analytical outcomes and (2) including only individuals who had a minimum of 15 years of follow-up to examine those who had the longest opportunity to age. Both of these sensitivity analyses allowed us to analyze how age influenced disability by focusing on those who were aging and those who had the opportunity to age. We conducted independent sample t-tests for continuous variables and chi-square tests for categorical variables to examine demographic and injury characteristic differences between individuals kept in the sensitivity analyses and those dropped from the analyses. All statistical analyses were conducted using SAS® software, version 9.3 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
We analyzed 1,660 individuals. To obtain this sample, 11,150 potential individuals were identified, and 6,333 individuals had a motor-FIM score. Of these individuals, 3,084 had only 1 completed motor-FIM measure and were subsequently excluded from the analyses. Also excluded were subjects who were missing other important covariates: motor-FIM at discharge from rehabilitation (n=1,524), level and severity of SCI at discharge from rehabilitation (n=41) and race (n=20). The sample size was limited due to the inclusion criteria required. Due to small sample sizes, 4 additional subjects who were classified as paraplegia, minimal deficit (n=2) and tetraplegia, minimal deficit (n=2) were also excluded. Participants who were used in the final analyses were significantly older (34.4 (14.6) vs. 31.7 (14.8) years; t(6331) = 6.40, p<0.0001), were less likely to be white (72% vs. 76%; χ2(1)=10.47, p=0.001), and had a different distribution of level and severity of SCI with more being likely to be paraplegia, complete (33% vs 28%; χ2(4)=26.46, p<0.0001 ), than individuals who were not included in the final analyses. No significant between-group sex differences were found.
Table 1 shows a breakdown of key demographic and injury characteristics for the 1,660 individuals used in the analyses. All had 2 or more motor-FIM scores and were not missing important covariates. The participants’ median age at injury was 32 years (range 6–88). Of those originally discharged to private location (94%, n=1,555), 72 individuals were subsequently in a non-private home location during one of the follow-up interviews. In the study sample, 167 (10%) individuals were reported as having died. The average time between FIM assessments was 6.6 years.
Table 1.
Demographics, Injury Characteristics (N = 1660)
| Sample Characteristics | n (%)* |
|---|---|
| Age at injury, X̄ ±SD | 34.4±14.6 |
| Male | 1304 (78.6) |
| Race | |
| white | 1198(72.2) |
| minority | 462(27.8) |
| Number of motor-FIM Scores, X̄ ±SD | 2.5±0.8 |
| First follow-up motor-FIM (years), X̄ ±SD | 2.8±3.3 |
| Time between first and last motor-FIM (years), x ±SD | 6.6(3.7) |
| Motor-FIM at Discharge from Rehab, X̄ ±SD | 52.5±21.8 |
| Level and severity at discharge | |
| Paraplegia, incomplete | 261(15.7) |
| Paraplegia, complete | 548(33.0) |
| Tetraplegia, incomplete | 497(29.9) |
| Tetraplegia, complete | 354(21.3) |
| Place of Discharge | |
| Private | 1555(93.7) |
| Non-private home location | 103(6.2) |
| Unknown | 2(0.1) |
| Death | |
| Dying | 167(10.06) |
| Years of death from injury, X̄ ±SD | 9.5±4.5,n=165 |
Note:
Unless noted.
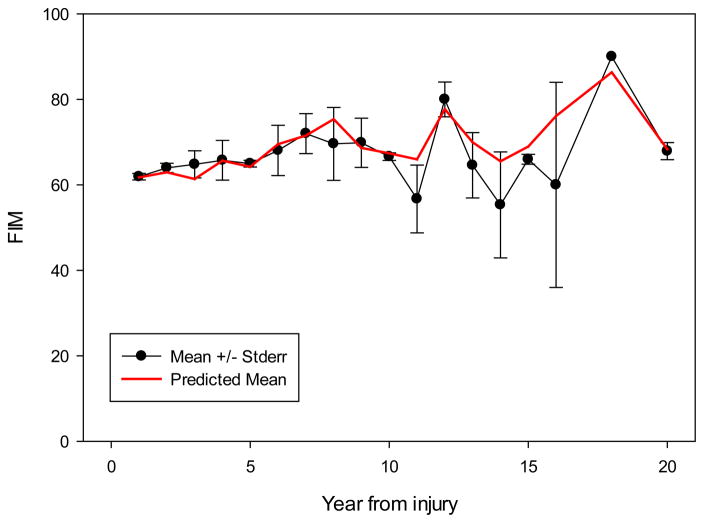
Figure 1 shows the raw and predicted data for the motor-FIM scores over time (mean ± standard error). The predicted line displays the model’s estimated motor-FIM score based on covariates, visually demonstrating the model’s accuracy. Table 2 presents the results of the mixed model analysis. When controlling for critical factors (motor-FIM at discharge from rehabilitation, level and severity of injury, age at injury, gender, and race), no significant Age × Time or Age × Time2 (p = 0.17; p = 0.07) interaction effects were detected. A significant relationship with age at injury was found (p = 0.001).
