Abstract
The photolysis of riboflavin (RF) in the presence of acetate buffer (pH 3.8–5.6) and carbonate buffer (pH 9.2–10.8) has been studied using a multicomponent spectrophotometric method for the simultaneous assay of RF and its photoproducts. Acetate and carbonate buffers have been found to catalyze the photolysis reaction of RF. The apparent first-order rate constants for the acetate-catalyzed reaction range from 0.20 to 2.86 × 10−4 s−1 and for the carbonate-catalyzed reaction from 3.33 to 15.89 × 10−4 s−1. The second-order rate constants for the interaction of RF with the acetate and the carbonate ions range from 2.04 to 4.33 × 10−4 M−1 s−1 and from 3.71 to 11.80 × 10−4 M−1 s−1, respectively. The k-pH profile for the acetate-catalyzed reaction is bell shaped and for the carbonate-catalyzed reaction a steep curve. Both HCO−3 and CO2 −3 ions are involved in the catalysis of the photolysis reaction in alkaline solution. The rate constants for the HCO−3 and CO2 −3 ions catalyzed reactions are 0.72 and 1.38 × 10−3 M−1 s−1, respectively, indicating a major role of CO2 −3 ions in the catalysis reaction. The loss of RF fluorescence in acetate buffer suggests an interaction between RF and acetate ions to promote the photolysis reaction. The optimum stability of RF solutions is observed in the pH range 5–6, which is suitable for pharmaceutical preparations.
KEY WORDS: acetate effect, carbonate effect, photolysis, riboflavin, spectrophotometric assay
INTRODUCTION
Buffer salts are commonly used in liquid pharmaceutical preparations to maintain the pH of the solutions. These salts may catalyze the degradation of drugs present in the medium. The theoretical concept of buffer catalysis and its effect on the stability of various drugs have been discussed in detail (1–7). Riboflavin (RF) is sensitive to light (8–10), and its photolysis in aqueous solution is influenced by general acid–base catalysis. The acetate buffer has been shown to exhibit a higher catalytic activity than the formate buffer (11). The rate of photolysis of RF is proportional to the concentration of the phosphate buffer (12). Extensive studies of the effect of phosphate (12–18), borate (19), and citrate species (20) on the kinetics of RF photolysis have been conducted. Phosphate ions promote the degradation of RF, whereas borate and citrate ions inhibit the reaction. Divalent phosphate ions also alter the normal photolysis pathway of RF in favor of the photoaddition pathway (13–18). So far, no work has been carried out to study the catalytic effect of acetate and carbonate buffers on the photodegradation of RF.
Acetate buffer catalyzes the degradation of several drugs including benzyl penicillin (21), carbenicillin (22), cefotaxime (23), epinephrine (24), methotrexate (25), mitomycin C (26), prostaglandin E2 (27), and thiamine hydrochloride (28). Carbonate buffer is also known to catalyze the degradation of methotrexate (25), cefotaxime (23), cisplatin (29), cyclophosphamide (30), and doxorubicin (31). In view of the importance of the buffers in pharmaceutical systems and their catalytic effects on the degradation of active ingredients, the present study has been undertaken to evaluate the effects of acetate and carbonate ions in the pH range of their buffer activity on the photolysis of RF in aqueous solution. It has been conducted using a specific spectrophotometric method for the simultaneous determination of RF and its photoproducts, formylmethylflavin, lumichrome, and lumiflavin. The rate constants for the acetate and carbonate catalyzed reactions have been determined and correlated with the pH and buffer concentration. The individual effects of bicarbonate and carbonate species have also been determined. The study would facilitate the formulator of liquid vitamin preparations to make a judicious decision in the choice of buffer for these preparations. The pH range of acetate buffer is most suitable for a majority of vitamin preparations, and this buffer at 0.1–0.2 M concentration exhibits minimum catalytic activity on the degradation of RF.
MATERIALS AND METHODS
RF, lumichrome (LC), and lumiflavin (LF) were obtained from Sigma Chemical Co. ST. Louis, MD, USA. Formylmethylflavin (FMF) and carboxymethylflavin (CMF) were prepared by the methods of Fall and Petering (32) and Fukumachi and Sakurai (33), respectively. These compounds were checked for purity using thin-layer chromatography. All reagents and solvents were of the purest form available from Merck & Co., Whitehouse Station, NJ. The following buffer systems were used: acetic acid-sodium acetate, pH 3.8–5.6 and sodium carbonate-sodium bicarbonate, pH 9.2–10.8. The ionic strength of the solutions was kept constant (0.6 M).
pH Measurements
The pH measurements of solutions were performed on an Elmetron LCD display pH meter (Model-CP501, sensitivity ±0.01 pH units, Poland) using a combination pH electrode. The electrode was automatically calibrated using phthalate (pH 4.008), phosphate (pH 6.865), and disodium tetraborate (pH 9.180) buffer solutions.
Photolysis
A 5 × 10−5 M solution of RF (100 ml) was prepared at the appropriate pH in a volumetric flask (Pyrex) using acetate (0.1–0.6 M) or carbonate (0.1–0.6 M) buffer and immersed in a water bath maintained at 25 ± 1°C. The solution was exposed in a dark chamber to Philips HPLN 125 W high pressure mercury vapor fluorescent lamp (emission bands at 405 and 435 nm; the 435 nm bands overlap the 445 nm band of RF) (14), placed at a distance of 30 cm from the center of the flask. The exposure time varied from 1 to 3 h, depending upon the pH of the solution. Samples of the photolysed solutions were withdrawn at various intervals to carry out thin-layer chromatography and spectrophotometric assay.
Thin-Layer Chromatography
The photolysed solutions of RF were subjected to thin-layer chromatography (TLC) using 250-μm cellulose plates (Whatman CC 40) and the solvent systems: (a) 1-butanol–acetic acid–water (40:10:50, v/v, organic phase), and (b) 1–butanol–1–propanol–acetic acid–water (50:30:2:18, v/v) (34). The compounds were detected by their characteristic fluorescence on exposure to UV (365 nm) light: RF, LF, FMF, CMF (yellow green), and LC (sky blue).
Spectral Measurements
The spectral and absorbance measurements on pure and photolysed solutions of RF were performed on a Shimadzu UV–1601 recording spectrophotometer using quartz cell of 10-mm path length.
Determination of Light Intensity
The intensity of the Philips HPLN 125 W lamp was determined using potassium ferrioxalate actinometry (35) as 1.21 ± 0.10 × 1017 quanta s−1.
