Abstract
Rapid influenza diagnostic tests (RIDTs) had low test sensitivity for detecting 2009 pandemic influenza A (H1N1pdm09) infection, causing public health authorities to recommend that treatment decisions be based primarily upon risk for influenza complications. We used multivariate Poisson regression analysis to estimate the contribution of RIDT results and risk for H1N1pdm09 complications to receipt of early antiviral (AV) treatment among 290 people with influenza-like illness (ILI) who received an RIDT ≤48 hours after symptom onset from May to December 2009 at four southwestern U.S. facilities. RIDT results had a stronger association with receipt of early AVs (rate ratio [RR] = 3.3, 95% confidence interval [CI] 2.4, 4.6) than did the presence of risk factors for H1N1pdm09 complications (age <5 years or high-risk medical conditions) (RR=1.9, 95% CI 1.3, 2.7). Few at-risk people (28/126, 22%) who had a negative RIDT received early AVs, suggesting the need for sustained efforts by public health to influence clinician practices.
Widespread reliance on rapid influenza diagnostic tests (RIDTs) complicated outbreak control and medical management during 2009 pandemic influenza A (H1N1pdm09).1 On April 29, 2009, the Centers for Disease Control and Prevention (CDC) advised clinicians to consider RIDT screening of patients with signs and symptoms compatible with influenza, but warned to interpret results with caution given limited test accuracy.2 CDC recommended antiviral (AV) treatment for children aged <5 years and people with certain medical conditions, two groups at higher risk for adverse influenza-related outcomes.3 In May 2009, CDC confirmed low sensitivity (40%–69%) in three different RIDTs for detecting H1N1pdm09, with sensitivities subsequently reported at 11%–88%.4–7
The Indian Health Service (IHS) is responsible for providing health care to eligible American Indian/Alaska Native (AI/AN) people through IHS, Tribal, and Urban Indian health facilities (collectively referred to as IHS). These facilities cared for approximately 60% of the U.S. AI/AN population, or 1.5 million beneficiaries, in 2009.8 On average, AI/ANs have lower income levels, education, and employment than the general U.S. population9 and are recognized to be at elevated risk of influenza complications and death.10
In June 2009, after multiple RIDT-negative cases of severe, probable H1N1pdm09 respiratory illness were reported, IHS issued clinical guidance recommending that patients with influenza-like illness (ILI) be tested for H1N1pdm09 virus and presumptively treated with AVs if they met CDC treatment criteria. To estimate the contribution of RIDT results to AV treatment decisions, IHS retrospectively analyzed ILI case data from four southwestern IHS facilities to measure the association among an RIDT result, presence of risk factors, and receipt of early AV therapy.
METHODS
In February 2010, IHS and CDC officials reviewed the charts of 819 people randomly selected from 8,132 people identified by laboratory records as having received an RIDT (BinaxNOW® Influenza A & B, Inverness Medical Professional Diagnostics, Princeton, New Jersey) from May to December 2009. Of these 819 people, 521 (64%) had documented ILI (temperature ≥100°F and cough or sore throat) and were considered for analyses. Clinical and sociodemographic variables included age group (<5, 5–24, or ≥25 years of age), sex, obesity (body mass index [BMI] ≥30 kilograms per meter squared), medical conditions conferring elevated risk for influenza-related adverse outcomes,11 ILI onset date, facility, RIDT result, and AV treatment status.
Early AV treatment (≤48 hours after symptom onset) has been shown to be most effective in reducing adverse influenza outcomes;12 thus, it was chosen as a suitable endpoint. Cases were excluded from analysis if chart review indicated a rapid test was performed >48 hours after symptom onset (n=186), symptom onset was missing (n=43), the RIDT date preceded symptom onset (n=1), or AVs were started before the RIDT (n=1). We used multivariate Poisson regression analysis to evaluate the association between RIDT result and receipt of AV treatment after adjusting for conditions conferring elevated risk for influenza-related adverse outcomes (age <5 years or medical risk factors). We compared results during the spring wave (ILI onset from May to August 2009) with the fall wave (ILI onset from September to December 2009). We conducted analyses using SAS® version 9.3.13
RESULTS
Overall, 308 (59%) of 521 people with ILI with an RIDT from May to December 2009 were female (Table 1). Of 379 people aged ≥2 years, 48% were obese. Of all 521 people, 151 (29%) were aged <5 years, 186 (36%) had medical risk factors for adverse outcomes, and 132 (25%) had a positive RIDT. AV treatment was initiated in 163 (31%) people: 111/309 (36%) people at elevated risk for adverse outcomes (either aged <5 years or with medical risk factors) and 52/212 (25%) people not at elevated risk for adverse outcomes.