Figure 1.
Motor-FIM ± Standard Error*
Note: *Figure depicts raw motor-FIM data. Only general progression overtime can be deduced from the figure. Please note the variability in number of individuals with SCI at each time point.
Table 2.
Effects of age and other covariates on motor-FIM (N=1660)
| Variable | Estimate | Standard Error | F(df),p |
|---|---|---|---|
| Intercept | 33.24 | 1.80 | -- |
| Motor-FIM at Discharge | 0.71 | 0.02 | F1,238=1169,p<0.0001 |
| Level/Severity at Discharge | F3,238=84.59,p<0.0001 | ||
| Paraplegia, complete | −2.78 | 0.93 | |
| Paraplegia, incomplete | 1.63 | 1.16 | |
| Tetraplegia, complete | −16.14 | 1.06 | |
| Age at injury | −0.14 | 0.03 | F1,238=22.49, p<0.0001 |
| Sex (Female) | 0.20 | 0.84 | F1,238=0.06, p=0.81 |
| Race (white) | 1.20 | 0.77 | F1,238=2.42, p=0.12 |
| Time Linear | 0.47 | 0.26 | F1,1659=3.20, p=0.07 |
| Time Squared | −0.02 | 0.01 | F1,646=1.59, p=0.21 |
| Age × Time | 0.010 | 0.007 | F1,238=1.90, p=0.17 |
| Age × Time2 | −0.0008 | 0.0004 | F1,238=3.23, p=0.07 |
The first sensitivity analyses excluded individuals who died or relocated to a non-private home location. In these analyses, only those who remained in a private home location were analyzed. Those who were included in the sensitivity analyses were significantly younger [32.8 (13.7) vs. 44.9 (16.3) years; t(264.9) = −10.40, p < 0.0001], had higher motor-FIM scores at discharge from rehabilitation [53.8 (21.4) vs. 43.3 (22.6); t(1658) = 6.70, p < 0.0001], and had a different distribution of level and severity of SCI with more being likely to have had lower level and less severe injuries (χ2 (3)=13.80, p=0.003; Table 3). No significant sex or race differences were found. However, the sensitivity analyses found significant interactions for both the Age × Time and Age × Time2 interaction effects (Table 4; p=0.03, p=0.01). These effects indicate that the trajectories of the curves are moderated by the age at the time of injury. The significant Age × Time2 term shows the average curve is more peaked for patients who were older at the time of injury, while the significant Age × Time term shows a shift of the curve (for patients who were older at the time of the injury) to the left.
Table 3.
Demographics, Injury Characteristics from Sensitivity Analyses
| Sample Characteristics | Examining those who were living and maintain a private residential status | Examining those who had 15 or more years of follow-up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n (%)* | n (%)* | |||||
|
| ||||||
| In Sensitivity Analyses (N=1442) | Dropped from Sensitivity Analyses (N=218) | In Sensitivity Analyses (N=481) | Dropped from Sensitivity Analyses (N=1179) | |||
| Age at injury, X̄ ±SD | 32.8 ±13.7 | 44.9 ± 16.3 | t(264.9+)=−10.40, p<0.0001 | 29.8 ± 12.3 | 36.2 ± 15.1 | t(1082.4+)=9.00, p<0.0001 |
| Male | 1126 (78.1) | 178(81.7) | χ2(1)=1.43, p=0.23 | 385 (80.0) | 919 (78.0) | χ2(1)=0.89, p=0.35 |
| Race (N missing 114) Minority | 408 (28.3) | 54(24.8) | χ2 (1)=1.17, p=0.28 | 116 (24.1) | 346 (29.4) | χ2(1)=4.65, p=0.03 |
| Motor-FIM at discharge from Rehab, X̄ ±SD | 53.8 ±21.4 | 43.3 ±22.6 | t(1658)=6.70, p<0.0001 | 57.7 ± 22.1 | 50.3 ± 21.4 | t(1658)= −6.30, p<0.0001 |
| Level/Severity at discharge (N missing 125) | χ2 (3)=13.80, p=0.003 | χ2(3)=8.23, p=0.04 | ||||
| Paraplegia, incomplete | 236(16.4) | 25(11.5) | 81 (16.8) | 180 (15.3) | ||
| Paraplegia, complete | 488(33.8) | 60(27.5) | 173 (36.0) | 375 (31.8) | ||
| Tetraplegia, incomplete | 429(29.8) | 68(31.2) | 120 (25.0) | 377 (32.0) | ||
| Tetraplegia, complete | 289(20.0) | 65(29.8) | 107 (22.3) | 247 (21.0) | ||
+Satterthwaite method used due to unequal variances.