Spectrophotometric Assay
RF and its major photoproducts, FMF and LC in the presence of acetate buffer (pH 3.8–5.6) and FMF, LC and LF in the presence of carbonate buffer (pH 9.2–10.8), in photolysed solutions were assayed using a multicomponent spectrophotometric method developed by Ahmad et al. (36). It is based on the adjustment of the pH of photolysed solutions to 2.0 (0.2 HCl–KCl buffer), followed by extraction with 3 × 10 ml chloroform to remove LC and LF. The chloroform is evaporated and the residue is dissolved in 0.2 M acetate buffer (pH 4.5). The concentration of LC and LF is determined by a two-component assay at 356 and 445 nm. FMF and RF in the aqueous phase are determined by a two-component assay at 385 and 445 nm. FMF (pKa 3.5) (37) is protonated at pH 2.0, giving a distinct absorption maximum (385 nm) from that of RF (445 nm) and thus making the two-component assay feasible. CMF is a minor photoproduct obtained when FMF is slightly oxidized in the presence of air (38,39). The amount of CMF formed in acidic and alkaline solutions has been estimated to be ∼0.2–0.4% of the degraded RF using TLC. This amount would not interfere with the assay method employed for RF and the photoproducts in this study. The assay method has been validated with respect to accuracy, precision, and linearity under the present experimental conditions.
Fluorescence Measurements
The fluorescence measurements on RF solutions were carried out at room temperature (∼25°C) with a Spectromax 5 fluorimeter (Molecular Devices, Sunnyvale, CA, USA) in the end point mode, using 374 nm as the excitation wavelength and 525 nm as the fluorescence emission wavelength (40). The fluorescence was measured in relative fluorescence units using a pure 0.05 mM RF solution as standard.
RESULTS AND DISCUSSION
Photoproducts of RF
In order to accurately determine RF and its photoproducts formed in degraded solutions in the presence of acetate or carbonate buffer, it was necessary to carry out TLC studies of the photolysed solutions during the reactions. At around 50% photolysis of RF in the presence of acetate buffer (pH 3.8–5.6) and carbonate buffer (pH 9.2–10.8), the compounds identified by their characteristic fluorescence on comparison with the standards included undegraded RF, FMF, LC, LF (all major), and CMF (minor) with Rf values of 0.47, 0.70, 0.66, 0.53 and 0.38, and 0.28 in solvent system (a) and 0.27, 0.70, 0.63, 0.41, and 0.20 in solvent system (b), respectively. The intensity of the spots of FMF, a major intermediate product in the degradation sequence of RF, decreased with the progress of the reactions indicating its conversion to LC in the acid medium and to LC and LF in the alkaline medium (10,36). An increase in the buffer concentration leads to an increase in the intensity of the spots of LC and LF, suggesting a greater yield as a result of the buffer effect. The formation of LC in the thermal (41) and bacterial degradation of RF (42) has also been reported.
Assay of RF and Photoproducts
In view of the TLC results indicating the presence of undegraded RF and its photoproducts, FMF and LC in acid media and FMF, LC, and LF in alkaline media as major component of the degraded solutions, the assay of RF, FMF, LC, and LF has been carried out according to the multicomponent spectophotometric method of Ahmad and Rapson (36), described under the methods section. The assay method was validated under the present experimental conditions, and the various validation data for RF, FMF, LC, and LF are given in Table I. The method is specific since the absorbance measurements on the mixtures of degraded solutions are carried out at the wavelengths corresponding to the absorption maxima of the four compounds reported previously (36). The amount of CMF formed in the photolysis reactions is too small (∼0.2–0.4%) for any accurate determination, and, therefore, its presence has not been considered in the assay method. Moreover, the accuracy of the method is within ±5%, which takes into consideration any minor impurities present in the solution. This assay method has extensively been employed to study the kinetics of RF photolysis under various conditions (10,14–20,36,43) and is appropriate for this present work.
Table I.
Validation Data for Multicomponent Spectrophotometric Assay of RF, FMF, LC, and LF
| Compound | RF | FMF | LC | LF |
|---|---|---|---|---|
| λmax nm (pH 2.0) | 445 | 385 | 356 | 445 |
| Molar absorptivity (ϵ) M−1 cm−1 | 12,530 | 16,380 | 10,800 | 10,400 |
| Linearity | ||||
| Concentration range (M × 105) | 0.5–5.0 | 0.5–5.0 | 0.5–5.0 | 0.5–5.0 |
| Slope × 10−4 | 1.253 ± 0.003 | 1.638 ± 0.005 | 1.080 ± 0.004 | 1.040 ± 0.004 |
| Intercept | 0.0032 | 0.0040 | 0.0042 | 0.0036 |
| SE of slope | 0.968 | 1.202 | 1.415 | 1.025 |
| Recovery range (%) | 98.6–101.1 | 98.4–101.0 | 98.2–100.6 | 98.0–101.8 |
| Accuracy (%) ± SD | 100.2 ±1.21 | 100.8 ±1.42 | 99.8 ± 1.58 | 99.7 ± 1.50 |
| RSD (%) | 1.21 | 1.41 | 1.51 | 1.59 |
| LOD (M × 106) | 1.197 | 1.025 | 1.257 | 1.264 |
| LOQ (M × 106) | 4.189 | 3.587 | 4.399 | 4.425 |
Values are mean of five determinations
RSD relative standard deviation, LOD limit of detection, LOQ limit of quantification
Kinetics of Photolysis
Effect of Acetate Buffer
It has been reported that the photolysis of RF in aqueous solution follows an apparent first-order kinetics (10). This reaction occurs through the formation of FMF as an intermediate to give LC as the final product (10,36,39,44,45). The photolysis of RF in acid solution can be represented by a reaction scheme involving a consecutive first-order reaction as follows:
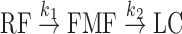 |
1 |
where k1 and k2 are the rate constants for the formation of FMF and LC, respectively.
The differential equations for the reactant and the products are
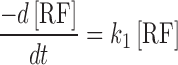 |
2 |
 |
3 |
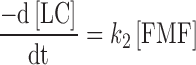 |
4 |
The differential equations can be solved (3,7,19) to obtain the molar concentrations of RF, FMF, and LC at time, t
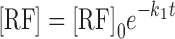 |
5 |
 |
6 |
and
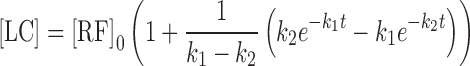 |
7 |
The rate constants k1 and k2 have been determined by solving the above equations (3,7,46). The value of the apparent first-order rate constant (kobs) (correlation coefficients, 0.996–0.999) for the photolysis of RF at pH 3.8–5.6 and the rate constants for the formation of FMF (k1) and LC (k2) in the presence of acetate buffer (0.1–0.6 M) are reported in Table I. The values of the rate constants indicate that the acetate buffer is exerting a catalytic effect on the photolysis of RF in the pH range studied. The effect of acetate species on the k1 can be expressed as
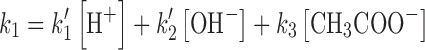 |
8 |
where k′1, k′2, and k3 are the rate constants for the H+ and OH− and CH3COO− catalyzed reactions and k0 is the rate constant for the photolysis of RF in the absence of the buffer. Under constant pH conditions, the terms k′1 [H+] and k′2 [OH−] are constant and Eq. (8) may be expressed as
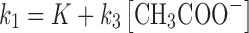 |
9 |
where
 |
In Eq. (9), k3 represents the second-order rate constant for the acetate catalyzed photolysis of RF in the acid solution. In order to determine the effect of acetate ions on the photolysis of RF, plots of k1versus acetate concentrations at various pH values have been constructed. The values of the second-order rate constants, k′ (correlation coefficients, 0.997–0.999), determined from the slopes of the linear curves, along with the values of k0 determined by extrapolation of the second-order plots to the vertical axis, are reported in Table II. The values of k0 at pH 3.8–5.6 are about 10–20 times slower that those of the highest buffer concentration (0.6 M), showing considerable effect of the buffer species on the rate of RF photolysis.
