Abstract
Objective
We validated cases of active tuberculosis (TB) recorded in the Indian Health Service (IHS) National Patient Information Reporting System (NPIRS) and evaluated the completeness of TB case reporting from IHS facilities to state health departments.
Methods
We reviewed the medical records of American Indian/Alaska Native (AI/AN) patients at IHS health facilities who were classified as having active TB using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes from 2006 to 2009 for clinical and laboratory evidence of TB disease. Individuals were reclassified as having active TB disease; recent latent TB infection (LTBI); past positive tuberculin skin test (TST) only; or as having no evidence of TB, LTBI, or a past positive TST. We compared validated active TB cases with corresponding state records to determine if they were reported.
Results
The study included 596 patients with active TB as per ICD-9-CM codes. Based on chart review, 111 (18.6%) had active TB; 156 (26.2%) had LTBI; 104 (17.4%) had a past positive TST; and 221 (37.1%) had no evidence of TB disease, LTBI, or a past positive TST. Of the 111 confirmed cases of active TB, 89 (80.2%) resided in participating states; 81 of 89 (91.2%) were verified as reported TB cases.
Conclusions
ICD-9-CM codes for active TB disease in the IHS NPIRS do not accurately reflect the burden of TB among AI/ANs. Most confirmed active TB cases in the IHS health system were reported to the state; the national TB surveillance system may accurately represent the burden of TB in the AI/AN population.
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis (M. tuberculosis) complex. Treatment for active TB disease requires months of combination drug therapy. Left untreated, TB can result in substantial morbidity and occasionally death. Although the number of TB cases in the United States has steadily declined during the past two decades, TB remains a major health concern within many subgroups, including American Indians/Alaska Natives (AI/ANs). The TB case rate among AI/ANs is estimated at 5.6 per 100,000 population, notably higher than the national average of 3.4 cases per 100,000 population.1
Surveillance of active TB disease is an important component of monitoring and controlling the spread of TB. Currently, annual rates of TB in the U.S. are calculated by the Centers for Disease Control and Prevention (CDC) National Tuberculosis Surveillance System (NTSS).1 The NTSS is an electronic database that relies on the collaboration of state and local health departments; each person diagnosed with TB disease is verified as an incident case of TB and reported using a standard TB case form. The criteria for TB disease surveillance are based on a laboratory case definition, clinical case definition, or provider diagnosis.1,2 The laboratory case definition requires isolation of M. tuberculosis complex in culture or detection of M. tuberculosis complex nucleic acids by amplification testing or demonstration of acid-fast bacilli in a clinical specimen when a culture cannot be obtained. The clinical case definition requires (1) a positive tuberculin skin test (TST), (2) signs and symptoms compatible with TB, (3) treatment with at least two anti-TB medications, and (4) a completed diagnostic evaluation. A provider diagnosis is used when the clinical presentation is consistent with TB but the criteria to meet laboratory or clinical case definitions are not met.
The Indian Health Service (IHS), an agency of the U.S. Department of Health and Human Services, provides comprehensive health-care services through IHS, Tribal, and Urban Indian facilities (collectively referred to hereafter as IHS) to eligible AI/AN people who are members of 566 federally recognized Tribes. IHS provides care for approximately 2.1 million (62%) of the nation's estimated 3.4 million AI/ANs.3 The IHS maintains a national database, the National Patient Information Reporting System (NPIRS).4 Within NPIRS, diseases and conditions are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).5 In addition, IHS is in the process of implementing an electronic health record (EHR) system.6 Electronic data collected by IHS have the potential to serve as a resource to better understand the burden and monitor trends of TB disease within the AI/AN population; yet, the accuracy of NPIRS for identifying people with TB disease has not been previously established. Several previous studies in other U.S. populations have cited wide variability (0%–77%) in the positive predictive value (PPV) of ICD-9-CM diagnostic codes for active TB disease.7–13
CDC provides guidance for IHS providers to report all nationally notifiable diseases, including TB, to local and state authorites.1,2 However, there are no explicit mechanisms for IHS to report cases of TB directly to the NTSS, and the extent to which IHS facilities collaborate with local authorities on case reporting is not well understood.
We validated active cases of TB disease within the AI/AN population by reviewing the medical charts of individuals assigned an active TB disease ICD-9-CM code in the inpatient and outpatient NPIRS visit data from 2006 to 2009 to determine the completeness of reporting TB disease by examining whether validated TB cases from IHS facilities were reported to state health departments.