Table 4.
Effects of age and other covariates on motor-FIM: Sensitivity Analyses
| Variable | Examining those who were living and maintain a private residential status
|
Examining those who had 15 or more years of follow-up
|
||||
|---|---|---|---|---|---|---|
| Standard Estimate | Error | F(df),p | Standard Estimate | Error | F(df),p | |
| Intercept | 34.67 | 1.90 | -- | 32.69 | 3.24 | -- |
| Motor-FIM at Discharge | 0.68 | 0.02 | F1,213=960,p<0.0001 | 0.72 | 0.03 | F1,415=437.10,p<0.0001 |
| Level/Severity at Discharge | F3,213=76.38,p<0.0001 | F3,415=35.74,p<0.0001 | ||||
| Paraplegia, complete | −2.73 | 0.98 | −3.30 | 1.61 | ||
| Paraplegia, incomplete | 1.90 | 1.21 | −1.22 | 1.95 | ||
| Tetraplegia, complete | −16.20 | 1.12 | −17.53 | 1.72 | ||
| Age at injury | −0.12 | 0.03 | F1,213=11.34, p=0.0009 | −0.18 | 0.07 | F1,415=5.66, p=0.02 |
| Sex (Female) | 0.12 | 0.88 | F1,213=0.02, p=0.90 | −0.75 | 1.41 | F1,415=0.29, p=0.59 |
| Race(white) | 1.13 | 0.81 | F1,213=1.94, p=0.17 | 3.27 | 1.32 | F1,415=6.12, p=0.01 |
| Time Linear | 0.27 | 0.27 | F1,1430=0.99, p=0.32 | −0.73 | 0.41 | F1,479=3.17, p=0.08 |
| Time Squared | −0.01 | 0.01 | F1,566=0.35, p=0.55 | 0.04 | 0.02 | F1,415=4.30, p=0.04 |
| Age × Time | 0.017 | 0.008 | F1,213=5.11, p=0.03 | 0.03 | 0.01 | F1,415=5.44, p=0.02 |
| Age × Time2 | −0.0011 | 0.0004 | F1,213=6.31, p=0.01 | −0.0017 | 0.0006 | F1,415=7.54, p=0.006 |
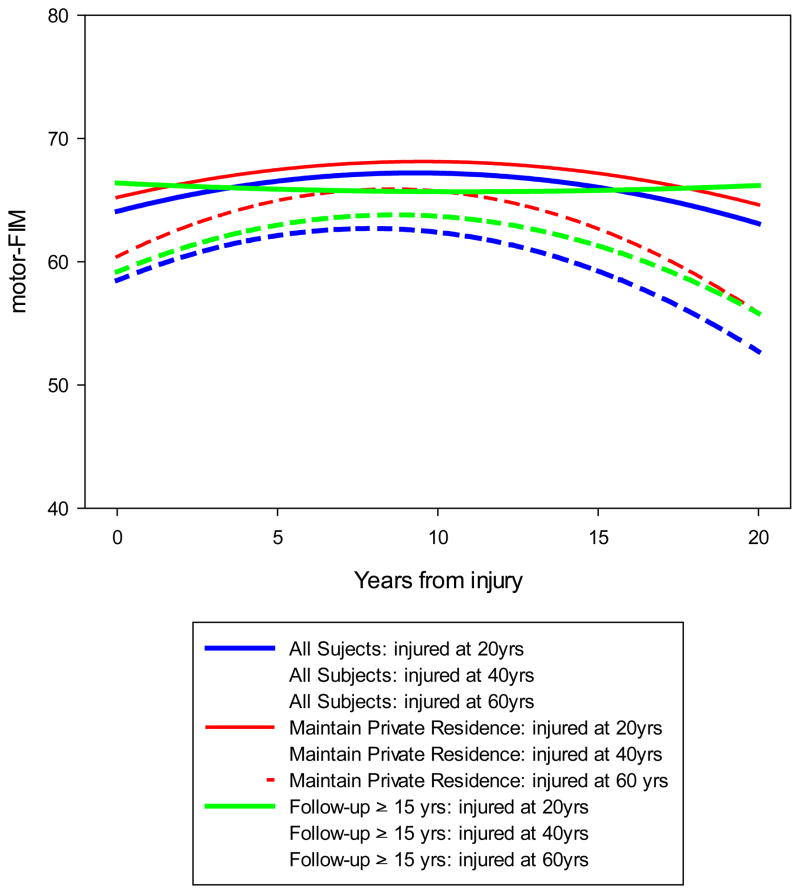
The second set sensitivity analyses only included individuals who had a minimum of 15 years of follow-up. Those who were included in these sensitivity analyses were significantly younger, less likely to be a minority, had higher motor-FIM scores at discharge from rehabilitation, and had a different distribution of level and severity of SCI with less likely to have tetraplegia, incomplete, than those who were excluded. No other differences were found. These sensitivity analyses found significant interactions for Time2, Age × Time and Age × Time2 interaction effects (Table 4; p=0.04, p=0.02, p=0.006). These effects, as the previously sensitivity analyses found, indicate that the trajectories of the curves are moderated by the age at the time of injury. The significant Age × Time2 term shows the average curve is more peaked patients who were older at the time of injury, while the significant Age × Time term shows a shift of the curve (for patients who were older at the time of the injury) to the left. Figure 2 shows the regression lines for three different age groups (20, 40, and 60 year olds) for all three models. Covariates are held constant in Figure 2.
Figure 2.
Predicted curves modeled using the baseline average covariates
DISCUSSION
The study findings indicate that among the entire sample of eligible individuals with SCI the rate of increase in disability was not associated with aging per se. However, a number of interesting and useful findings emerged from the analyses. First, we found that age at injury is associated with motor-FIM score. The significant association showed that, excluding the process of aging, age influences motor-FIM score. We, also, found that excluding individuals who died or moved to a non-private home location or only analyzing those who were followed for at least 15 years (seen in sensitivity analyses), age at time of injury did have a moderating influence on the association between disability and time. In these analyses, the motor-FIM scores had a quadratic shape, indicating that an individual’s level of independence in daily activities actually increases before it decreases. This suggests that individuals make gains in their independence in activities of daily living after they are living in the community.