Table II.
Apparent First-Order Rate Constants (k obs) for the Photolysis of Riboflavin (k obs) in the Presence of Acetate Buffer (pH 3.8–5.6), for the Formation of Formylmethyflavin (k 1) and Lumichrome (k 2) and Second-Order Rate Constants for the Interaction of Riboflavin and Acetate Buffer (k′)
| pH | Concentration (M) | k obs × 104 s−1 ± SD | k 0 × 104 s−1a ± SD | k 1 × 10−4 s−1 ± SD | k 2 × 104 s−1 ± SD | k′ × 104 (M−1 s−1) ± SD |
|---|---|---|---|---|---|---|
| 3.8 | 0.1 | 0.60 ± 0.03 | 0.24 ± 0.01 | 0.44 ± 0.02 | 0.16 ± 0.01 | 4.10 ± 0.20 |
| 0.2 | 1.01 ± 0.05 | 0.71 ± 0.03 | 0.17 ± 0.02 | |||
| 0.3 | 1.42 ± 0.08 | 0.95 ± 0.05 | 0.23 ± 0.02 | |||
| 0.4 | 1.85 ± 0.07 | 1.22 ± 0.06 | 0.30 ± 0.04 | |||
| 0.6 | 2.40 ± 0.16 | 1.60 ± 0.08 | 0.40 ± 0.02 | |||
| 4.1 | 0.1 | 0.65 ± 0.03 | 0.25 ± 0.02 | 0.50 ± 0.03 | 0.22 ± 0.01 | 4.33 ± 0.22 |
| 0.2 | 1.12 ± 0.06 | 0.76 ± 0.04 | 0.37 ± 0.02 | |||
| 0.3 | 1.51 ± 0.08 | 1.05 ± 0.06 | 0.50 ± 0.03 | |||
| 0.4 | 1.93 ± 0.08 | 1.33 ± 0.07 | 0.63 ± 0.04 | |||
| 0.6 | 2.85 ± 0.10 | 1.98 ± 0.20 | 0.88 ± 0.06 | |||
| 4.3 | 0.1 | 0.66 ± 0.03 | 0.24 ± 0.01 | 0.51 ± 0.02 | 0.15 ± 0.01 | 4.31 ± 0.26 |
| 0.2 | 1.14 ± 0.06 | 0.80 ± 0.04 | 0.35 ± 0.02 | |||
| 0.3 | 1.70 ± 0.09 | 1.10 ± 0.05 | 0.47 ± 0.03 | |||
| 0.4 | 2.23 ± 0.11 | 1.40 ± 0.06 | 0.63 ± 0.03 | |||
| 0.6 | 2.88 ± 0.15 | 2.24 ± 0.20 | 0.98 ± 0.05 | |||
| 4.6 | 0.1 | 0.43 ± 0.02 | 0.22 ± 0.02 | 0.48 ± 0.02 | 0.15 ± 0.01 | 3.27 ± 0.18 |
| 0.2 | 0.63 ± 0.03 | 0.76 ± 0.04 | 0.26 ± 0.02 | |||
| 0.3 | 1.02 ± 0.05 | 1.00 ± 0.05 | 0.37 ± 0.02 | |||
| 0.4 | 1.10 ± 0.06 | 1.20 ± 0.07 | 0.48 ± 0.03 | |||
| 0.6 | 2.20 ± 0.11 | 1.87 ± 0.12 | 0.71 ± 0.03 | |||
| 4.9 | 0.1 | 0.39 ± 0.02 | 0.12 ± 0.01 | 0.37 ± 0.02 | 0.04 ± 0.03 | 3.10 ± 0.15 |
| 0.2 | 0.44 ± 0.03 | 0.56 ± 0.03 | 0.14 ± 0.05 | |||
| 0.3 | 0.97 ± 0.05 | 0.73 ± 0.04 | 0.32 ± 0.02 | |||
| 0.4 | 0.83 ± 0.05 | 0.95 ± 0.05 | 0.35 ± 0.02 | |||
| 0.6 | 1.62 ± 0.10 | 1.26 ± 0.08 | 0.59 ± 0.03 | |||
| 5.1 | 0.1 | 0.24 ± 0.02 | 0.10 ± 0.01 | 0.27 ± 0.01 | 0.17 ± 0.01 | 2.58 ± 0.13 |
| 0.2 | 0.44 ± 0.03 | 0.41 ± 0.02 | 0.22 ± 0.02 | |||
| 0.3 | 0.83 ± 0.05 | 0.54 ± 0.03 | 0.37 ± 0.02 | |||
| 0.4 | 0.83 ± 0.05 | 0.66 ± 0.03 | 0.41 ± 0.03 | |||
| 0.6 | 1.20 ± 0.07 | 0.89 ± 0.04 | 0.48 ± 0.03 | |||
| 5.3 | 0.1 | 0.20 ± 0.02 | 0.09 ± 0.04 | 0.14 ± 0.06 | 0.11 ± 0.05 | 2.04 ± 0.10 |
| 0.2 | 0.38 ± 0.02 | 0.16 ± 0.07 | 0.14 ± 0.06 | |||
| 0.3 | 0.67 ± 0.03 | 0.39 ± 0.02 | 0.27 ± 0.02 | |||
| 0.4 | 0.78 ± 0.04 | 0.49 ± 0.04 | 0.45 ± 0.03 | |||
| 0.6 | 0.95 ± 0.06 | 0.67 ± 0.06 | 0.79 ± 0.04 | |||
| 5.6 | 0.1 | 0.18 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.03 | 0.03 ± 0.02 | 1.75 ± 0.08 |
| 0.2 | 0.34 ± 0.02 | 0.16 ± 0.07 | 0.14 ± 0.01 | |||
| 0.3 | 0.62 ± 0.03 | 0.34 ± 0.02 | 0.23 ± 0.02 | |||
| 0.4 | 0.76 ± 0.04 | 0.42 ± 0.03 | 0.33 ± 0.02 | |||
| 0.6 | 0.60 ± 0.04 | 0.64 ± 0.04 | 0.53 ± 0.03 |
a k 0 is the first-order rate constant for the photolysis of riboflavin in the absence of acetate buffer
Effect of Carbonate Buffer
The photolysis of RF in the presence of carbonate buffer (9.2–10.8) is much faster than that of the acetate buffer. The photolysis of RF in alkaline solution (10,19,34,36,44,45) leads to the formation of LC and LF through FMF as an intermediate product and may be represented by the following reaction scheme:
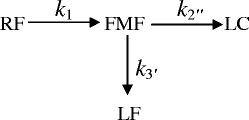 |
10 |
where k1, k〞2 and k′3 are the rate constants for the formation of FMF, LC, and LF, respectively.
