Abstract
In the present study, nonionic surfactant vesicles (niosomes) formulated with Span 20, cholesterol, and novel synthesized spermine-based cationic lipids with four hydrocarbon tails in a molar ratio of 2.5:2.5:1 were investigated as a gene carrier. The effects of the structure of the cationic lipids, such as differences in the acyl chain length (C14, C16, and C18) of the hydrophobic tails, as well as the weight ratio of niosomes to DNA on transfection efficiency and cell viability were evaluated in a human cervical carcinoma cell line (HeLa cells) using pDNA encoding green fluorescent protein (pEGFP-C2). The niosomes were characterized both in terms of morphology and of size and charge measurement. The formation of complexes between niosomes and DNA was verified with a gel retardation assay. The transfection efficiency of these cationic niosomes was in the following order: spermine-C18 > spermine-C16 > spermine-C14. The highest transfection efficiency was obtained for transfection with spermine-C18 niosomes at a weight ratio of 10. Additionally, no serum effect on transfection efficiency was observed. The results from a cytotoxicity and hemolytic study showed that the cationic niosomes were safe in vitro. In addition, the cationic niosomes showed good physical stability for at least 1 month at 4°C. Therefore, the cationic niosomes offer an excellent prospect as an alternative gene carrier.
KEY WORDS: cationic lipid, cationic niosomes, gene carrier, gene transfection, nonionic surfactant vesicles, spermine derivatives
INTRODUCTION
Gene therapy has been widely endorsed as a promising therapeutic approach for many incurable diseases related to gene function, such as genetic diseases, cancer, cardiovascular diseases, and autoimmune diseases (1). Successful gene therapy requires not only therapeutically suitable genes but also a safe and efficient gene carrier (2). To avoid severe side effects resulting from viral vectors, such as immunogenicity, mutagenesis, and carcinogenesis, nonviral vectors offer an attractive alternative. Cationic liposomes, a vesicular system widely investigated as effective gene carriers, are one of the preferred nonviral vectors (3,4). In addition, several studies have reported on the use of another vesicular system, nonionic surfactant vesicles (niosomes), as a gene carrier that can potentially be substituted for liposomes (4–6).
Niosomes are nonionic surfactant vesicles formed by the self-assembly of nonionic amphiphiles into a bilayer structure in an aqueous medium (7). The nonionic surfactants preferably used to prepare niosomes include alkyl ethers and alkyl glyceryl ethers (Brij), sorbitan fatty acid esters (Span), and polyoxyethylenefatty acid esters (Tween) (7–9). Cholesterol is usually combined with a nonionic surfactant in a 1:1 molar ratio in niosome formulations (10,11). Niosomes can be prepared in the same way as liposomes and possess similar structure and physical properties. However, niosomes have several advantages over liposomes, including low production costs, high purity, uniform content, greater stability, and the ease of storing nonionic surfactants (12). Cationic niosomes used as gene carriers are usually composed of nonionic surfactants (i.e., Tween and Span), cholesterol, and cationic lipids (10,13). One of the major factors affecting gene transfection mediated by cationic niosomes is niosome composition, including the types of surfactants and cationic lipids used (5,6).
The cationic lipids used as transfection reagents usually contain three parts: a hydrophobic group, a linker group, and a positively charged head group that can interact with DNA and cause DNA condensation. The polyamines furnish one of the most effective cationic lipid head groups (14). Among the polyamines, spermine, a well-known polyamine consisting of a tetraamine with two primary and two secondary amino groups, plays an important role as a gene carrier (15). Spermine-derivative cationic lipids commercially available for gene delivery applications include dioctadecylamidoglycylspermine (DOGS) and dipalmitoylphosphatidyl ethanolamidospermine (DPPES) (16–18). In the present study, cationic niosomes formulated with Span 20, cholesterol, and novel synthesized spermine-based cationic lipids with four hydrocarbon tails, namely, Tetra-(N1,N1,N14,N14-myristeroyloxyethyl)-spermine (spermine-C14), Tetra-(N1,N1,N14,N14-palmitoyloxyethyl)-spermine (spermine-C16), and Tetra-(N1,N1,N14,N14-steroyloxyethyl)-spermine (spermine-C18) (Fig. 1), in a molar ratio of 2.5:2.5:1 were investigated as gene carriers. Factors affecting transfection efficiency and cell viability, including cationic lipid structure (i.e., differences in the acyl chain length (C14, C16, and C18) of the hydrophobic tails as well as the weight ratio of niosomes to DNA) were evaluated in a human cervical carcinoma cell line (HeLa cells) using pDNA-encoded green fluorescent protein (pEGFP-C2). The morphology, size, and charge of these niosomes/DNA complexes were characterized, and agarose gel electrophoresis was performed. Moreover, the physical stability of these cationic niosomes was evaluated with size and charge measurements.
Fig. 1.