METHODS
Study population
IHS is divided into six regions: Alaska, East, Northern Plains, Southern Plains, Southwest, and West.3 Each region is split into service units (SUs) containing hospitals and clinics that provide health care for the local AI/AN population. Currently, there are 168 SUs in the U.S.
Using estimates of the 2007 AI/AN user population (i.e., eligible AI/ANs who have used services within the past three years),14 we used population proportional to size sampling to select 20 IHS SUs for inclusion in the study. The study population included all AI/ANs with an ICD-9-CM code indicating active TB disease (010.0–018.9) (hereafter referred to as presumptive TB cases) in NPIRS from 2006 to 2009. The study sample was supplemented by including the four SUs with the greatest number of presumptive TB cases during the study period, for a total of 24 SUs. All health facilities in each selected SU that had at least five patients with an active TB code during the study period were included. We generated a list of inpatients and outpatients at each selected facility, and randomly selected 10% or 30% presumptive TB cases (whichever was higher) from each list. All patients were selected if a list contained ≤30 patients. We identified and selected for inclusion all state health departments containing the selected SUs to allow evaluation of reporting completeness by SUs to state health authorities.
Data collection and definitions
Validation of active TB disease.
We reviewed medical charts for the sampled individuals for clinical and laboratory evidence of active TB disease, including clinical symptoms, history of TB exposure or disease, TST results, chest x-rays, and sputum smear and culture results. We reviewed both paper and electronic medical charts for the time period six months prior to and six months following the date of the initial active TB ICD-9-CM code in NPIRS to identify the first coding for the current episode or diagnosis. We also collected information on patient demographics, medical history, comorbidities, and social and behavioral characteristics. All data were abstracted by trained research staff using standardized data collection forms.
Based on the medical record review, each presumptive case of active TB disease was reclassified into one of four categories: active TB disease; recent diagnosis of latent TB infection (LTBI); past positive TST only; or no evidence of TB, LTBI, or a previous positive TST. A diagnosis of LTBI applies to individuals who have an indication of exposure to TB (e.g., a positive TST that was previously negative) but do not have any clinical evidence of active TB disease. Patients with documentation of a positive TST more than one year preceding the diagnostic code were not considered to have recent LTBI and were recorded as having a past positive TST only. For patients defined as valid active TB cases based on record review, we collected additional information on whether the patient was alive at the time of diagnosis, the site of TB disease (pulmonary or extrapulmonary), whether the patient initiated anti-TB treatment, and the treatment regimen. We also collected information on LTBI treatment for patients classified with a recent diagnosis of LTBI.
Information on TB symptoms and test results for screening and testing for TB disease was coded as either documented in the chart as present or positive at the time of evaluation (e.g., documentation of cough) or as no indication of presence or positivity (e.g., no cough or absence of any transcription indicating cough in the chart). We calculated a summary value for TB symptoms by tallying all symptoms affirmatively documented in the chart.
Cross-reference of chart-verified TB diagnosis with state health departments.
We compared all presumptive TB cases validated by chart review as having active TB disease with state records by directly linking name, date of birth, and year of diagnosis. From this linkage, people were classified as either a verified TB case, not a verified TB case but recorded in the state database for a TB evaluation, or lacking a record in the state database. For IHS facilities servicing people from more than one state, multiple states were contacted.
Statistical analysis
We used frequencies and proportions to describe the sociodemographic and clinical characteristics of the population included in the medical chart review and by verified disease category (verified active TB disease, recent LTBI, past positive TST only, or neither TB nor LTBI). We calculated the PPV and 95% confidence intervals (CIs) of ICD-9-CM diagnostic codes for identifying cases of active TB. We used logistic regression to calculate odds ratios (ORs) and 95% CIs for the association between symptoms and tests used to screen for and diagnose TB disease and whether the individual was classified as having active TB disease based on chart review. We considered factors to be statistically significant at p<0.05. We conducted all data analyses using SAS® version 9.3.15
RESULTS
Study population
We randomly selected 661 patients with active TB disease ICD-9-CM codes from 24 SUs for chart review (Figure 1). Ninety percent (n=596) of the medical charts were available and reviewed at IHS facilities.
Figure 1.