This would require an additional differential equation in the reaction scheme involving Eqs. (2) to (4)
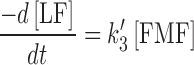 |
11 |
In order to determine the value of k〞2 and k′3 (k2 in Eq. (1)) for the formation of LC and LF, respectively, the ratios of the concentrations of the two products have been considered, which depend upon the ratios of the corresponding rate constants.
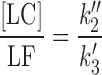 |
12 |
The rate constant k〞2 and k′3 have been determined using the Eq. (12) as in the case of the photolysis reactions of RF in the presence of phosphate buffer (18). The same method for the determination of these rate constants has been described by some authors (6,7). The values of the apparent first-order rate constants (kobs) (correlation coefficients 0.996–0.999) for the photolysis of RF at pH 9.2–10.8 in the presence of carbonate buffer and the rate constants for the formation of FMF (k1), LC (k〞2), and k′3 (LF) are reported in Table II. Similar to the effect of acetate ions, the carbonate ions also exert a catalytic effect on the photolysis of RF at pH 9.2–10.8. The effect of carbonate ions and the bicarbonate ions (depending upon the pH of the solution) on the values of k1 can be written as
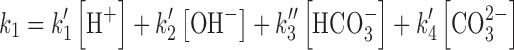 |
13 |
where k〞3 and k′4 are the rate constants for the HCO−3 and CO2 −3 catalyzed reactions, respectively, k0 is the rate constant in the absence of the buffer species. At a constant pH, the k1 could be expressed as
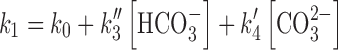 |
14 |
where
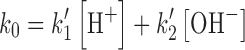 |
In Eq. (14), the values of k3′′ and k′4 indicate the second-order rate constants for the catalytic effect of HCO−3 and CO2 −3 ions on the photolysis of RF in alkaline solution. The second-order rate constants (correlation coefficients 0.997–0.999), derived from the slopes of the straight lines of the plots of k1versus carbonate concentrations at various pH values, along with the values of k0 are reported in Table III. The values of k0 at pH 9.2–10.8 are about 1.3–1.4 times slower than those of the 0.6 M buffer concentration, indicating a little effect of the buffer on the photolysis of RF. This catalytic effect is much smaller than that observed in the presence of acetate buffer.
Table III.
Apparent First-Order Rate Constants (k obs) for the Photolysis of Riboflavin (k obs) in the Presence of Carbonate Buffer (pH 9.2–10.8), for the Formation of Formylmethylflavin (k 1), Lumichrome (k ′2), and Lumiflavin (k ′3) and Second-Order Rate Constants for the Interaction of Riboflavin and Carbonate Buffer (k′)
| pH | Concentration (M) | k obs × 104 s−1 ± SD | k 0 × 104 s−1a ± SD | k 1 × 104 s−1 ± SD | k ′ ′2 × 104 s−1 ± SD | k ′3 × 104 s−1 ± SD | k′ × 104 (M−1 s−1) ± SD |
|---|---|---|---|---|---|---|---|
| 9.2 | 0.1 | 2.90 ± 0.14 | 2.60 ± 0.13 | 1.70 ± 0.08 | 0.73 ± 0.04 | 0.42 ± 0.02 | 3.71 ± 0.18 |
| 0.2 | 3.33 ± 0.16 | 2.22 ± 0.11 | 0.91 ± 0.05 | 0.51 ± 0.02 | |||
| 0.3 | 3.71 ± 0.18 | 2.90 ± 0.14 | 1.01 ± 0.05 | 0.66 ± 0.03 | |||
| 0.4 | 4.02 ± 0.20 | 3.42 ± 0.18 | 1.22 ± 0.06 | 0.92 ± 0.05 | |||
| 0.6 | 4.71 ± 0.24 | 4.64 ± 0.25 | 1.51 ± 0.07 | 1.31 ± 0.07 | |||
| 9.4 | 0.1 | 4.44 ± 0.22 | 3.70 ± 0.18 | 2.90 ± 0.14 | 1.30 ± 0.08 | 0.22 ± 0.01 | 7.40 ± 0.39 |
| 0.2 | 5.10 ± 0.24 | 3.42 ± 0.17 | 1.42 ± 0.07 | 0.43 ± 0.02 | |||
| 0.3 | 5.92 ± 0.27 | 3.94 ± 0.11 | 1.51 ± 0.06 | 0.51 ± 0.03 | |||
| 0.4 | 6.77 ± 0.26 | 4.44 ± 0.26 | 1.63 ± 0.09 | 0.62 ± 0.03 | |||
| 0.6 | 8.11 ± 0.46 | 6.32 ± 0.28 | 1.71 ± 0.09 | 0.84 ± 0.05 | |||
| 9.8 | 0.1 | 7.60 ± 0.43 | 6.80 ± 0.36 | 4.62 ± 0.22 | 1.60 ± 0.08 | 0.39 ± 0.02 | 8.51 ± 0.41 |
| 0.2 | 8.33 ± 0.53 | 5.11 ± 0.27 | 1.94 ± 0.09 | 0.53 ± 0.02 | |||
| 0.3 | 9.01 ± 0.56 | 5.73 ± 0.30 | 2.32 ± 0.12 | 0.82 ± 0.05 | |||
| 0.4 | 9.72 ± 0.52 | 6.31 ± 0.31 | 2.61 ± 0.13 | 1.03 ± 0.05 | |||
| 0.6 | 11.12 ± 0.66 | 9.44 ± 0.37 | 3.22 ± 0.17 | 1.44 ± 0.08 | |||
| 10.2 | 0.1 | 8.92 ± 0.42 | 8.20 ± 0.40 | 7.83 ± 0.42 | 0.33 ± 0.02 | 0.21 ± 0.01 | 9.65 ± 0.46 |
| 0.2 | 9.90 ± 0.55 | 8.88 ± 0.41 | 0.64 ± 0.03 | 0.43 ± 0.02 | |||
| 0.3 | 10.75 ± 0.56 | 9.92 ± 0.52 | 1.02 ± 0.05 | 0.66 ± 0.04 | |||
| 0.4 | 11.58 ± 0.67 | 10.91 ± 0.45 | 1.33 ± 0.06 | 0.85 ± 0.04 | |||
| 0.6 | 13.21 ± 0.66 | 12.93 ± 0.62 | 2.14 ± 0.10 | 1.32 ± 0.06 | |||
| 10.6 | 0.1 | 9.84 ± 0.06 | 8.70 ± 0.46 | 8.02 ± 0.33 | 1.33 ± 0.06 | 0.36 ± 0.02 | 11.21 ± 0.64 |
| 0.2 | 10.80 ± 0.54 | 9.32 ± 0.34 | 1.84 ± 0.09 | 0.53 ± 0.03 | |||
| 0.3 | 12.02 ± 0.65 | 10.74 ± 0.46 | 2.50 ± 0.13 | 0.88 ± 0.04 | |||
| 0.4 | 13.01 ± 0.63 | 12.12 ± 0.58 | 3.01 ± 0.15 | 1.05 ± 0.06 | |||