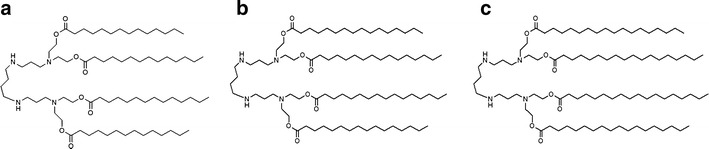
Chemical structure of a Tetra-(N 1,N 1,N 14,N 14-myristeroyloxyethyl)-spermine (spermine-C14), b Tetra-(N 1,N 1,N 14,N 14-palmitoyloxyethyl)-spermine (spermine-C16), and c Tetra-(N 1,N 1,N 14,N 14-steroyloxyethyl)-spermine (spermine-C18)
MATERIALS AND METHODS
Materials
Sorbitan monolaurate (Span 20) was obtained from Sigma-Aldrich, MO, USA. Cholesterol was purchased from Carlo Erba Reagenti, MI, Italy. Spermine-based cationic lipids as shown in Fig. 1a–c were synthesized via solid phase synthesis. The synthesis and characterization procedure of these compounds were described in the previous report (19). HeLa cells, the human cervical cancer cell line, were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Modified Eagle’s medium (MEM), trypsin-EDTA, penicillin–streptomycin antibiotics, and fetal bovine serum (FBS) were purchased from GIBCO-Invitrogen, NY, USA. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Sigma-Chemical Co., MO, USA. The pEGFP-C2 plasmid DNA, encoding green fluorescent protein (GFP), was obtained from Clontech, CA, USA. The λHindIII were obtained from Promega, CA, USA. LipofectamineTM 2000 was purchased from Invitrogen, NY, USA. The human leukocyte-poor packed red cells (LPRC) preserved with SAGM red cell preservation solution (dextrose monohydrate 0.9 g, sodium chloride 0.877 g, mannitol 0.525 g, and adenine 0.0169 g dissolved in 100 ml water for injection) was donated from National Blood Centre Thai Red Cross Society, Bangkok, Thailand. All other reagents employed were of cell culture and molecular biology quality.
Plasmid Preparation
pEGFP-C2 was propagated in Escherichia coli DH5-α and purified by using the Qiagen endotoxin-free plasmid purification kit (Qiagen, Santa Clarita, CA, USA). DNA concentration was quantified by measuring UV absorbance at 260 nm using a GeneRay UV Photometer (Biometra®, Goettingen, Germany). The purity of the plasmid was verified by gel electrophoresis (1% agarose gel) in Tris acetate-EDTA buffer, pH 8.0 (TAE buffer) using λDNA/HindIII as a DNA marker.
Preparation of Cationic Niosomes
The cationic niosomes used in the present study were prepared using a sonication method (20). Briefly, nonionic surfactants and cholesterol were dissolved in an ethanol/chloroform mixture (1:1 v/v ratio), whereas cationic lipid was dissolved in a chloroform/methanol mixture (2:1 v/v ratio). The cationic lipid solution was then added to the solution of surfactant and cholesterol to obtain a mixed solution of surfactant/cholesterol/cationic lipid in a molar ratio of 2.5:2.5:1. The solvents were evaporated under N2 gas flow to generate a thin film at the bottom of the test tubes. The thin film was left in a desiccator overnight to remove the remaining organic solvents, and thin film hydration was performed using a Tris buffer (20 mM Tris and 150 mM NaCl, pH 7.4). After hydration, the dispersion was sonicated using a bath sonicator for 30 min followed by a probe sonicator (Vibra-CellTM Ultrasonic Processor, Sonics & Materials, Inc., USA), each for 30 min in two cycles.
Preparation and Characterization of the Niosomes/DNA Complexes
The niosomes/DNA complexes were prepared at variable-weight ratios of niosomes to DNA by adding the niosome solution to a DNA solution (the amount of DNA was fixed at 0.5 μg). The mixture was gently mixed by pipetting up and down for 3–5 s to initiate complex formation. To complete the complex formation process, the mixture was left at room temperature for 30 min. Agarose gel electrophoresis was performed to ensure complex formation using 1% agarose gels (1 g agarose in 100 ml TAE buffer containing ethidium bromide 0.5 μg/ml) and TAE as a running buffer. The electrophoresis was performed for 45 min at 100 V. Ten microliters of niosomes/DNA complexes containing 0.5 μg of DNA were loaded per well.
Size and Zeta Potential Measurements
The particle size and surface charge of the cationic niosomes and niosomes/DNA complexes were determined by photon correlation spectroscopy using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK). The niosomes and the niosomes/DNA complexes at varying weight ratios were diluted with distilled water that had been filtered through a 0.22-μm membrane filter to obtain the volume required for each measurement (approximately 1 ml). All samples were measured in triplicate at room temperature.
In Vitro Transfection of Niosomes/DNA Complexes into HeLa Cells
On the day before transfection, HeLa cells were seeded into a 48-well plate at a density of 2 × 104 cells/well in 0.25 ml of complete medium (MEM containing 10% fetal bovine serum and supplemented with 1% l-glutamine, 1% nonessential amino acid solution, and 100 U/ml penicillin and 100 μg/ml streptomycin) and were incubated at 37°C with 5% CO2 overnight. Prior to transfection, the medium was removed, and the cells were incubated with 0.25 ml of the niosomes/DNA complexes in serum-free medium at variable-weight ratios containing 0.5 μg of pEGFP-C2 for 24 h at 37°C and with 5% CO2. Cells transfected with naked pEGFP-C2 were used as negative controls, and cells transfected with LipofectamineTM 2000 complexed with DNA at a weight ratio of 2 were used as positive controls. On the day after transfection, the cells were washed with pH 7.4 phosphate-buffered saline (PBS) and replaced with complete medium. GFP expression was directly observed under a fluorescence microscope (model: GFP-B; wavelengths: excitation filter 480/40 and emission filter 535/50). All transfection experiments were performed in triplicate. A complete medium containing 10% FBS was used to investigate the effect of serum on transfection efficiency.