Additionally for the subgroups examined in the sensitivity analyses, the interaction indicates that the steepness of the effect age at injury has on disability is less for individuals who were younger when they sustained their SCI and becomes steeper for individuals who were older when they were injured. Thus, individuals who sustain a SCI at an older age show a different course of disability than those who are younger at the time of injury. We found that the older an individual is when they sustain a SCI, the more rapidly their disability increases over time. On the other hand, the younger an individual is when they sustain a SCI the less slowly their disability increases as they age. These findings may be due to the possibility that those who sustain a SCI at a younger age have adapted to their disability whereas those who are older do not have that opportunity to adapt as they are already experiencing physical changes with age.
Both sensitivity analyses examined a sample that was significantly younger at time of injury, had higher motor-FIM scores when discharged from rehabilitation, and had lower level and less severe SCI. Given their younger age and higher motor-FIM scores, there may have been more opportunity for these individuals to experience the effects of aging and functional decline. They had a longer time to age and demonstrate the relationship between age and disability. However, the older adults who sustained SCI or had higher level and more severe injuries may have been more likely to die or move to a non-private home location, producing a ceiling effect for this subgroup. These analyses were statistically significant but have relatively small effect sizes.
While the effect size is relatively small, these unique findings addressing aging and disability are not surprising given the robust research that demonstrates individuals with SCI experience “accelerated aging”12 and health-related complications (e.g., bowel complications, diminished renal function, higher rates of ventilator use, increased rates of hospitalizations and skilled nursing home admissions, increase cardiovascular disease, increased healthcare costs, and increased prevalence of pressure sores) as they age.4–11 Our findings, when considered in light of the previous findings, provide additional evidence for the need for assessments and interventions that address long-term health outcomes. Current practice ensures that acute health outcomes are met; however, additional follow-up or screening may be needed to ensure that adults aging with SCI maintain their health and independence. Special consideration and interventions may be needed for adults who sustain SCI at an older age, given their higher risk for more rapid declines in disability over time.