Flow chart of AI/AN patients with an ICD-9-CM code for active TB disease (010.0–018.9) in the IHS NPIRS database selected for medical chart review: U.S., 2006–2009
AI/AN = American Indian/Alaska Native
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
TB = tuberculosis
IHS = Indian Health Service
NPIRS = National Patient Information Reporting System
SU = service unit
Approximately one-quarter (25.2%) of the 596 patients were ≥65 years of age and 6.0% were children or adolescents <15 years of age (Table 1). Almost half (47.3%) of patients were from IHS facilities in the Southwest region and the majority (83.6%) were outpatients. Most patients (86.2%) were diagnosed with pulmonary TB, and the most common reasons for TB evaluation were because they needed to be screened for employment or administrative purposes (26.1%), presented with TB symptoms (21.0%), or had been in recent contact with an active TB case (10.9%). Nearly one-third of patients with information available had diabetes (31.6%), had used alcohol excessively in the past year (31.7%), or currently smoked (30.3%).
Table 1.
Sociodemographic and clinical characteristics of AI/AN patients at IHS facilities included in the medical chart review to validate active TB disease: U.S., 2006–2009


aAll characteristics assessed as documented in the medical chart; if no information was identified in the chart and the characteristic could not be characterized as yes or no, it was coded as missing. Percentages reflect the proportion of those with documentation for the characteristic (excluding missing).
bThe IHS East Region was not included due to logistic constraints.