Evaluation of Cell Viability
Cell viability was investigated using an MTT assay. HeLa cells were seeded into a 96-well plate at a density of 8 × 103 cells/well in 100 μl of complete medium and were incubated at 37°C and 5% CO2 overnight. Prior to the cytotoxicity study, the medium was removed, and the cells were treated with the niosomes/DNA complexes under the same conditions as in the transfection experiment. On the day after treatment, the cells were rinsed with PBS and incubated in 100 μl MTT-containing medium (1 mg/ml) for 4 h. The formazan crystals formed in living cells were dissolved in 100 μl dimethyl sulfoxide per well. Relative cell viability (in percent) was calculated based on the absorbance observed at 550 nm using a microplate reader (Universal Microplate Analyzer, models AOPUS01 and AI53601, Packard BioScience, CT, USA) and compared with nontreated cells. The viability of nontreated cells was defined as 100%.
Hemolytic Assay of Cationic Niosomes and Niosomes/DNA Complexes
Hemolytic activity was evaluated using human LPRC according to (21). Briefly, LPRC was centrifuged at 3,800 rpm for 5 min to remove the plasma and additive solution. The red blood cells (RBCs) were then washed three times with PBS. The RBCs were distributed in a 96-well plate (100 μl/well), and an equal volume of cationic niosomes or niosomes/DNA complexes under the same concentration as in the transfection experiment (both diluted with PBS) was added. After incubation at 37 ºC for 1 h, the plates were centrifuged at 800×g for 10 min, and the supernatant was collected. The absorbance of the supernatant was measured at 550 nm using a microplate reader (Universal Microplate Analyzer, models AOPUS01 and AI53601, Packard BioScience, CT, USA). RBCs treated with PBS were used as a negative control (normal hemolysis), whereas RBCs treated with sterile water were used as a positive control (100% hemolysis). Positive and negative control wells were added on each plate. The percentage of hemolytic activity of each sample was estimated from the absorbance at 550 nm using the following equation: [(A − A0) / (Amax − A0)] × 100, where A0 is the absorbance of the negative control and Amax is the absorbance of the positive control, corresponding to 100% hemolysis. The percentages of hemolysis (Y axis) were plotted against the log of niosome concentration (X axis), and the 50% hemolytic concentration (HC50) value of the niosomes (the niosome concentration that lyses 50% RBCs) was estimated.
Transmission Electron Microscopy
The morphological analysis of cationic niosomes and niosomes/DNA complexes was performed with a transmission electron microscope (JEOL JEM-1230, JEOL, Tokyo, Japan). A small drop of sample was placed on a 200-mesh carbon-coated copper grid. The excess sample was removed using a filter paper, and the sample was then air-dried and viewed at 80 kV.
Physical Stability of Cationic Niosomes
The physical stability of the cationic niosomes after storage for 30 days at 4°C and 25°C was determined by monitoring the particle size and zeta potential.
Statistical Analysis
The significance of differences in transfection efficiency and cell viability was statistically evaluated with a one-way analysis of variance (ANOVA) followed by an LSD post hoc test for analyzing transfection efficiency and a Tukey post hoc test for determining cell viability. The significance level was set at p < 0.05.
RESULTS AND DISCUSSION
Characterization of Cationic Niosomes and Niosomes/DNA Complexes
Cationic niosomes composed of Span 20, cholesterol (Cho), and spermine-cationic lipids with four hydrocarbon tails (Fig. 1; A = Tetra-(N1,N1,N14,N14-myristeroyloxyethyl)-spermine (spermine-C14), B = Tetra-(N1,N1,N14,N14-palmitoyloxyethyl)-spermine (spermine-C16), and C = Tetra-(N1,N1,N14,N14-steroyloxyethyl)-spermine (spermine-C18)) in a molar ratio of 2.5:2.5:1 were prepared. The formulations of niosomes, particle sizes, and zeta potentials are shown in Table I. The particle size of the cationic niosomes ranged from 385 to 876 nm in the following order: niosomes-C14 > niosomes-C16 > niosomes-C18. The spermine-cationic lipids with a long carbon chain may increase the rigidity of the vesicle due to deeper insertion into the bilayer, thus increasing the carbon chain length produced a decrease in vesicle size. The zeta potential of the cationic niosomes ranged from 42 to 54 mV in the following order: niosomes-C18 > niosomes-C16 > niosomes-C14. These findings might result from the intrinsic properties of each spermine-cationic lipid. The hydrophobic strength of the long-chain carbon was greater than that of the short-chain carbon. Accordingly, the long-chain carbon increased the solubility of the cationic lipid in the niosome bilayer. The amount of long-chain carbon in the niosome bilayer was higher than that of the short-chain carbon. Accordingly, the niosomes-C18 showed a higher zeta potential than the niosomes-C14. These results were consistent with the findings of a previous study that the membrane rigidity and stability of polymer-hybridized liposomes (PHL) increased with the alkyl chain length of poly(asparagines) grafted with alkyl chains at the same polymer concentration. It is expected that longer alkyl chains will have stronger hydrophobic interactions with PC lipids, producing a tighter PHL lipid bilayer (22).
Table I.