Table 1.
Sociodemographic and clinical characteristics of patients (n=521) screened for pandemic influenza A (H1N1) with an RIDT at four IHS facilities in the southwestern U.S., May–December 2009
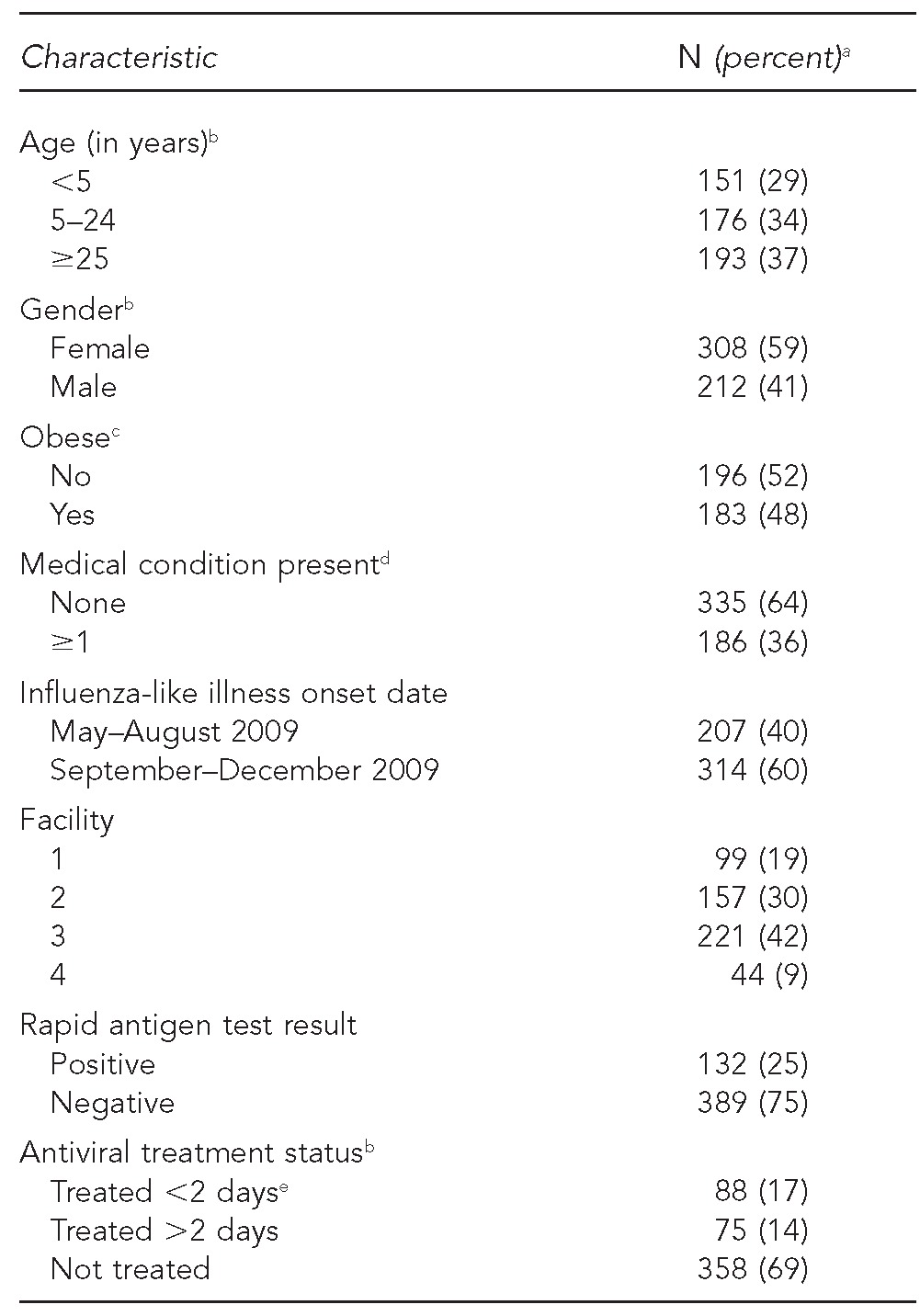
aTotal percentages may not equal 100 due to rounding.