cICD-9-CM codes 012 = other respiratory TB; 013 = TB of meninges and central nervous system; 014 = TB of intestines and peritoneum and mesenteric glands; 015 = TB of bones and joints; 016 = TB of genitourinary system; 017 = TB of other organs; and 018 = military TB.
AI/AN = American Indian/Alaska Native
IHS = Indian Health Service
TB = tuberculosis
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
NPIRS = National Patient Information Reporting System
HIV = human immunodeficiency virus
Of the patient charts documenting screening and tests for TB, 390 patients (65.4%) did not have any documented TB symptoms (Table 2). However, among patients with information available, 41.6% had a cough and 22.8% noted weakness or fatigue. A total of 177 patients (29.7%) reported a TST within a year preceding diagnosis; 152 of those patients (85.9%) were TST positive. Nearly one-third (29.6%) of individuals with documentation of a chest x-ray had abnormal findings. Additionally, 50/486 patients (10.3%) had a positive sputum smear result and 51/472 patients (10.8%) had a positive sputum culture result.
Table 2.
Frequency and proportion of clinical characteristics and tests used to validate active TB disease among selected AI/AN patients with an ICD-9-CM code for active TB disease at IHS facilities: U.S., 2006–2009
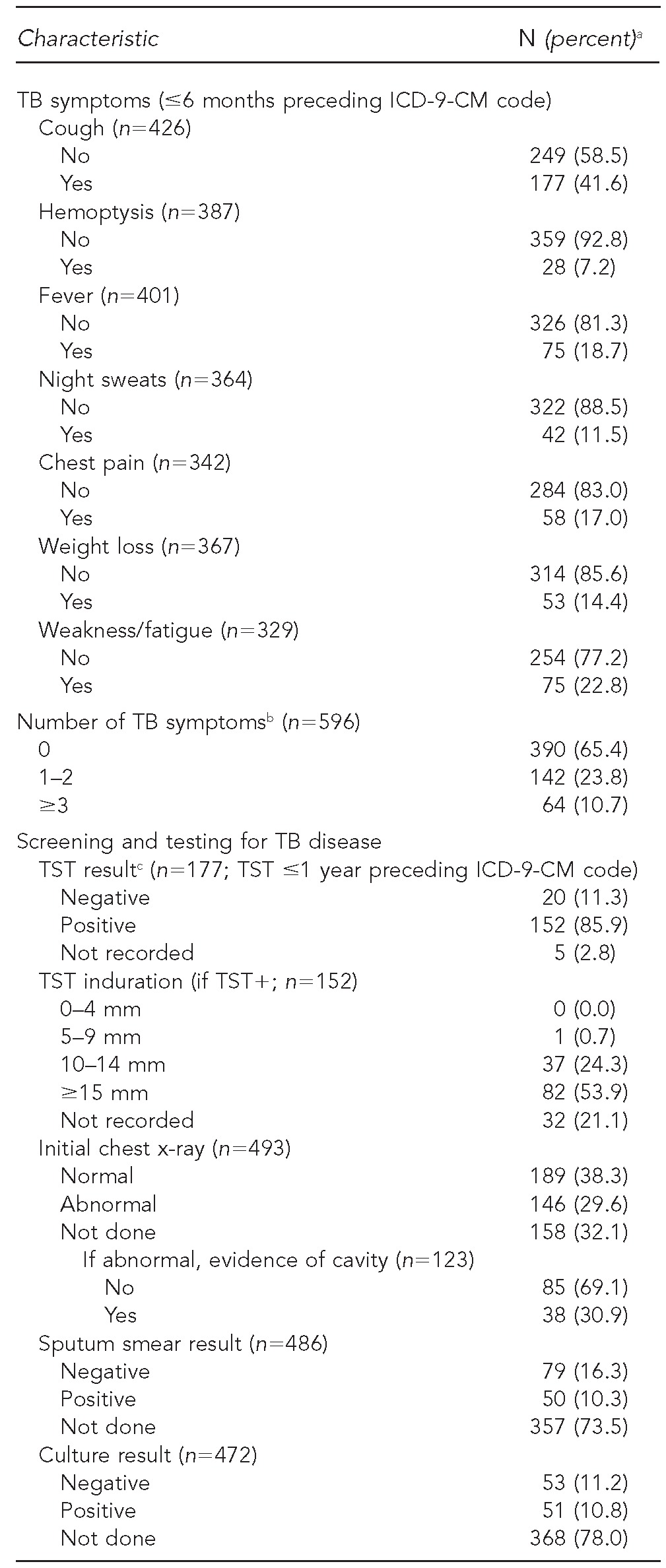

aPercentages reflect proportion of those with documentation for the characteristic (excluding missing).
bEach TB symptom was assigned as present (1) or absent/not noted in chart (0) to calculate summary.
cAs noted per written comment or electronic notation in the chart (not based on induration)
TB = tuberculosis
AI/AN = American Indian/Alaska Native