Physical Stability of Cationic Niosomes Formulated with Span 20/Cholesterol/Spermine-Cationic Lipid in a Molar Ratio of 2.5:2.5:1 After Storage at 4°C and 25°C for 30 Days. Each Value Represents the Mean ± Standard Deviation (S.D.) from Three Independent Experiments
| Formulations | Particle size (nm) | Zeta potential (mV) | ||||
|---|---|---|---|---|---|---|
| Initial (day 0) | Day 30 | Initial (day 0) | Day 30 | |||
| 4°C | 25°C | 4°C | 25°C | |||
| Niosomes-C14 (Span 20/Cho/spermine-C14) | 876 ± 25 | 893 ± 14 | 2,459 ± 348 | +42 ± 7 | +38 ± 5 | +1 ± 3 |
| Niosomes-C16 (Span 20/Cho/spermine-C16) | 462 ± 10 | 472 ± 2 | 2,130 ± 744 | +49 ± 1 | +47 ± 2 | +2 ± 2 |
| Niosomes-C18 (Span 20/Cho/spermine-C18) | 385 ± 12 | 395 ± 3 | 2,796 ± 616 | +54 ± 1 | +53 ± 3 | +15 ± 1 |
The conditions for complex formation were optimized through the use of a gel retardation assay to determine the degree of binding between the cationic niosomes and DNA at varying weight ratios. DNA migration will be retarded if the carrier can form complexes with DNA. Moreover, no DNA migration is observed if complex formation is complete (23). In this study, the weight ratios of the cationic niosomes to DNA were altered by varying the amount of cationic niosomes and fixing the amount of DNA. The complex formation demonstrated by agarose gel electrophoresis is shown in Fig. 2. The results showed that niosomes-C14, niosomes-C16, and niosomes-C18 achieved complete complex formation at weight ratios greater than 130, 100, and 60, respectively (Fig. 2a–c). It can be concluded that the cationic niosomes containing spermine-cationic lipids can condense DNA at different binding affinities.
Fig. 2.
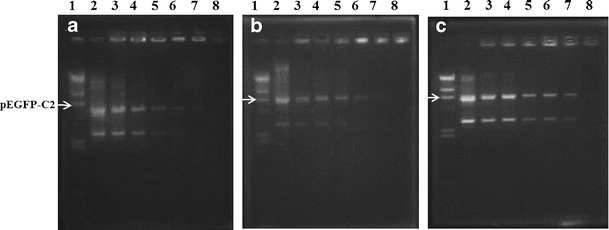
Gel retardation assay of niosomes/DNA complexes. Lane 1, λHind III DNA marker; lane 2, pEGFP-C2; lanes 3–8, niosomes/DNA complexes at weight ratios of 80, 90, 100, 110, 120, and 130 for a niosomes-C14, weight ratios of 50, 60, 70, 80, 90, and 100 for b niosomes-C16, and weight ratios of 35, 40, 45, 50, 55, and 60 for c niosomes-C18
Further investigations of the particle size and zeta potential of the niosomes/DNA complexes were performed at weight ratios of 2.5, 10, 20, 30, 40, and 100. The particle size and zeta potential were plotted against the weight ratios of the niosomes/DNA complexes (Fig. 3). The particle size of all formulations of niosomes/DNA complexes did not obviously change when weight ratio increased from 2.5 to 30, whereas further increasing the weight ratio from 30 to 100 resulted in an increase in particle size. A negative value of the zeta potential of niosomes/DNA complexes in all niosome formulations was observed for weight ratios ranging from 2.5 to 40. The zeta potential changed from negative to neutral as the weight ratio increased from 40 to 100. It can be assumed from these findings that the lack of variation in particle size as well as the negative value of the zeta potential observed with the increasing weight ratios cited might be due to the adsorption of anionic DNA molecules at the cationic surface of the niosomes through electrostatic interactions.
Fig. 3.
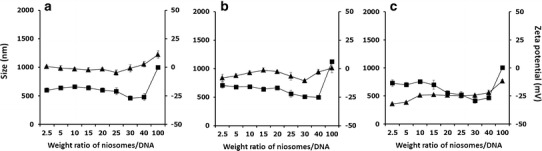
Particle size (black triangle) and zeta potential (black square) at varying weight ratios of niosomes/DNA complexes; a niosomes-C14, b niosomes-C16, and c niosomes-C18. Each value represents the mean ± S.D. of three measurements
In Vitro Transfection in HeLa Cells
An effective gene delivery system should provide high transfection efficiency. To investigate the in vitro transfection efficiency of cationic niosomes, cationic niosomes carrying pEGFP-C2 were transfected into a human cervical carcinoma cell line (HeLa cells). Gene transfection mediated by cationic niosomes is affected by numerous factors, including the types of surfactants and cationic lipids that compose the niosomes (5,6). In this component of the study, the effects of multiple factors, including the acyl chain length (C14, C16, and C18) of cationic lipids and the niosomes/DNA weight ratio, on transfection efficiency were evaluated in comparison with LipofectamineTM 2000 at a weight ratio of 2 (positive control) and naked pDNA (negative control) (Fig. 4). The niosomes-C16/DNA complexes and niosomes-C18/DNA complexes showed significantly higher transfection efficiency compared with naked DNA transfection at any weight ratio (Fig. 4b, c), whereas the transfection efficiency of niosomes-C14/DNA complexes was only significantly higher than the negative control at weight ratios of 10–40 (Fig. 4a). In general, higher weight ratios were correlated with higher transfection efficiencies. However, a decrease in transfection efficiency was observed if the weight ratio was increased above that yielding the highest transfection efficiency. This finding is in agreement with our previous reports (24,25). The highest transfection efficiency observed for the niosomes-C14, niosome-C16, and niosomes-C18 was at weight ratios of 20, 30, and 10, respectively. The transfection efficiency decreased as follows: niosomes-C18 (7,993 ± 94 cells/cm2) > niosomes-C16 (5,958 ± 197 cells/cm2) > niosomes-C14 (2,082 ± 63 cells/cm2).