| 0.6 | 15.24 ± 0.86 | 14.44 ± 0.79 | 4.32 ± 0.24 | 1.52 ± 0.08 | |||
| 10.8 | 0.1 | 10.02 ± 0.59 | 8.80 ± 0.42 | 8.32 ± 0.31 | 0.66 ± 0.03 | 0.52 ± 0.02 | 11.80 ± 0.51 |
| 0.2 | 11.14 ± 0.51 | 9.71 ± 0.45 | 1.22 ± 0.07 | 0.64 ± 0.03 | |||
| 0.3 | 12.32 ± 0.63 | 10.89 ± 0.41 | 2.01 ± 0.11 | 0.86 ± 0.04 | |||
| 0.4 | 13.54 ± 0.66 | 12.31 ± 0.62 | 2.63 ± 0.15 | 1.02 ± 0.06 | |||
| 0.6 | 15.89 ± 0.87 | 15.13 ± 0.81 | 3.91 ± 0.19 | 1.38 ± 0.07 |
a k 0 is the first-order rate constant for the photolysis of riboflavin in the absence of carbonate buffer
The values of the rate constants reported for these reactions have been determined under constant irradiation conditions (i.e., light intensity and wavelengths) and temperature to avoid any variations in the data. The values of the rate constants are relative and could be used for comparative evaluation.
Effect of pH
The effect of pH on the rates of photodegradation of RF has been shown by plotting k1 values (for the formation of FMF) in the absence of acetate and carbonate buffers as a function of pH and discussed accordingly.
Acid Range (pH 3.8–5.6)
The k-pH profile (Fig. 1) for the reactions carried out in the pH range 3.8–5.6 is somewhat similar to those observed for the photolysis of RF (10,44) and FMF (39) in this range. The rate of photolysis is gradually decreasing and is about 2–3 times lower at pH 5.6 than that of pH 3.8. RF (pKa1, 1.7) (47) exists 99% in the neutral form at pH 3.8–5.6 and, therefore, the decrease in the rate is not due to any change in the ionized state of the molecule. The photolysis of RF in aqueous solution basically involves intramolecular photoreduction of the isoalloxazine ring followed by cleavage of the ribityl side chain to produce FMF and subsequent photoproducts (LC, LF) (17,18,44,48,49). The decrease in the rate of RF photolysis could be ascribed to the redox behavior of RF in the pH range 3.8–5.6. The dependency of the redox potentials of RF on pH (7,50,51) would determine the rate of the reaction in a particular pH range. The redox potentials of RF are lowest in the pH range 5–6 (E° pH 5.0 = –0.117 V) (7,52), and hence its rate of photolysis is expected to be the lowest in this range. The k-pH profile (Fig. 1) for the photolysis of RF is in agreement with the decrease in the redox potentials of RF, and it exhibits maximum stability to photolysis in this range. The increase in the rate at pH 7.0 (E° = –0.207 V) (51,52) and above is in accordance with the increase in the redox potentials of RF (10).
Fig. 1.
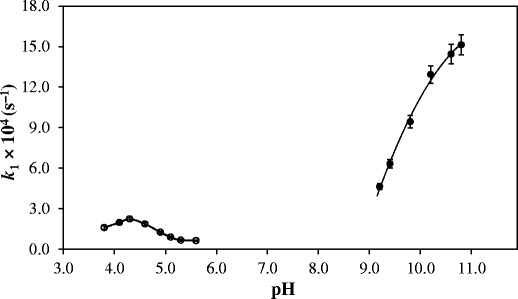
k-pH profiles for the photolysis of riboflavin at pH 3.8–5.6 and pH 9.2–10.8
Alkaline Range (pH 9.2–10.8)
The k-pH profile (Fig. 1) for the photolysis of RF in the pH range 9.2–10.8 shows an initial sharp increase in the rate followed by a reduced enhancement above pH 10.0. This may result from the ionization of RF (pKa2 N-3, 10.2) (47) and the low susceptibility of the RF anion to photolysis as observed in a previous study (10). The more than threefold increase in the rate constant from pH 9.2 to 10.8 is probably due to the higher reactivity of the flavin excited triplet state as suggested by Carins and Metzler (44). According to these authors, the high pKa (6.5) and the increased reactivity of the excited triplet state at higher pH is due to its existence in a bent, diradical form. Carbonic acid (pKa1 6.4, pKa2 10.4) (53) gives rise to two species i.e., HCO−3 and CO2 −3 ions in the alkaline solution. The catalytic effects of these ions on the kobs for the photolysis of RF in the pH range 9.2–10.8 may be described by Eq. (14). In the presence of carbonate buffer, kobs may be expressed as
 |
15 |
where k′ is the overall rate constant for the photolysis of RF in the presence of carbonate ions and CB is the total concentration of carbonate buffer. A plot of k1versus CB gives an intercept k0 and a gradient k′. The two rate constants, k′3 and k′4 (Eq. (13), may be obtained by rearranging the Eq. (13) into a linear form (16).
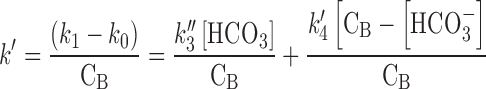 |
16 |
A plot of k′ versus the fraction of HCO−3, i.e., [HCO−3]/CB would give the values of k〞3 and k′4. The intercept at [HCO−3]/CB = 0 is equal to the rate constant k′4 (Fig. 2). The k value at [HCO−3] CB = 1 gives the rate constant k〞3. In the present study, the values k〞3 and k′4 for the HCO−3 and CO2 −3 anion-catalyzed photolysis of RF are 0.72 and 1.38 × 10−3 M−1 s−1, respectively. The value of k′4 being greater than k′ ′3 indicates that the CO2 −3 ions have a greater catalytic effect on the photolysis of RF in the pH range studied.
Fig. 2.