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
IHS = Indian Health Service
TST = tuberculin skin test
mm = millimeter
Validation of TB cases
Overall, 111 of 596 (18.6%, 95% CI 15.6, 22.0) patient charts reviewed had documentation of active TB disease (Figure 2). Another 156 (26.2%) patients were determined to have a diagnosis of recent LTBI. One hundred and four (17.4%) patient charts indicated a past positive TST but no current evidence of TB or LTBI. More than one-third of patients (n=221, 37.1%) had no evidence in their chart of active TB disease, LTBI, or a past positive TST.
Figure 2.
Flow chart of verified TB diagnoses based on chart review among selected AI/AN patients at IHS facilities and reporting of chart-verified TB cases to the state health department: U.S., 2006–2009
TB = tuberculosis
AI/AN = American Indian/Alaska Native
IHS = Indian Health Service
LTBI = latent tuberculosis infection
TST = tuberculin skin test
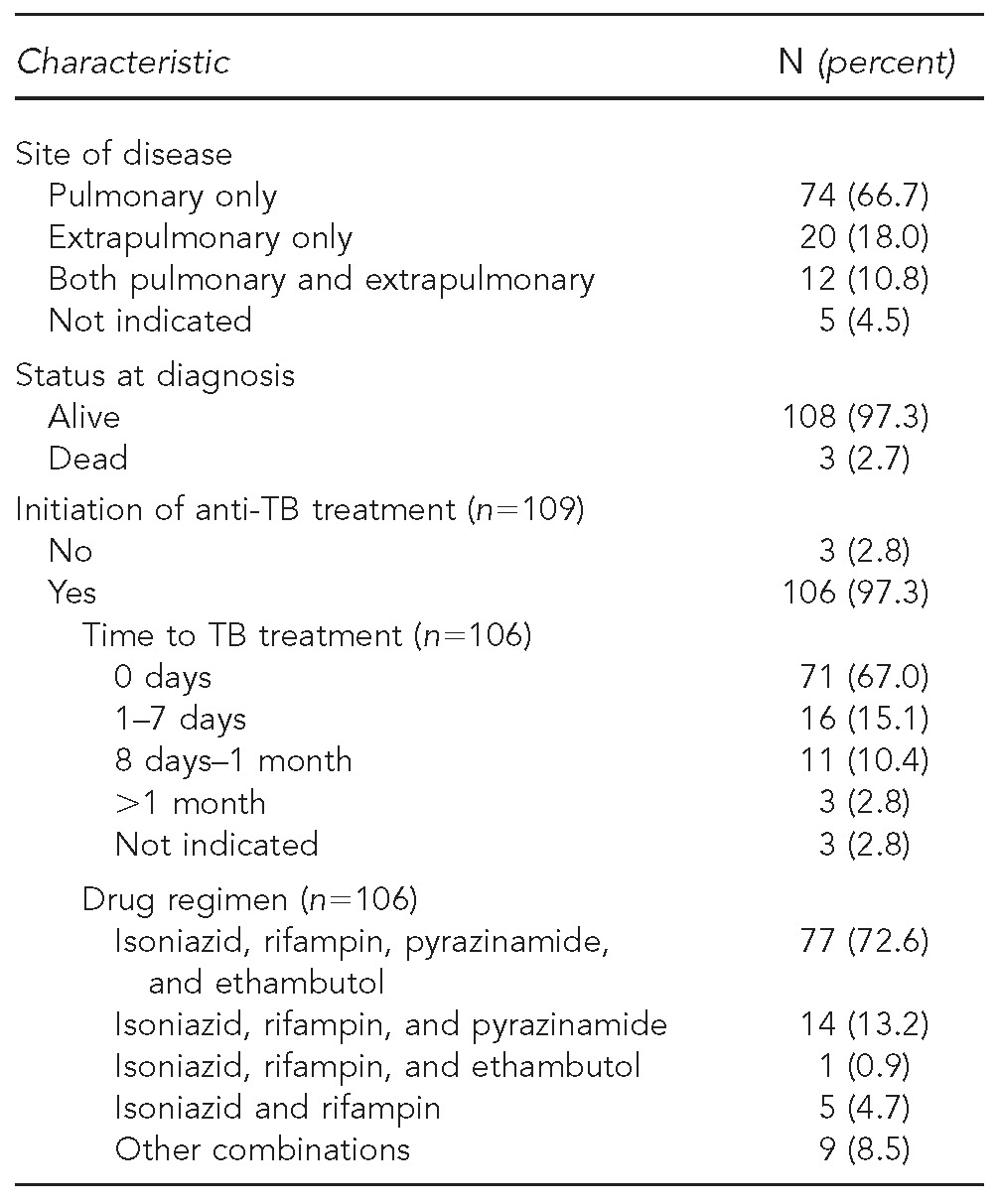
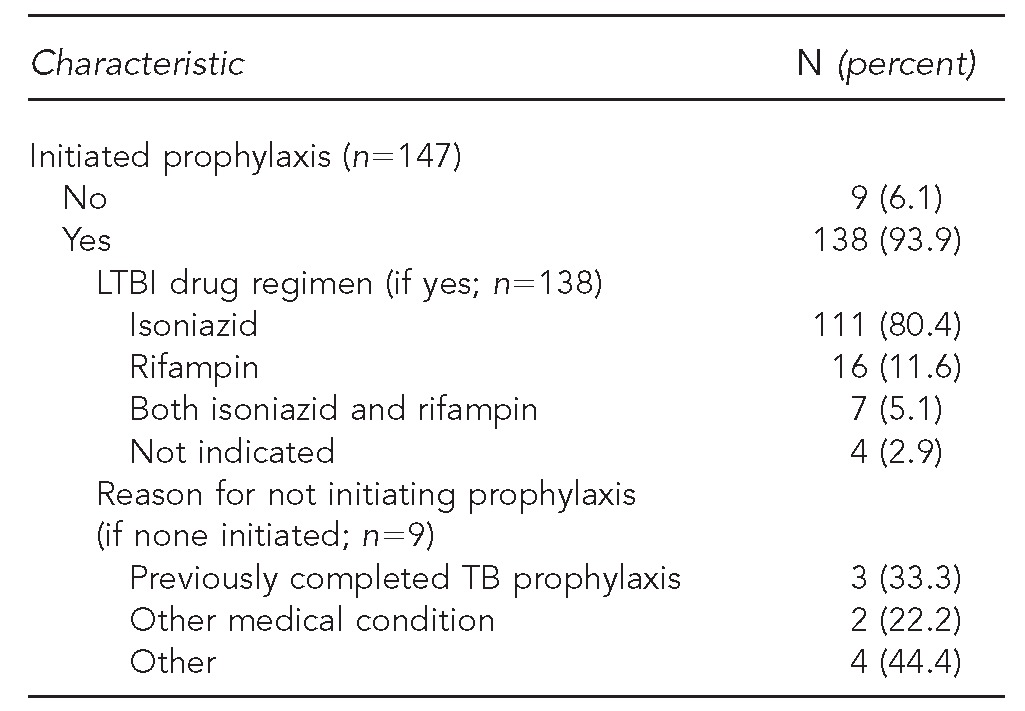
Of the 111 patients verified as having active TB disease based on chart review, 74 (66.7%) had pulmonary TB disease only, 20 (18.0%) had extrapulmonary TB only, and 12 (10.8%) had both pulmonary and extrapulmonary disease. Nearly all (n=106, 97.3%) verified TB patients had documentated initiation of anti-TB therapy; the standard first-line treatment regimen of isoniazid, rifampin, pyrazinamide, and ethambutol was indicated in the charts of 77 of these patients (72.6%) (Table 3a). Most patients (n=138, 93.9%) classified as current LTBI had initiated LTBI treatment, with isoniazid most commonly prescribed (n=111, 80.4%) (Table 3b).