Fig. 4.
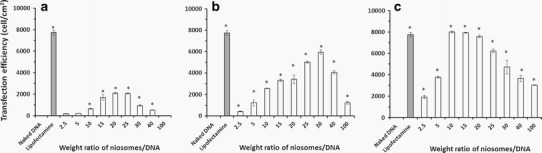
In vitro transfection efficiency of niosomes/DNA complexes with weight ratios of 2.5–100 at pH 7.4 in HeLa cells; a niosomes-C14, b niosomes-C16, and c niosomes-C18. Each value represents the mean ± S.D. of triplicate experiments (Asterisk indicates p < 0.05 compared with naked DNA)
Among the niosomes, the highest transfection efficiency was observed in the niosomes-C18 formulation at a weight ratio of 10. In addition, no significant difference was observed between the transfection efficiency of the niosomes-C18 formulation at weight ratios from 10 to 20 and that of the LipofectamineTM 2000 positive control (p > 0.05) (data not shown). These results showed that not only the weight ratio but also the alkyl chain length of the spermine-cationic lipids affected the efficiency of gene transfection. Increasing the alkyl chain length increased the efficiency of gene transfection. The high transfection efficiency of niosomes-C18 might be attributed to the small particle size (385 ± 12 nm), high zeta potential (+54 ± 1 mV), and complete DNA complex formation at lower weight ratios (above 60) relative to niosomes-C16 (above 100) and niosomes-C14 (above 130). Accordingly, the niosomes-C18 (composed of spermine-C18 cationic lipid) at a weight ratio of 10 serves as an effective gene carrier in vitro. However, niosomes-C18 achieved complete complex formation at weight ratios above 60 (Fig. 2c). Thus, at this weight ratio of 10, where the highest transfection efficiency was obtained, complex formation was not complete. This finding is in accordance with our previous report (20) on gene transfection using cationic niosomes containing another spermine derivative. In this previous study, the highest gene transfection efficiency was observed at a weight ratio below that at which complete complex formation occurred (partial complex formation). In contrast, our previous study on cationic liposomes formulated with the same spermine-C18 cationic lipid and phosphatidylcholine (PC) (liposome-C18) found that the highest transfection efficiency was obtained at a weight ratio of 15 (greater than the weight ratio needed for complete complex formation) (24). This finding might result from differences between liposomes and niosomes in intracellular processing or cellular uptake mechanisms. Experiments on complex formation effects and the possible cellular >uptake mechanisms of niosomes will be conducted in the future. The surface modification of cationic liposomes using sorbitan ester (Span®) can enhance the cellular uptake of oligonucleotides through an endocytosis-dependent pathway. The hydrophobicity of cationic liposomes must be appropriate for the cellular uptake process. Excessively strong hydrophobicity may impede carrier access to the cell surface, whereas a decrease in hydrophobicity to a certain level may hinder adhesion and phagocytosis. Additionally, Span may destabilize the cell membrane by disordering the liquid crystal structures of lipid bilayers and could thus facilitate entrance by the cells of xenobiotics (26). The mechanism of cellular internalization of surfactant vesicles has also been investigated (27). The cited study suggested that surfactant vesicles were primarily internalized through the cholesterol-dependent clathrin-mediated endocytosis pathway. It is reasonable to suppose that surfactant vesicles bind nonspecifically to clustered cellular receptors in coated regions and are then internalized. After cellular internalization, effective gene carriers should promote endosomal escape. Zhou et al. investigated the mechanism of cellular internalization of niosomes-siRNA complexes using cationic niosomes (SPANosome) formulated with DOTAP/Span 80/TPGS in a molar ratio of 50:49:1. The niosomes-siRNA complexes were shown to be internalized by tumor cells primarily through the caveolae-mediated pathway, which does not result in lysosomal delivery and is thus less degradative. In contrast, the pathway used by lipofectamine–siRNA was primarily clathrin-mediated endocytosis (28). Accordingly, the present study investigated cationic niosomes containing Span 20 niosomes and spermine-cationic lipids. The high transfection efficiency found in this study might have occurred because cationic lipids can be protonated at an intralysosomal acidic pH, producing endosomal membrane destabilization, subsequent cytosolic release of the delivered DNA, and, finally, gene transcription and translation. Lysosomotropic detergents, such as lipophilic amines, can become protonated at an intralysosomal acidic pH level, resulting in enhanced endosomal escape (29). The role of cationic niosomes in gene delivery, as well as the cellular uptake mechanism and intracellular process, is interesting and is currently under investigation in our laboratory.
One of the major factors to be considered regarding transfection reagents is their ability to function in serum-supplemented cell culture medium. A crucial drawback hindering the clinical success of cationic transfection lipids is their serum incompatibility. The in vitro gene transfection efficiency of cationic amphiphiles is usually adversely affected in the presence of serum. Generally, gene transfection mediated by cationic liposomes is inhibited by serum (30). Such serum incompatibility of cationic transfection lipids is hypothesized to begin through the adsorption of negatively charged serum proteins onto the positively charged cationic liposome surfaces, preventing the efficient interaction of these proteins with the cell surface and/or their internalization (31). Additional investigations of the effect of serum on the transfection efficiency of cationic niosomes (niosomes-C14, niosomes-C16, and niosomes-C18) at the weight ratios that provided the highest gene transfection were conducted (Fig. 5). The transfection efficiency of all of the niosome formulations did not differ significantly in the presence (10% FBS) and absence of serum (p > 0.05). This presence of a serum effect appears to be another advantageous property of these cationic niosomes that facilitates their use as gene carriers.