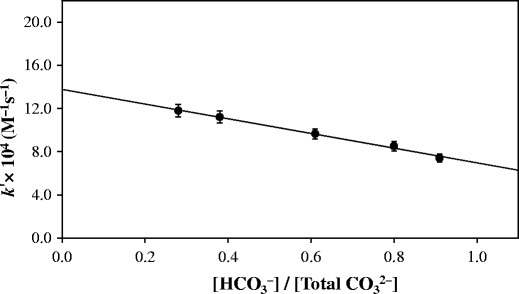
Plot of k′ for the photolysis of riboflavin versus [HCO−3]/[Total CO2 −3]
Effect of Acetic Acid
The effect of acetic acid (pKa 4.76) concentration on the rate of photolysis of RF has also been examined by constructing a plot of kobsversus the fraction of [H+]/[CH3COOH] in the presence of acetate buffer (pH 3.8–5.6) (Fig. 3), similar to that reported above in the presence of carbonate buffer (Fig. 2). It has been observed that the k value for the general acid catalyzed reaction ([H+]/[CH3COOH] = 1) is around zero and the acetate anion-catalyzed rate constant ([H+]/[CH3COOH] = 0), 5.61 × 10−4 M−1 s−1. This indicates the absence of any general acid catalysis by acetic acid on the photolysis of RF and the enhancement in the rate of the reaction is due to the catalytic effect of acetate ions only.
Fig. 3.
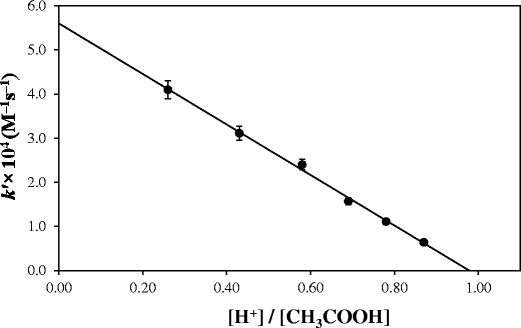
Plot of k 1 for the photolysis of riboflavin versus [H+]/[CH3COOH]
Fluorescence Studies
Aqueous RF solutions exhibit intense yellow green fluorescence which is destroyed by mineral acids or alkalis (54–56). In the present study, fluorescence measurements on RF have been carried out in acetate buffer (pH 4.3) and in carbonate buffer (pH 9.8). Acetate ions (0.6 M) have been found to decrease the fluorescence intensity of RF to the extent of about 4% (Table IV). Since the acetate ions enhance the photodegradation of RF in the pH range 3.8–5.6 (Table II), the loss of fluorescence could not be attributed to the quenching of the excited singlet state (1RF), which would inhibit the reaction. There is a possibility of the formation of a complex between 1RF state and the acetate ions, as suggested in the case of 1RF state and the monovalent or divalent phosphate ions to cause the photodegradation of RF (13–18). This is substantiated by a decrease in fluorescence emission of RF solutions in the presence of these species. The participation of the 1RF state in the intramolecular photodegradation of RF leading to the direct formation of LC has been reported (44,57–60). Thus LC may also originate from the 1RF state, in addition to that formed from the excited triplet state [3RF] through FMF (62,63).
Table IV.
Fluorescence Intensity of 5 × 10−5 M Riboflavin Solution in the Presence of Acetate and Carbonate Buffers
| pH | Acetate concentration (M) | Carbonate concentration (M) | Relative fluorescence intensity at 525 nm |
|---|---|---|---|
| 4.3 | 0.00 | 0.00 | 100.0 |
| 0.20 | 0.00 | 98.3 | |
| 0.40 | 0.00 | 96.8 | |
| 0.60 | 0.00 | 95.1 | |
| 9.8 | 0.00 | 0.00 | 100.0 |
| 0.00 | 0.20 | 99.7 | |
| 0.00 | 0.40 | 99.4 | |
| 0.00 | 0.60 | 99.1 |
Measurements were made at ambient temperature (∼25°C) under aerobic conditions
There is no significant decrease in the fluorescence of RF solutions in the presence of carbonate ions (Table II). This indicates that these ions do not quench the 1RF state and probably interact with the 3RF state to facilitate the photodegradation of RF in alkaline range.
Mode of Photodegradation
The photodegradation of RF in the presence of acetate/carbonate buffers may be represented by the following overall reaction scheme.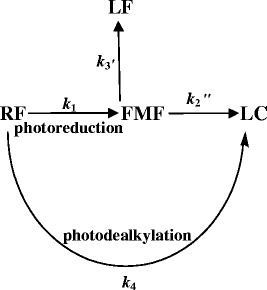
According to the proposed reaction scheme, RF on the absorption of light undergoes photoreduction (k1) through the [3RF] state to form FMF (17,40,58). It may also be converted directly to LC (k4) by photodealkylation through the [1RF] state (44,57,61). FMF undergoes hydrolysis to LC (k2) and LF (k3) in aqueous media (34,38,39). The equilibria of RF, 1RF, and 3RF are not the rate determined steps in the reaction. Although the photoreduction is the main reaction involved in the photolysis of RF, the overall loss of RF (kobs) could be considered as a combination of the rate constants, k1 + k4.
CONCLUSION
The photolysis of riboflavin in aqueous solution follows first-order kinetics and is catalyzed by the acetate and carbonate buffers. The rate constants for the reactions in the alkaline range (9.2–10.8) are ∼5–15 times greater than those of the acid range (3.8–5.6). The flavin excited triplet state shows higher reactivity in the alkaline solution, resulting in an increase in the rate of the reaction, with pH. The lower rates in the pH range 5–6, compared to that of pH 4.3 (pHmax), are due to the lowest redox potentials of riboflavin in that range. The k-pH profiles indicate that the highest rate of RF photolysis is at ∼ pH 4.3 and 10.8. Both HCO−3 and CO2 −3 ions participate in the catalytic activity of the photolysis of riboflavin in alkaline solution. The loss of fluorescence of riboflavin in the acid range probably is due to quenching of the excited singlet state, indicating an interaction between riboflavin and the buffer species. The participation of both the excited singlet and the excited triplet stated in the photolysis of riboflavin has been reported. The study suggests that a careful approach should be adopted in the selection of a buffer to minimize its catalytic effect on the degradation of the drug molecule in a particular pH range.
References
- 1.Lachman L, DeLuca P, Askers MJ. Kinetic principles and stability testing. In: Lachman L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 2. Philadelphia: Lea & Febiger; 1986. pp. 760–803. [Google Scholar]
- 2.Connors KA, Amidon GL, Stella VJ. Chemical stability of pharmaceuticals a handbook for the pharmacist. 2. New York: Wiley; 1986. [Google Scholar]
- 3.Laidler KJ. Chemical kinetics. 3. New York: Harper & Row; 1987. pp. 384–399. [Google Scholar]
- 4.Carstensen JT. Catalysis, complexation and photolysis. In: Carstensen JT, Rhodes CT, editors. Drug stability principles and practices. 3. New York: Marcel Dekker; 2000. pp. 73–84. [Google Scholar]
- 5.Yoshioka S, Stella VJ. Stability of drugs and dosage forms. New York: Klumer Academic/Plenuim Publisher; 2000. pp. 97–99. [Google Scholar]
- 6.Florence AT, Attwood D. Drug stability. In: Physiochemical principles of pharmacy. 4th ed. London: Pharmaceutical Press; 2006. p. 93–138.