Table 3a.
Clinical and treatment characteristics of selected AI/AN patients at IHS facilities with presumptive TB based on ICD-9-CM diagnostic codes, verified as having active TB disease (n=111): U.S., 2006–2009
AI/AN = American Indian/Alaska Native
IHS = Indian Health Service
TB = tuberculosis
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
Table 3b.
Clinical and treatment characteristics of selected AI/AN patients at IHS facilities with presumptive TB based on ICD-9-CM diagnostic codes, determined to have a current diagnosis of LTBI (n=156): U.S., 2006–2009
AI/AN = American Indian/Alaska Native
IHS = Indian Health Service
TB = tuberculosis
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
LTBI = latent tuberculosis infection
Clinical factors and TB screening test results associated with validated active TB disease
Patients with presumptive TB with chart documentation of cough, hemoptysis, fever, night sweats, chest pain, weight loss, or fatigue were more likely to be verified as active TB cases than patients who did not have these symptoms (Table 4). There were significantly increased odds of being verified as an active TB case with an increasing number of TB symptoms, wherein people with documentation of only 1–2 symptoms had three times greater odds (OR=3.1, 95% CI 1.9, 5.0; p<0.001) and those with ≥3 symptoms had more than five times greater odds (OR=5.7, 95% CI 3.2, 10.3; p<0.001) of being an active case compared with people who had no TB symptoms. There was no significant association between positive TST results and receiving an ICD-9-CM diagnostic code for active TB disease.
Table 4.
Univariate associations between clinical symptoms and results from tests used to screen for TB disease and classification of AI/AN patients as verified active TB disease based on medical chart review: U.S., 2006–2009 (n=596)

aClinical TB symptoms and testing results dichotomized as those with known documentation of a yes/positive (e.g., documentation of cough present) or as no/not documented (documentation of no cough or no written documentation in chart for cough).
bPercentage of patients with a symptom present or positive test that are TB cases is equal to the positive predictive value.
cStatistically significant at p<0.001
TB = tuberculosis
AI/AN = American Indian/Alaska Native
OR = odds ratio
CI = confidence interval
Ref. = reference group
Results were similar when the analysis was restricted to patients with complete documentation for each TB symptom or test result (e.g., documented as TST negative or TST positive, excluding patients with no documentation on TST) (data not shown).
Reporting of active TB disease to state health departments
Six of eight state health departments invited for participation completed the evaluation. Of the active TB cases verified by chart review, 89 cases were in these six states (Figure 2). Overall, the state health departments confirmed that 81 (91.2%) of these cases had been reported to NTSS as cases of active TB, with a range of 75%–100% of cases reported by IHS across the six states (data not shown).
DISCUSSION
Our study revealed that ICD-9-CM codes were grossly inaccurate for identifying AI/ANs in the IHS population with active TB disease; only 18.6% of patients with presumptive TB based on diagnostic codes in electronic data were verified as TB cases during a review of medical charts. Previous evaluations of other medical conditions have reported similarly low PPVs (0%,6 1%,10 9%,9 and 15%8), but the accuracy of ICD-9-CM codes for TB disease has been higher in other subpopulations (38%,5 54%,9 and 77%7).13 In our study, ICD-9-CM codes were more accurate in identifying patients with active TB disease when patients had TB symptoms; there was an increasing likelihood of the coding to accurately reflect a case of active TB with an increased number of symptoms. Integrating a symptom checklist, or routine review of TB symptoms using medical records, may help to better identify true cases of active TB. Furthermore, patients with abnormal chest x-rays were also more likely to be active cases of TB; using an algorithm that includes chest x-ray results may also help to better refine the identification of active TB cases.16 A similar strategy was applied to electronic influenza surveillance in IHS, where the addition of a single variable (patient temperature) to a set of ICD-9 codes contributed to the system's high sensitivity and PPV.17 In our study, anti-TB treatment was initiated in most cases of verified TB; integrating NPIRS pharmacy information may further improve the capacity to identify actual cases of TB disease.11,12
We found that most (91.2%) verified cases of TB from IHS facilities were reported to the respective state health department and reflected in the database provided to the NTSS for compilation of national statistics; however, failure to report eight (8.8%) cases does raise some concern. Our results fall between previous evaluations in Puerto Rico, Massachusetts, and a seven-state study, which had reported rates of 80.5%,18 100.0%,10 and 95.5%,19 respectively.