Fig. 5.
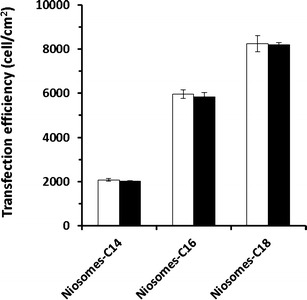
In vitro transfection efficiency of niosomes/DNA complexes in HeLa cells at pH 7.4 in the absence of serum (white bars) and presence of 10% serum (black bars) with weight ratios of 20, 30, and 10 for niosomes-C14, niosomes-C16, and niosomes-C18, respectively. Each value represents the mean ± S.D. of triplicate experiments
Morphological Analysis of Cationic Niosomes and Niosomes/DNA Complexes
The cationic niosomes and niosomes/DNA complexes were morphologically characterized with transmission electron microscopy (TEM). The TEM images demonstrated that the cationic niosomes had spherical shapes with a smooth surface and a detached border (Fig. 6a–c). The morphology of the niosomes/DNA complex at the weight ratio providing the highest gene transfection is illustrated in Fig. 6d–f. This complex also had a spherical shape, but the surface of the complex was rough or hairy, and the particle size was larger. The hairy surface of the complex in the images shows that anionic DNA molecules interacted electrostatically with the cationic niosome surfaces.
Fig. 6.
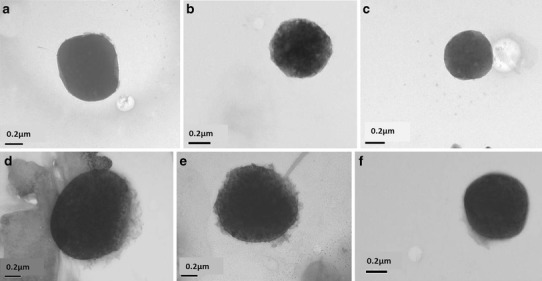
Transmission electron microscope (TEM) images at magnification ×50 k of the cationic niosomes; a niosomes-C14; b niosomes-C16; c niosomes-C18 and niosomes/DNA complexes; d niosomes-C14/DNA complexes at weight ratio of 20; e niosomes-C16/DNA complexes at weight ratio of 30; and f niosomes-C18/DNA complexes at a weight ratio of 10
Effect of Niosomes/DNA Complexes on Cell Viability
Suitable gene delivery systems require not only high transfection efficiency but also safety. To assure the safety of the gene carriers, an MTT assay was performed in HeLa cell culture (Fig. 7). The cell viability of the niosomes/DNA complex-treated cells was compared with the nontreated cells (controls). Safe formulations should result in cell viabilities similar to those of untreated cells. The results showed that niosomes-C14 was safe for use at a weight ratio of 2.5–25, whereas niosomes-C16 and niosomes-C18 were safe at weight ratios of 2.5–30 and 2.5–15, respectively. Cytotoxicity has been observed to be correlated with increasing carrier-to-DNA weight ratios (23). These cationic niosomes possess low toxicity at the weight ratios that provided the highest transfection efficiency (weight ratios of 20, 30, and 10 for niosome-C14, niosome-C16, and niosome-C18 formulations, respectively). Therefore, it can be assumed that these cationic niosomes are safe for in vitro use.
Fig. 7.
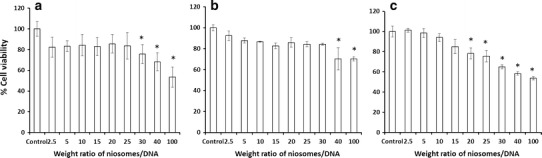
The percentage cell viability of niosomes/DNA complexes with weight ratios of 2.5–100 at pH 7.4 in HeLa cells; a niosomes-C14, b niosomes-C16, and c niosomes-C18. Each value represents the mean ± S.D. of triplicate experiments (Asterisk indicates p < 0.05 compared with control)
Hemolytic Assay
The hemolytic activity of cationic niosomes and niosomes/DNA complexes was determined using RBCs as a marker of general membrane toxicity (21). To evaluate the hemolytic activity of cationic niosomes in terms of HC50, the percentage of hemolysis was plotted against the log niosome concentration. All niosome formulations in the concentrations of 0.2 to 2,000 μg/ml used in this study had a low hemolytic effect, and the estimated HC50 of all formulations was greater than 2,000 μg/ml (the concentration of niosomes prepared in this study was approximately 2,000 μg/ml) (Fig. 8a). In addition, the niosomes/DNA complexes at the weight ratios used in the transfection experiment (weight ratios of 2.5 to 100) also showed a low hemolytic effect (Fig. 8b).
Fig. 8.