- 7.Sinko PJ. Chemical kinetics and stability. In: Martin’s physical pharmacy and pharmaceutical sciences. 5th ed. Philadelphia, PA: Lippincott Willams & Wilkins, 2006, p. 416–20.
- 8.British Pharmacopoeia, London: Her Majesty’s Stationary Office; 2012, Electronic version.
- 9.United States Pharmacopoeia, 30-National Formulary 25. Rockville, MD: United States Pharmacopoeial Convention, Inc., 2013. Electronic version.
- 10.Ahmad I, Fasiullah Q, Noor A, Ansari IA, Ali QNM. Photolysis of riboflavin in aqueous solution: a kinetic study. Int J Pharm. 2004;280:199–208. doi: 10.1016/j.ijpharm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Halwer M. The photochemistry of riboflavin and related compounds. J Am Chem Soc. 1951;73:4870–4874. doi: 10.1021/ja01154a118. [DOI] [Google Scholar]
- 12.Holmstrom B, Oster G. Riboflavin as an electron donor in photochemical reactions. J Am Chem Soc. 1961;83:1867–1871. doi: 10.1021/ja01469a022. [DOI] [Google Scholar]
- 13.Schuman Jorns M, Sehollnhammer G, Hemmerich P. Intramolecular addition of the riboflavin side chain. Anion-catalyzed neutral photochemistry. Eur J Biochem. 1975;57:35–48. doi: 10.1111/j.1432-1033.1975.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad I, Fasihullah Q, Vaid FHM. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2004;75:13–20. doi: 10.1016/j.jphotobiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Fasihullah Q, Vaid FHM. Effect of phosphate buffer on photodegradation reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2005;78:229–234. doi: 10.1016/j.jphotobiol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad I, Fasihullah Q, Vaid FHM. Effect of light intensity and wavelength on photodegradation reactions riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2006;82:21–27. doi: 10.1016/j.jphotobiol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad I, Vaid FHM. Photochemistry of flavins in aqueous and organic solvents. In: Silva E, Edwards AM, editors. Flavins photochemistry and photobiology. Cambridge: Royal Society of Chemistry; 2006. pp. 13–40. [Google Scholar]
- 18.Ahmad I, Ahmed S, Sheraz MA, Vaid FHM, Ansari IA. Effect of divalent anions on photodegradation kinetics and pathways of riboflavin in aqueous solution. Int J Pharm. 2010;390:174–182. doi: 10.1016/j.ijpharm.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad I, Ahmed S, Sheraz MA, Vaid FHM. Effect of borate buffer on the photolysis of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2008;93:82–87. doi: 10.1016/j.jphotobiol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad I, Sheraz MA, Ahmed S, Hafeez SK, Mirza T, Aminuddin M. Stabilizing effect of citrate buffer on the photolysis of riboflavin in aqueous solution. Result Pharma Sci. 2011;1:11–15. doi: 10.1016/j.rinphs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou JP, Poole JW. Lactam antibiotics: their physicochemical properties and biological activities in relation to structure. J Pharm Sci. 1971;60:503–532. doi: 10.1002/jps.2600600402. [DOI] [PubMed] [Google Scholar]
- 22.Wood RW. Carbenicillin monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2. New York: Wiley; 1986. pp. 290–294. [Google Scholar]
- 23.Fabre H, Eddine NH, Berge G. Degradation kinetics in aqueous solution of cefotaxime sodium, a third-generation cephalosporin. J Pharm Sci. 1984;73:611–618. doi: 10.1002/jps.2600730508. [DOI] [PubMed] [Google Scholar]
- 24.Hajratwala BR. Kinetics of sulfite-induced anaerobic degradation of epinephrine. J Pharm Sci. 1975;64:45–48. doi: 10.1002/jps.2600640108. [DOI] [PubMed] [Google Scholar]
- 25.Hansen J, Kreilgard B, Nielsen, Veje J. Kinetics of degradation of methotrexate in aqueous solution. Int J Pharm. 1983;16:141–152. doi: 10.1016/0378-5173(83)90051-0. [DOI] [Google Scholar]
- 26.Underberg WJM, Lingeman H. Aspects of the chemical stability of mitomycin and profiromycin in acidic solution. J Pharm Sci. 1983;72:549–553. doi: 10.1002/jps.2600720518. [DOI] [PubMed] [Google Scholar]
- 27.Stehle RG, Smith RW. Relative’s aqueous stabilities of dinoprostone free acid (prostaglandin E2) and its carbamomylmethyl ester. J Pharm Sci. 1976;65:1844–1845. doi: 10.1002/jps.2600651238. [DOI] [PubMed] [Google Scholar]
- 28.Windheuser JJ, Higuchi T. Kinetics of thiamine hydrolysis. J Pharm Sci. 1962;51:354–364. doi: 10.1002/jps.2600510415. [DOI] [PubMed] [Google Scholar]
- 29.Pertzer D. Cis-platin monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2. New York: Wiley; 1986. pp. 356–364. [Google Scholar]
- 30.Reid JM. Cyclophosphamide monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2. New York: Wiley; 1986. pp. 385–393. [Google Scholar]
- 31.Konorev EA, Zhang A, Joseph J, Kennedy MC, Kalyanaraman B. Bicarbonate exacerbates oxidative injury induced by antitumor antibiotic doxorubicin in cardiomyocytes. AJP-Heart. 2000;279:2424–2430. doi: 10.1152/ajpheart.2000.279.5.H2424. [DOI] [PubMed] [Google Scholar]
- 32.Fall HH, Petering HG. Metabolic inhibitors.1. 6,7-dimethyl-9-formylmethylisoalloxazine, 6,7-dimethyl-9-(12-hydroxyethyl)-isoalloxazine and derivatives. J Am Chem Soc. 1956;78:377–381. doi: 10.1021/ja01583a035. [DOI] [Google Scholar]
- 33.Fukumachi C, Sakurai Y. Vitamin B2 photolysis. V. The photolytic formation of 6, 7-dimethylflavin-9-acetic acid ester from riboflavin. Vitamins (Kyoto) 1954;7:939–943. [Google Scholar]
- 34.Ahmad I, Rapson HDC, Heelis PF, Phillips GO. Alkaline hydrolysis of 7, 8-dimethyl-10(formylmethyl)-isoalloxazine. A kinetic study. J Org Chem. 1980;45:31–33. doi: 10.1021/jo01292a040. [DOI] [Google Scholar]