In most IHS facilities visited, medical coders were responsible for selecting the diagnostic code for clinical encounters; physicians assigned diagnostic codes in some smaller facilities. Inaccuracies may increase when coding personnel are responsible for all conditions and disease states or when they may be unaware of key distinctions among active disease, latent infection, and process codes (e.g., for TST placement and reading). Furthermore, clinician notation is not always clearly scripted (e.g., “isoniazid for TB disease” was noted in several charts).20 The rollout of EHRs bestows an additional layer of complexity for medical coders,21,22 as there may be new challenges to coder-physician communication to clarify diagnoses.23 Finally, although diagnostic ICD-9-CM codes are intended for reporting morbidity statistics and billing, the emphasis may be on the latter rather than the former in many health-care settings.
Limitations
A few limitations in our evaluation are worth noting. As with any study that relies upon review of medical records, our determinations were dependent upon the completeness and accuracy of information in the medical charts. If any TB-related information was incorrectly filed or was not transcribed in the chart, it would have been missed in our review, potentially distorting our results. A few individuals selected for review had charts that could not be located. Individuals may seek care at more than one IHS health facility, making it possible that information at the current facility was incomplete. Finally, this study took place at the time EHRs were being adopted; as such, despite the diligence of chart reviewers in reviewing both sources to locate test results, some information may have been missed.
In the second stage of our analysis linking validated TB cases from IHS to state facilities, we were unable to compare all validated cases of TB with state records because two states chose not to participate. The state-level verification was complicated by the fact that IHS regions and SUs are independent of state boundaries, making it difficult to know which state may have received the report of diagnosis. Notifiable diseases are to be reported in the state where the patient resides, but it is possible that some addresses of patients in the charts do not reflect their current residence. However, when possible, we attempted to link the validated TB cases with records in multiple states.
CONCLUSIONS
Our analysis demonstrates that ICD-9-CM codes for active TB in national IHS electronic data are inaccurate as a sole measure to describe trends in the burden of TB among AI/AN people, largely due to miscoding errors that used an active TB code for TB screening and documentation of a history of a positive TST. The error seems to be in the coding rather than the diagnosis, however, as most cases of verified TB and patients diagnosed with recent LTBI were started on appropriate therapy. Further, our findings suggest that most providers at IHS facilities are collaborating well with local and state authorities to report cases of active TB disease, and that the NTSS may provide an accurate representation of the TB burden in the AI/AN population. Further work will attempt to determine whether the addition of other electronic data available locally (e.g., symptoms such as cough and prescriptions such as ethambutol) to ICD-9-CM codes may be successful in creating a system able to more rapidly detect local outbreaks of TB and provide useful data to public health officials working with Tribes.
Footnotes
This study was reviewed and approved by the Ethics Committee of the Indian Health Service (IHS). The study was also reviewed by the U.S. Centers for Disease Control and Prevention (CDC) and determined to be non-research, thereby not requiring approval by the Institutional Review Board for research on human subjects. Permissions were granted by the IHS facility and state health department authorities prior to study commencement.
The authors thank all of the personnel at the IHS facilities and offices who were instrumental in coordinating and facilitating this evaluation, and to the state health department representatives who participated in the evaluation and provided invaluable information that enabled our team to link records. The authors also thank Dr. Lara Jacobs for her contribution to the design, implementation, and synthesis of data in the initial stages of the project; and Jennifer Giroux, James Hayslett, Bevin Moon, and Greg Welch for their help with coordination and data collection.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of CDC.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2012. [cited 2013 Mar 5]. Reported tuberculosis in the United States, 2011. Also available from: URL: http://www.cdc.gov/tb/statistics/reports/2011/pdf/report2011.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention (US) National Notifiable Diseases Surveillance System (NNDSS): case definitions. [cited 2013 Mar 5]. Available from: URL: http://wwwn.cdc.gov/nndss/script/casedefDefault.aspx.