bOne person was missing information on age and gender; nine people were missing information on treatment status.
cObesity only calculated for patients ≥2 years of age. Obesity defined as BMI ≥30 kilograms per meter squared for people aged >18 years and as ≥95th percentile of gender-specific BMI for age for people aged 2–18 years.
dBased on assessment of the following conditions identified by the Advisory Committee on Immunization Practices as increasing the risk for influenza-related complications: pregnant, asthma, cancer, cardiovascular disease, chronic lung disease, chronic obstructive pulmonary disease, diabetes, immunosuppression, kidney disorder, liver disorder, or neurological or neurodevelopmental disorder.
eThree of 88 people receiving early antiviral treatment were treated prior to receiving an RIDT and, thus, were not included in the analysis of the association between RIDT result and antiviral treatment.
RIDT = rapid influenza diagnostic test
IHS = Indian Health Service
BMI = body mass index
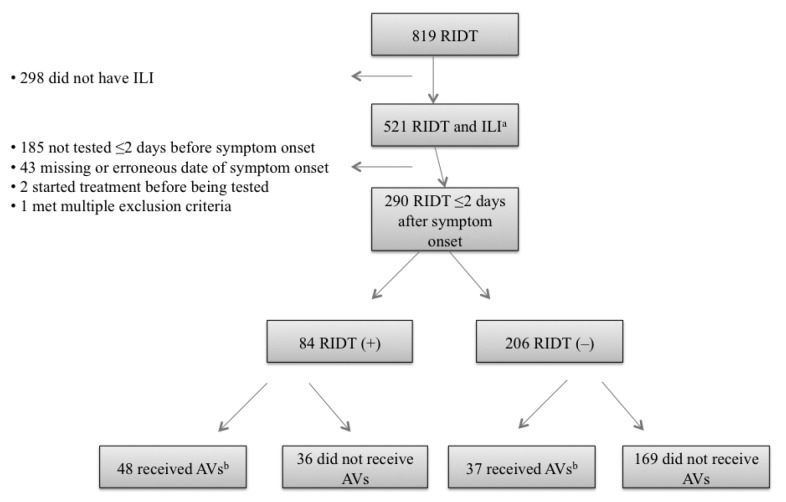
Of 290 people with ILI with an RIDT ≤48 hours after symptom onset (but not before symptom onset), 84 (29%) were RIDT positive and 206 (71%) were RIDT negative. Early AVs were received by 48/84 (57%) people with a positive RIDT and by 37/206 (18%) people with a negative RIDT (Figure). During the spring wave, early AVs were received by 13/35 (37%) people with a positive RIDT and by 5/96 (5%) people with a negative RIDT (Table 2). During the fall wave, early AVs were received by 35/49 (71%) people with a positive RIDT and by 32/110 (29%) people with a negative RIDT. During the entire period, after adjusting for conditions conferring elevated risk for adverse outcomes (aged <5 years or with medical risk factors), an RIDT result had a stronger association with receipt of early AVs (rate ratio [RR] = 3.3, 95% confidence interval [CI] 2.4, 4.6) than did the presence of these risk factors (RR=1.9, 95% CI 1.3, 2.7). These differences were more striking in the spring wave (RR=7.4, 95% CI 2.9, 18.9 vs. RR=2.5, 95% CI 0.9, 6.7) than in the fall wave (RR=2.5, 95% CI 1.8, 3.5 vs. RR=1.8, 95% CI 1.2, 2.5) (Table 2). Overall, 33 of 46 people (72%) at risk for adverse outcomes with a positive RIDT received early AVs, whereas 28 of 126 people (22%) at risk for adverse outcomes with a negative RIDT received early AVs (Table 3).
Figure.
People with ILI who received an RIDT during the 2009 influenza A (H1N1) pandemic (n=521), according to status of treatment with AV therapy and RIDT result, at four IHS facilities in the southwestern U.S., May–December 2009
aDefined as documentation of temperature >100°F plus sore throat or cough
bFor example, oseltamivir or zanamivir were used ≤2 days after symptom onset.