- 35.Hatchard CG, Parker CA. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical antinometer. Proc Roy Soc (Lond) 1956;A 235:518–536. doi: 10.1098/rspa.1956.0102. [DOI] [Google Scholar]
- 36.Ahmad I, Rapson HDC. Multicomponent spectrophotometeric assay of riboflavin and photoproducts. J Pharm Biomed Anal. 1990;8:217–223. doi: 10.1016/0731-7085(90)80029-O. [DOI] [PubMed] [Google Scholar]
- 37.Suelter CH, Metzler DE. The oxidation of a reduced pyridine nucleotide analog by flavins. Biochem Biophys Acta. 1960;44:22–23. doi: 10.1016/0006-3002(60)91518-3. [DOI] [Google Scholar]
- 38.Ahmad I, Fasihullah Q, Vaid FHM. Photolysis of formylmethylflavin in aqueous and organic solvents. Photochem Photobiol Sci. 2006;5:680–685. doi: 10.1039/b602917e. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad I, Mirza T, Iqbal K, Ahmed S, Sheraz MA, Vaid FHM. Effect of pH, buffer and viscosity on the photolysis of formylmethylflavin: a kinetic study. Aust J Chem. 2013;66:579–585. [Google Scholar]
- 40.Song PS, Metzler DE. Photochemical degradation of flavins. IV. Studies of the anaerobic photolysis of riboflavin. Photochem Photobiol. 1967;6:691–709. doi: 10.1111/j.1751-1097.1967.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 41.Farrer KTH, Macewan JL. The thermal destruction of riboflavin. Aust J Biol Sci. 1954;7:73–84. [PubMed] [Google Scholar]
- 42.Harkness DR, Stadman ER. Bacterial degradation of riboflavin. J Biol Chem. 1965;240:4089–4096. [PubMed] [Google Scholar]
- 43.Ahmad I, Ahmed S, Sheraz MA, Aminuddin M, Vaid FHM. Effect of caffeine complexation on the photolysis of riboflavin in aqueous solution: a kinetic study. Chem Pharm Bull. 2009;57:1363–1370. doi: 10.1248/cpb.57.1363. [DOI] [PubMed] [Google Scholar]
- 44.Cairns WL, Metzler DE. Photochemical degradation of flavins. VI. A new photoproduct and its use in studying the photolytic mechanism. J Am Chem Soc. 1971;93:2772–2777. doi: 10.1021/ja00740a031. [DOI] [PubMed] [Google Scholar]
- 45.Heelis PF, Phillips GO, Ahmad I, Rapson HDC. The photodegradation of formylmethylflavin—a steady state and laser flash photolysis study. Photochem Photobiophys. 1980;1:125–130. [Google Scholar]
- 46.Rodiguin NM, Rodiguina EN. Consecutive chemical reactions. Princelin: Van Nostand Co., Inc; 1964. pp. 84–96. [Google Scholar]
- 47.O’Neil MJ, editor. The Merck Index. 15th ed. Cambridge, UK: The Royal Society of Chemistry; 2013. Electronic Version.
- 48.Moore WM, Spence JT, Raymond FA, Colson SD. Photochemistry of riboflavin. I. The hydrogen transfer process in the anaerobic photobleaching of flavins. J Am Chem Soc. 1963;85:3367–3372. doi: 10.1021/ja00904a013. [DOI] [Google Scholar]
- 49.Smith EC, Metzler DE. The photochemical degradation of riboflavin. J Am Chem Soc. 1963;85:3285–3288. doi: 10.1021/ja00903a051. [DOI] [Google Scholar]
- 50.Clark WM. Oxidation-reduction potentials of organic systems. Baltimore: Williams & Wilkins; 1960. p. 442. [Google Scholar]
- 51.Mayhew SG. The effects of pH and semiquinone formation on the oxidation-reduction potentials of flavin mononucleotide. A reappraisal. Eur J Biochem. 1999;265:698–702. doi: 10.1046/j.1432-1327.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 52.Wells JI. Pharmaceutical formulation: the physicochemical properties of drug substances. New York: Wiley; 1988. p. 171. [Google Scholar]
- 53.Albert A, Serjent EP. Ionization constants of acids and bases. A laboratory mannual. London: Methuen & Co; 1962. p. 151. [Google Scholar]
- 54.Weber G. Fluorescence of riboflavin and flavin adenine dinucleotide. Biochem J. 1950;47:114–121. doi: 10.1042/bj0470114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heelis PF. The photophysical and photochemical properties of flavins (isoalloxazines) Chem Soc Rev. 1982;11:15–39. doi: 10.1039/cs9821100015. [DOI] [Google Scholar]
- 56.Drossler P, Holzer W, Penzkoffer A, Hegemann P. Fluorescence quenching of aqueous solutions of riboflavin by methionin and cystein. Chim Phys. 2003;286:409. doi: 10.1016/S0301-0104(02)00969-2. [DOI] [Google Scholar]
- 57.Song PS. Chemistry of flavins in their excited states. In: Kamin H, editor. Flavins and flavoproteins. Baltimore: University Park Press; 1971. pp. 37–61. [Google Scholar]
- 58.Heelis PF. The photochemistry of flavins. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. Boca Raton, FL: CRC Press; 1991. pp. 171–193. [Google Scholar]
- 59.Gladys M, Knappe WR. Photochemie des (Iso) alloxazins III. Intramolekulare photodealkylierung von 10-alkylisoalloxazinen, eine mofellreaktion fur den riboflavin-photoabbau. Chem Ber. 1974;107:3658–3673. doi: 10.1002/cber.19741071118. [DOI] [Google Scholar]
- 60.Knappe WR. Photochemie des (Iso) Alloxazins IV. Dealkylierung und decarboxylierung kurzkettiger isoalloxazin-10-alkancarbonsauren. Chem Ber. 1975;108:2422–2438. doi: 10.1002/cber.19751080730. [DOI] [Google Scholar]
- 61.Sheraz MA, Kazi SH, Ahmad S, Mirza T, Ahmad I, Evstigneev MP. Effect of phosphate buffer on the complexation and photochemical interaction of riboflavin and caffeine in aqueous solution: a kinetic study. J Photochem Photobiol A Chem. 2014;273:17–22. doi: 10.1016/j.jphotochem.2013.09.007. [DOI] [Google Scholar]
- 62.Ahmad I, Tollin G. Flavin triplet quenching and semiquinone formation by aliphatic α-substituted acetic acids: intermediates in flavin-sensitized photodecarboxylation. Photochem Photobiol. 1981;34:441–445. doi: 10.1111/j.1751-1097.1981.tb09022.x. [DOI] [Google Scholar]
- 63.Ahmad I, Tollin G. Solvent effects on flavin electron transfer reactions. Biochemistry. 1981;20:5925–5928. doi: 10.1021/bi00523a042. [DOI] [PubMed] [Google Scholar]