- 3.Indian Health Service (IHS) Indian Health Service: quick look. [cited 2014 Jan 31]. Available from: URL: http://www.ihs.gov/newsroom/factsheets/quicklook.
- 4.Indian Health Service (US) Albuquerque (NM): IHS; 2009. Direct/CHS inpatient data: all diseases, fiscal years 1998–2006, National Patient Information Reporting System. [Google Scholar]
- 5.National Center for Health Statistics, Centers for Medicare & Medicaid Services (US) International classification of diseases, ninth revision, clinical modification (ICD-9-CM) [cited 2013 Sep 14]. Available from: URL: http://www.cdc/gov/nchs/icd/icd9cm.htm.
- 6.Indian Health Service (US) IHS EHR GUI deployment status. [cited 2013 Apr 26]. Available from: URL: http://www.ihs.gov/ehr/index.cfm?module=gui_facilities.
- 7.Trepka MJ, Beyer TO, Proctor ME, Davis JP. An evaluation of the completeness of tuberculosis case reporting using hospital billing and laboratory data: Wisconsin, 1995. Ann Epidemiol. 1999;9:419–23. doi: 10.1016/s1047-2797(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Martin C, Saag KG, Patkar NM, Kramer J, Shatin D, et al. Confirmation of administrative claims-identified opportunistic infections and other serious potential adverse events associated with tumor necrosis factor α antagonists and disease-modifying antirheumatic drugs. Arthritis Rheum. 2007;57:343–6. doi: 10.1002/art.22544. [DOI] [PubMed] [Google Scholar]
- 9.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's Affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Calderwood MS, Platt R, Hou X, Malenfant J, Haney G, Kruskal B, et al. Real-time surveillance for tuberculosis using electronic health record data from an ambulatory practice in eastern Massachusetts. Public Health Rep. 2010;125:843–50. doi: 10.1177/003335491012500611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winthrop KL, Baxter R, Liu L, McFarland B, Austin D, Varley C, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf. 2011;20:229–35. doi: 10.1002/pds.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiske CT, Griffin MR, Mitchel E, Sterling TR, Grijalva CG. Accuracy of pharmacy and coded-diagnosis information in identifying tuberculosis in patients with rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 2012;21:666–9. doi: 10.1002/pds.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber C, Lacaille D, Fortin PR. A systematic review of validation studies of the use of administrative data to identify serious infections. Arthritis Care Res (Hoboken) 2013;65:1343–57. doi: 10.1002/acr.21959. [DOI] [PubMed] [Google Scholar]
- 14.Indian Health Service (IHS) Indian Health Service glossary. [cited 2013 Mar 5]. Available from: URL: http://www.ihs.gov/index_old.cfm?module=ihsGlossary.
- 15.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2012. SAS®: Version 9.3 for Windows. [Google Scholar]
- 16.Redd JT, Susser E. Controlling tuberculosis in an urban emergency department: a rapid decision instrument for patient isolation. Am J Public Health. 1997;87:1543–7. doi: 10.2105/ajph.87.9.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keck JW, Redd JT, Cheek JE, Layne LJ, Groom AV, Kitka S, et al. Influenza surveillance using electronic health records in the American Indian and Alaska Native population. J Am Med Inform Assoc. 2014;21:132–8. doi: 10.1136/amiajnl-2012-001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driver CR, Braden CR, Nieves RL, Navarro AM, Rullan JV, Valwy SE, et al. Completeness of tuberculosis case reporting, San Juan and Caguas regions, Puerto Rico, 1992. Public Health Rep. 1996;111:157–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis AB, McCray E, McKenna M, Onorato IM. Completeness and timeliness of tuberculosis case reporting: a multistate study. Am J Prev Med. 2001;20:108–12. doi: 10.1016/s0749-3797(00)00284-1. [DOI] [PubMed] [Google Scholar]
- 20.Language is key in clinical documentation. Hosp Case Manag. 2011;19:84–6. [PubMed] [Google Scholar]
- 21.Batres J. EHRs offer coders opportunities, challenges. J AHIMA. 2012;83:76–7. [PubMed] [Google Scholar]
- 22.Taylor JM. Coding in an EMR: impact on clinicians and coders. Revenue-cycle Strateg. 2010;7:1–3. [PubMed] [Google Scholar]
- 23.Featheringham M. Lowering the language barrier: data quality manager helps coders and physicians understand each other. J AHIMA. 2009;80:86. [PubMed] [Google Scholar]