ILI = influenza-like illness
RIDT = rapid influenza diagnostic test
AV = antiviral
IHS = Indian Health Service
Table 2.
Likelihood of receiving early antiviral treatment, by RIDT result and by being at riska for adverse influenza-related outcomes, at four IHS facilities in the southwestern U.S., May–December 2009 (n=290)
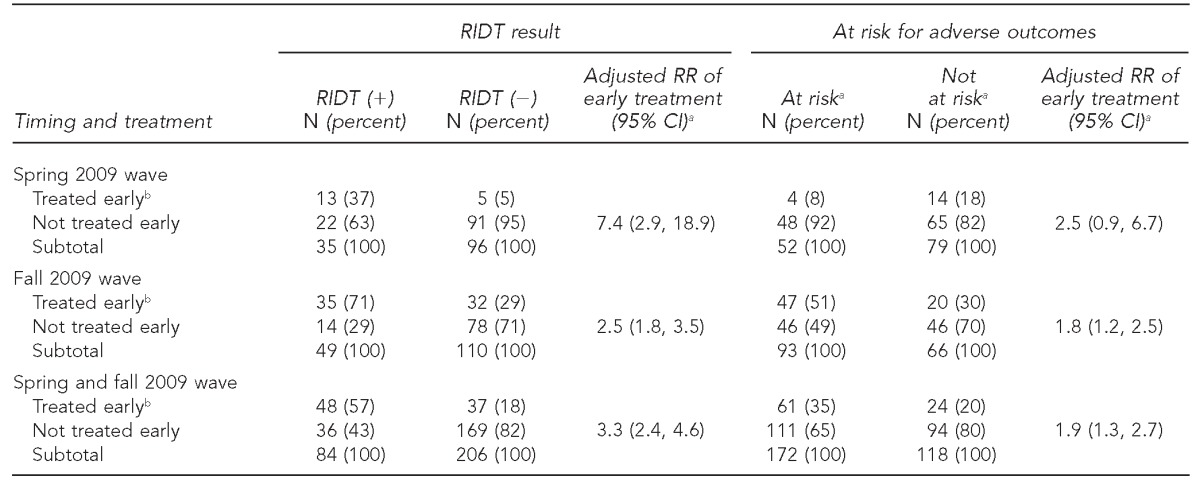
At-risk conditions were considered to be age <5 years or the presence of medical conditions conferring an increased risk for influenza-related complications: pregnant, asthma, cancer, cardiovascular disease, chronic lung disease, chronic obstructive pulmonary disease, diabetes, immunosuppression, kidney disorder, liver disorder, or neurological or neurodevelopmental disorder.
bTreated early refers to receipt of antiviral treatment within 48 hours of illness onset.
RIDT = rapid influenza diagnostic test
IHS = Indian Health Service
RR = relative risk
CI = confidence interval
Table 3.
Number and proportion of people at riska for adverse outcomes, by RIDT result, among people treated early vs. people not treated earlyb at four IHS facilities in the southwestern U.S., May–December 2009 (n=290)
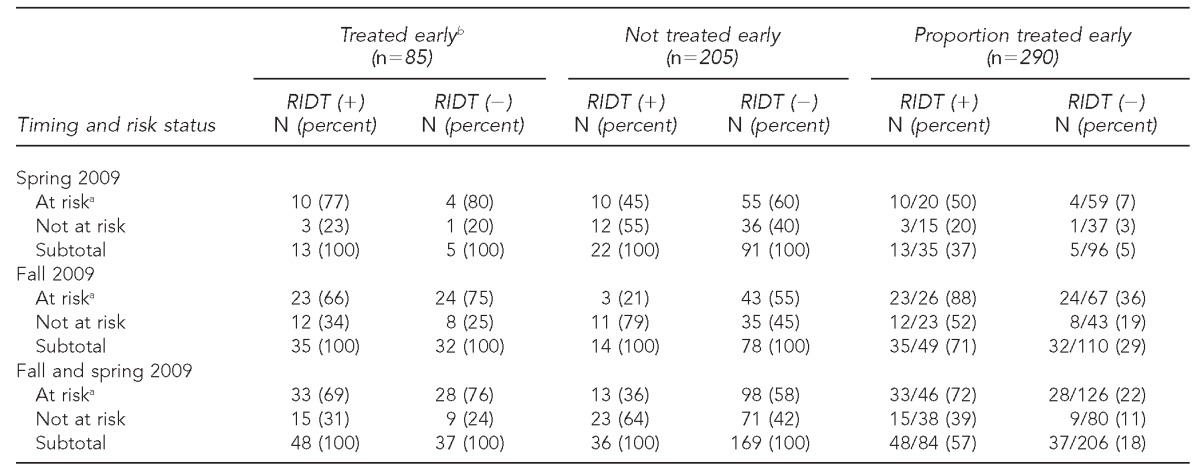
At-risk conditions were considered to be age <5 years or the presence of medical conditions conferring increased risk for influenza-related complications: pregnant, asthma, cancer, cardiovascular disease, chronic lung disease, chronic obstructive pulmonary disease, diabetes, immunosuppression, kidney disorder, liver disorder, or neurological or neurodevelopmental disorder.