Study Limitations
Although the findings provide a new perspective for aging with a SCI and represent one of the few longitudinal studies in this area, they should be interpreted with caution for a number of reasons. First, the study sample is not necessarily representative of the population of all individuals with SCI. Those included in the analyses were significantly older, less likely to be white, and had a different distribution of level and severity of SCI than those who were excluded from the primary analyses. These differences may introduce bias in the results reported. Caution is also advised in making definitive conclusions based on these analyses as the average follow-up time for the sample was relatively short. This is partly because collection of discharge motor-FIM data began in 1988 and follow-up motor-FIM began in 1995. Also of note, there are differences in the collection methods between discharge and follow-up FIM data. Discharge Motor-FIM was collected by observation and follow-up Motor-FIM was collected by interview. Information on the reliability of the Motor-FIM collected by interview is limited. However, one study found intraclass correlation coefficient between ratings by nurses and by patient or caregiver interviews of 0.93 for the total FIM,23 while another found that self-reported total-FIM scores to be 2-points higher than clinical-rated scores.24 Advising caution in generalizing results from these analyses is also consistent with evidence that there is enrollment bias for the NSCISC database.25 It should be noted that longer or shorter follow-up intervals in the statistical analyses may result in different data patterns and relationships between age and disability. Replicating these analyses in the future will allow for a determination of the generalizability of the findings.
In addition, the primary outcome used in these analyses was the motor-FIM. Although it is a valid measure of the functional status of individuals, it has limitations. First, it does not assess other important quality of life variables such as pain, social functioning, or general health. Second, occurrence of temporary changes in bowel and bladder function or adaptive technology may influence the motor-FIM scores. For example, an individual with a Urinary Tract Infection would report a lower motor-FIM score than if they did not have the infection. Future analyses should consider these limitations with the motor-FIM and examine other outcomes or factors that might be impacted by aging in individuals with SCI.
CONCLUSION
The findings from this study help achieve the long-term objective of having a greater understanding of how age might influence disability for individuals with SCI. The results findings provide important information that can contribute to the evaluation and development of interventions designed to buffer the effects of accelerated aging experienced by individuals with SCI. For example, recognizing that age at time of injury moderates the impact of time on disability can be used to develop preventative interventions to slow the progression of accelerated aging experienced by individuals with SCI, especially among those who are older when they are injured.
Acknowledgments
Acknowledgement of presentation
None.
Acknowledgement of financial support
Supported by the National Institute of Disability and Rehabilitation Research of the US Department of Education (H133N110011; H133B080024), the National Center for Medical Rehabilitation Research (T32 HD049307), and the National Institute of Mental Health (P30 MH090333; T32 MH019986). However, the contents of the article do not necessarily represent the policy of the Departments of Education or National Institutes of Health, and readers should not assume endorsement by the Federal Government.
Acknowledgement of contribution
Susan Charlifue, PhD at Craig Hospital contributed suggestions to the development of the manuscript.