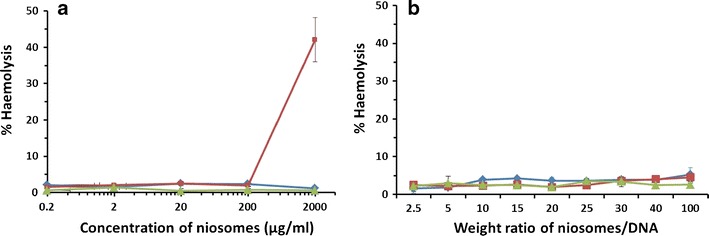
Hemolytic activity of a cationic niosome concentrations of 0.2–2,000 μg/ml and b niosomes/DNA complexes with weight ratios of 2.5–100 at pH 7.4 in human leukocyte-poor packed red cells (LPRC); niosomes-C14 ( ), niosomes-C16 (
), niosomes-C16 ( ), niosomes-C18 (
), niosomes-C18 ( ). Each value represents the mean ± S.D. of triplicate experiments
). Each value represents the mean ± S.D. of triplicate experiments
Due to safety concerns, the internal use of Span is limited because of its surfactant properties. In this study, however, the concentration of Span 20 in the niosome formulation that provided the highest transfection efficiency showed low hemolytic activity. Additionally, successful topical genetic immunization against hepatitis B in mice using niosomes composed of Span 85 and cholesterol encapsulating DNA encoding hepatitis B surface antigen (HBsAg) has been reported (12). Consequently, it might be assumed that Span-niosomes represent a promising alternative for in vivo application.
Physical Stability of Cationic Niosomes
Particle size is an important indicator of the stability response of colloids. Increases in particle size usually result from the aggregation and fusion of colloidal vesicles, correlated with an unstable colloidal system. After storage of the cationic niosomes at 4°C and 25°C for 30 days, the particle size and zeta potential of the niosomes were determined (Table I). The particle size of all niosome formulations stored at 25°C increased markedly compared with the initial value (day 0), whereas no alterations in particle size were observed at 4°C for any formulation. The zeta potential of all niosome formulations stored at 25°C decreased markedly compared with the initial value, whereas almost no changes in zeta potential were observed at 4°C for any formulation.
To prevent aggregation, vesicles must have a high surface charge to provide sufficient electrostatic repulsion (13). Cationic niosomes only show good physicochemical stability after refrigeration (4°C). This result implies that the stability of cationic niosomes is primarily affected by temperature. Temperature is associated with energy input, which can induce changes in the crystalline structure of lipids. This finding was in agreement with a previous observation (32) that rapid growth of solid lipid nanoparticles (SLNs) stored at 50°C occurred in association with a decrease in the zeta potential of the SLNs. Additionally, high temperatures can induce destabilization due to changes in zeta potential (33). In conclusion, the cationic niosomes were physically stable for at least 1 month at 4°C.
Although the particle size of the niosomes-DNA complexes that provided the highest transfection efficiency was relatively large (approximately 500 nm), this system might be used for topical DNA delivery. For cutaneous gene therapy, several niosome formulations have been successfully reported to deliver genes to the skin and provide excellent transfection efficiency (10). The current study shows that niosomes can potentially be used as a gene carrier in vitro. Several studies have reported that niosomes can facilitate siRNA delivery in vitro as well as gene delivery (28,34). Our study also supported the view that Span 20 niosomes show a low hemolytic effect and might serve as a promising alternative in vivo. However, further investigations of intracellular uptake mechanisms as well as the in vivo efficacy and safety of this system are required.
CONCLUSIONS
Cationic niosomes formulated from Span 20, cholesterol, and novel spermine-based cationic lipids with differences in the acyl chain length (C14, C16, and C18) of the hydrocarbon tails at a molar ratio of 2.5:2.5:1 were successfully prepared to enhance transfection efficiency in vitro. The niosomes composed of spermine cationic lipid with an acyl chain length of C18 demonstrated the highest transfection efficiency as well as low cytotoxicity and a low hemolytic effect in vitro. Additionally, the cationic niosomes had good physical stability for at least 1 month at 4°C. Consequently, these cationic niosomes show an excellent potential as an alternative gene carrier.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Commission of Higher Education (Thailand), the Thailand Research Funds through the Golden Jubilee Ph.D. Program (grant nos. PHD/0092/2551 and PHD/0217/2552), the Office of the Higher Education Commission, Ministry of Education, the Thailand Research Fund (RMU5480003), and the Silpakorn University Research and Development Institute (SURDI 57/01/24) for financial support.
Contributor Information
Boon-ek Yingyongnarongkul, Email: boonek@ru.ac.th.
Praneet Opanasopit, Phone: +66-34-255800, FAX: +66-34-255801, Email: praneet@su.ac.th.