Treated early refers to receipt of antiviral treatment within 48 hours of illness onset.
RIDT = rapid influenza diagnostic test
IHS = Indian Health Service
DISCUSSION
We found that an RIDT result had a stronger association with receipt of early AV treatment than did the presence of risk factors for H1N1pdm09 complications, particularly during the spring wave. The low sensitivity (11%–88%) of conventional RIDTs in detecting H1N1pdm09 is now well documented.4–7 Our data indicate that people with a negative RIDT were frequently left untreated, despite having indications for AV treatment and having presented within 48 hours of illness onset. This undertreatment of people with a negative RIDT occurred despite public health guidance that clinicians rely on the risk status of people with ILI rather than on an RIDT result when making treatment decisions.2 These findings are especially important when considering that AI/ANs are a recognized influenza complication risk group.10
The low rate of AV treatment among RIDT-negative people with ILI may represent missed treatment opportunities. Compared with AV treatment decisions made during the spring wave, decisions made during the fall wave were less reliant on RIDT results, likely due to the effect of public health messaging discouraging their use in making treatment decisions. A retrospective cohort study in Los Angeles, California, described AV treatment practices at variance with CDC guidance for treatment of high-risk groups, but treatment among high-risk patients (94% of inpatients and 54% of outpatients) was more common than we observed.14 The role of RIDTs in clinical decision-making during the pandemic was not explored in their analysis and has been seldom studied (for any epidemic).
Our findings also suggest missed treatment opportunities, even with positive RIDT results, as 28% of at-risk people with a positive RIDT still did not receive early AV treatment. We speculate that IHS providers might have restricted AV treatment based on current or anticipated shortages, particularly early in the pandemic. Understanding the relative contributions of RIDTs and risk factors in providers' treatment decisions can help inform future influenza treatment recommendations.
Weak rapid diagnostic tests (RDTs) are not unique to influenza and demonstrate the conflict between maximizing rapid, accurate diagnosis and minimizing missed or false diagnoses. For example, Dinnes et al. identified limitations to the performance of various RDTs in tuberculosis conditions.15 The performance of any RDT is dependent on a variety of community, host, and microbial factors, such as the disease prevalence in a community and the test setting.
Limitations
This study was subject to several limitations. One limitation was that reliance on retrospective chart review limited analyses to data recorded in clinical charts. Another limitation was that the cohort size precluded accurate assessment of some clinical endpoints (e.g., hospitalization, intensive care unit hospitalization, or death). A third limitation was that we focused on the experience of four facilities serving an AI/AN population; as such, this study might not be generalizable to all AI/ANs in the U.S. Lastly, our study population contained a larger proportion of younger people than among overall U.S. H1N1pdm09 cases,16 which is reflective of our service population, but which may impact the generalizability of these findings to the general U.S. population.9
CONCLUSIONS
Studies in the United States, China, and Mexico indicate that during H1N1pdm09, early AV treatment was associated with a reduction in severe influenza complications, especially intensive care unit admission and death.16–18 While early AV treatment is most effective, treatment can still be beneficial up to <5 days after symptom onset.17 In 2011, the Advisory Committee on Immunization Practices updated its recommendations for prescribing AVs in the case of clinically suspected or confirmed influenza to include all people who are hospitalized; have severe, complicated, or progressive disease; or are at higher risk for influenza complications.10 High-risk groups now include AI/ANs.