Abbreviations
- SCI
Spinal Cord Injury
- Motor-FIM
Motor-Functional Independence Measure
Footnotes
Conflicts of Interest
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
References
- 1.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S. Life expectancy after spinal cord injury: a 50 year study. Spinal Cord. 2012;50:803–811. doi: 10.1038/sc.2012.55. [DOI] [PubMed] [Google Scholar]
- 2.Lidal IB, Snekkevik H, Aamodt G, Hjeltness N, Biering-Sorensen f, Stanghelle JK. Mortality after spinal cord injury in Norway. J Rehabil Med. 2007;39:145–51. doi: 10.2340/16501977-0017. [DOI] [PubMed] [Google Scholar]
- 3.Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Menter R, Weitzenkamp D, Cooper D, Bingley J, Charlifue S, Whiteneck G. Bowel management outcomes in individuals with long-term spinal cord injuries. Spinal Cord. 1997;35:608–12. doi: 10.1038/sj.sc.3100461. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 6.DeVivo MJ, Shewchuk RM, Stover SL, Black KJ, Go BK. A cross-sectional study of the relationship between age and current health status for persons with spinal cord injuries. Paraplegia. 1992;30:820–7. doi: 10.1038/sc.1992.158. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, DeVivo MJ, Jackson AB. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil. 2005;86:1208–1213. doi: 10.1016/j.apmr.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Groah SL, Kehn ME. The state of aging and public health for people with spinal cord injury: Lost in transition? Top Spinal Cord Inj Rehabil. 2010;15:1–10. [Google Scholar]
- 9.Menter RR, Whiteneck GG, Charlifue SW, Gerhart K, Solnick SJ, Brooks CA, et al. Impairment, disability, handicap, and medical expenses of persons aging with spinal cord injury. Paraplegia. 1991;29:613–9. doi: 10.1038/sc.1991.90. [DOI] [PubMed] [Google Scholar]
- 10.Hitzig SL, Tonack M, Campbell KA, McGillvray CF, Boschen KA, Richards K, et al. Secondary health complications in an aging Canadian spinal cord injury sample. Am J Phys Med Rehabil. 2008;87:545–55. doi: 10.1097/PHM.0b013e31817c16d6. [DOI] [PubMed] [Google Scholar]
- 11.Groah SL, Charlifue S, Tate D, Jensen MP, Molton IR, Forchheimer M, et al. Spinal Cord Injury and Aging: Challenges and Recommendations for Future Research. Am J Phys Med Rehabil. 2012;91:80–93. doi: 10.1097/PHM.0b013e31821f70bc. [DOI] [PubMed] [Google Scholar]
- 12.Charlifue S, Jha A, Lammertse D. Aging with spinal cord injury. Phys Med Rehabil Clin N Am. 2010;21:383–402. doi: 10.1016/j.pmr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Krause JS, Crewe NM. Chronologic age, time since injury, and time of measurement: effect on adjustment after spinal cord injury. Arch Phys Med Rehabil. 1991;72:91–100. [PubMed] [Google Scholar]
- 14.Charlifue SW, Weitzenkamp DA, Whiteneck GG. Longitudinal outcomes in spinal cord injury: aging, secondary conditions, and well-being. Arch Phys Med Rehabil. 1999;80:1429–34. doi: 10.1016/s0003-9993(99)90254-x. [DOI] [PubMed] [Google Scholar]
- 15.DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011;92:332–8. doi: 10.1016/j.apmr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Krause JS. Aging and life adjustment after spinal cord injury. Spinal Cord. 1998;36:320–8. doi: 10.1038/sj.sc.3100540. [DOI] [PubMed] [Google Scholar]
- 17.Whiteneck G, Tate D, Charlifue S. Predicting community reintegration after spinal cord injury from demographic and injury characteristics. Arch Phys Med Rehabil. 1999;80:1485–91. doi: 10.1016/s0003-9993(99)90262-9. [DOI] [PubMed] [Google Scholar]
- 18.Krause JS, Saunders LL, Acuna J. Gainful employment and risk of mortality after spinal cord injury: effects beyond that of demographic injury and socioeconomic factors. Spinal Cord. 2012;50:784–788. doi: 10.1038/sc.2012.49. [DOI] [PubMed] [Google Scholar]
- 19.Stover SL, DeVivo MJ, Go BK. History, implementation, and current status of the National Spinal Cord Injury Database. Arch Phys Med Rehabil. 1999;80:1365–71. doi: 10.1016/s0003-9993(99)90246-0. [DOI] [PubMed] [Google Scholar]
- 20.Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, Granger CV. The Functional Independence Measure: tests of scaling assumptions, structure and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77:1101–1108. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- 21.Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of disability in patients with acute traumatic spinal cord injury: A systematic review of the literature. J Neurotrauma. 2011;28:1413–1430. doi: 10.1089/neu.2009.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, New Jersey: John A. Wiley & Sons, Inc; 2006. [Google Scholar]
- 23.Jaworski DM, Kult T, Boynton PR. The Functional Independence Measure: a pilot study comparison of observed and reported ratings. Rehabil Nurs Res. 1994;3:141–7. [Google Scholar]
- 24.Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self ratings. Paraplegia. 1993;31(7):457–61. doi: 10.1038/sc.1993.74. [DOI] [PubMed] [Google Scholar]
- 25.Go BK, DeVivo MJ, Richards JS. The epidemiology of spinal cord injury. In: Stover SL, DeLisa JA, Whiteneck GC, editors. Spinal cord injury: Clinical outcomes from the model systems. Gaithersburgh: Aspen; 1995. pp. 21–55. [Google Scholar]