REFERENCES
- 1.Mhashilkar A, Chada S, Roth JA, Ramesh R. Gene therapy: therapeutic approaches and implications. Biotechnol Adv. 2001;19:279–97. doi: 10.1016/S0734-9750(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 2.Gabor MR. The future of human gene therapy. Mol Aspects Med. 2001;22:113–42. doi: 10.1016/S0098-2997(01)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.Serikawa T, Suzuki N, Kikuchi H, Tanaka K, Kitagawa T. A new cationic liposome for efficient gene delivery with serum into cultured human cells: a quantitative analysis using two independent fluorescent probes. Biochim Biophys Acta. 2000;1467:419–30. doi: 10.1016/S0005-2736(00)00239-X. [DOI] [PubMed] [Google Scholar]
- 4.He CX, Tabata Y, Gao JQ. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386:232–42. doi: 10.1016/j.ijpharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Chen J, Chen X, Gao J, Liang W. PEGylated synthetic surfactant vesicles (Niosomes): novel carriers for oligonucleotides. J Mater Sci. 2008;19:607–14. doi: 10.1007/s10856-007-3193-4. [DOI] [PubMed] [Google Scholar]
- 6.Manosroi A, Thathang K, Werner RG, Schubert R, Manosroi J. Stability of luciferase plasmid entrapped in cationic bilayer vesicles. Int J Pharm. 2008;356:291–9. doi: 10.1016/j.ijpharm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 8.Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmacol Sin B. 2011;1:208–19. doi: 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- 9.Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interface Sci. 2012;183–184:46–54. doi: 10.1016/j.cis.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Geusens B, Strobbe T, Bracke S, Dynoodt P, Sanders N, Gele MV, et al. Lipid-mediated gene delivery to the skin. Eur J Pharm Sci. 2011;43:199–211. doi: 10.1016/j.ejps.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. J Colloids Surf B: Biointerfaces. 2003;30:129–38. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 12.Vyas SP, Singh RP, Jain S, Mishra V, Mahor S, Singh P, et al. Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int J Pharm. 2005;296:80–6. doi: 10.1016/j.ijpharm.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Rao Y, Chen J, Yang VC, Liang W. Polysorbate cationic synthetic vesicle for gene delivery. J Biomed Mater Res A. 2011;96:513–9. doi: 10.1002/jbm.a.32999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ram IM. Water insoluble and soluble lipids for gene delivery. Adv Drug Deliv Rev. 2005;57:699–712. doi: 10.1016/j.addr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Azzama T, Eliyahua H, Makovitzki A, Linial M, Domb AJ. Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. J Control Release. 2004;96:309–23. doi: 10.1016/j.jconrel.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Gaucheron J, Santaella C, Vierling P. Transfection with fluorinated lipoplexes based on fluorinated analogues of DOTMA, DMRIE and DPPES. Biochim. Biophys Acta. 2002;1564:349–58. doi: 10.1016/S0005-2736(02)00469-8. [DOI] [PubMed] [Google Scholar]
- 17.Jean PB, Barbara D, Jean PL, Jose PM. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989;86:6982–6. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–96. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Yingyongnarongkul B, Radchatawedchakoon W, Krajarng A, Watanapokasin R, Suksamrarn A. High transfection efficiency and low toxicity cationic lipids with aminoglycerol-diamine conjugate. Bioorg Med Chem. 2009;17:176–88. doi: 10.1016/j.bmc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Paecharoenchai O, Niyomtham N, Ngawhirunpat T, Rojanarata T, Yingyongnarongkul B, Opanasopit P. Cationic niosomes composed of spermine-based cationic lipids mediate high gene transfection efficiency. J Drug Target. 2012;20:783–92. doi: 10.3109/1061186X.2012.716846. [DOI] [PubMed] [Google Scholar]
- 21.Lopes RM, Luísa CM, Eleutério CV, Carvalheiro MC, Scoullica E, Cruz ME. Formulation of ORZ liposomes: in vitro studies and in vivo fate. Eur J Pharm Biopharm. 2012;82:281–90. doi: 10.1016/j.ejpb.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Park SI, Lee EO, Yang HM, Park CW, Kim JD. Polymer-hybridized liposomes anchored with alkyl grafted poly(asparagine) J Colloid Interface Sci. 2011;364:31–8. doi: 10.1016/j.jcis.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Weecharangsan W, Opanasopit P, Ngawhirunpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm. 2008;348:161–8. doi: 10.1016/j.ijpharm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Paecharoenchai O, Niyomtham N, Apirakaramwong A, Yingyongnarongkul B, Opanasopit P. Effect of acyl chain length of spermine derivatives on transfection efficiency. Adv Mater Res. 2012;506:445–8. doi: 10.4028/www.scientific.net/AMR.506.445. [DOI] [Google Scholar]
- 25.Paecharoenchai O, Niyomtham N, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Yingyongnarongkul B, et al. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech. 2012;13:1302–8. doi: 10.1208/s12249-012-9857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YZ, Gao JQ, Chen JL, Liang WQ. Cationic liposomes modified with non-ionic surfactants as effective non-viral carrier for gene transfer. Colloids Surf B: Biointerfaces. 2006;49:158–64. doi: 10.1016/j.colsurfb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Marzio DL, Marianecci C, Cinque B, Nazzarri M, Cimini AM, Cristiano L, et al. pH-sensitive non-phospholipid vesicle and macrophage-like cells: binding, uptake and endocytotic pathway. Biochim Biophys Acta. 2008;1778:2749–56. doi: 10.1016/j.bbamem.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Zhou C, Zhang Y, Yu B, Phelps MA, Lee LJ, Lee RJ. Comparative cellular pharmacokinetics and pharmacodynamics of siRNA delivery by SPANosomes and by cationic liposomes. Nanomedicine. 2013;9(4):504–13. doi: 10.1016/j.nano.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang E, Hughes J. Characterization of a pH-sensitive surfactant, dodecyl-2-(1′-imidazolyl) propionate (DIP), and preliminary studies in liposome mediated gene transfer. Biochim Biophys Acta. 1998;1369:39–50. doi: 10.1016/S0005-2736(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan: effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22:2075–80. doi: 10.1016/S0142-9612(00)00385-9. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–94. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 32.Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN) dispersions. Int J Pharm. 1998;168:221–9. doi: 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]
- 33.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24:4283–300. doi: 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 34.Paecharoenchai O, Teng L, Yung BC, Teng L, Opanasopit P, Lee RJ. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine. 2013;8(11):1865–73. doi: 10.2217/nnm.13.155. [DOI] [PMC free article] [PubMed] [Google Scholar]