Our findings highlight the challenges involved in making clinical decisions during a rapidly evolving public health emergency. In the case of influenza pandemics and other major public health events, public health authorities are a crucial source of guidance for clinical providers who are trying to keep up with increased patient load while simultaneously trying to follow rapidly changing recommendations. These results emphasize the importance of sustained efforts by public health authorities to educate clinical providers to consider not just the results of rapid testing, but a patient's entire risk profile as well.
Footnotes
The authors thank the tribal officials, infection control practitioners, laboratory personnel, and health-care providers who helped during the site visits and data gathering, especially Dr. Mark Traeger, Ms. Deborah Farrell, and Dr. Carol Barlage for their assistance with the logistics of this investigation. The authors also thank Dr. Julie Magri for helpful comments and revisions during the editorial process.
This study was supported by the Indian Health Service (IHS) and the Centers for Disease Control and Prevention (CDC). This investigation was part of an emergency public health response to the pandemic and underwent human subjects review by IHS, tribal authorities, and CDC. It was deemed not to be research in accordance with Federal Regulations 46.101c and 46.102d and CDC's Guidelines for Defining Public Health Research and Public Health Non-Research.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC.
REFERENCES
- 1.Jernigan DB, Lindstrom SL, Johnson JR, Miller JD, Hoelscher M, Humes R, et al. Detecting 2009 pandemic influenza A (H1N1) virus infection: availability of diagnostic testing led to rapid pandemic response. Clin Infect Dis. 2011;52(Suppl 1):S36–43. doi: 10.1093/cid/ciq020. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2009. [cited 2013 Feb 4]. Interim guidance for the detection of novel influenza A virus using rapid influenza diagnostic tests. Also available from: URL: http://www.cdc.gov/h1n1flu/guidance/rapid_testing.htm. [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2009. [cited 2013 Feb 4]. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. Also available from: URL: http://www.cdc.gov/h1n1flu/recommendations.htm. [Google Scholar]
- 4.Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(30):826–9. [PubMed] [Google Scholar]
- 5.Chu H, Lofgren ET, Halloran ME, Kuan PF, Hudgens M, Cole SR. Performance of rapid influenza H1N1 diagnostic tests: a meta-analysis. Influenza Other Respir Viruses. 2012;6:80–6. doi: 10.1111/j.1750-2659.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (swine) influenza A/H1N1. Clin Infect Dis. 2009;49:1090–3. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uyeki TM, Prasad R, Vukotich C, Stebbins S, Rinaldo CR, Ferng YH, et al. Low sensitivity of rapid diagnostic test for influenza. Clin Infect Dis. 2009;48:e89–92. doi: 10.1086/597828. [DOI] [PubMed] [Google Scholar]
- 8.Indian Health Service (US) Rockville (MD): IHS; 2010. [cited 2013 Feb 4]. IHS fiscal year 2010 user population estimates. Also available from: URL: http://www.ihs.gov/california/assets/File/Training/FY2010-MemoFinalUserPop.pdf. [Google Scholar]
- 9.Indian Health Service (US) Rockville (MD): Department of Health and Human Services (US); 2003. Trends in Indian health: 2002–2003 edition. [Google Scholar]
- 10.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;60(RR-1):1–24. [PubMed] [Google Scholar]
- 11.Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Morb Mortal Wkly Rep. 2009;58(RR-10):1–8. [PubMed] [Google Scholar]
- 12.Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66:959–63. doi: 10.1093/jac/dkr090. [DOI] [PubMed] [Google Scholar]
- 13.SAS Institute Inc. Cary (NC): SAS Institute Inc.; 2011. SAS®: Version 9.3. [Google Scholar]
- 14.Vijayan V, Jing J, Zangwill KM. Evaluation of diagnostic and therapeutic approaches for suspected influenza A(H1N1)pdm09 infection, 2009–2010. Emerg Infect Dis. 2012;18:1414–21. doi: 10.3201/eid1809.111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Feng Z, Uyeki TM, Liao Q, Zhou L, Feng L, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52:457–65. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuera Iglesias AL, Kudo K, Manabe T, Corcho Berdugo AE, Corrales Baeza A, Alfaro Ramos L, et al. Reducing occurrence and severity of pneumonia due to pandemic H1N1 2009 by early oseltamivir administration: a retrospective study in Mexico. PLoS One. 2011;6:e21838. doi: 10.1371/journal.pone.0021838. [DOI] [PMC free article] [PubMed] [Google Scholar]