Abstract
This study investigated the processing parameters and formulation factors on the bioadhesive properties, temperature stability properties, and drug release properties of miconazole in PolyOx® and Klucel® matrix systems produced by Hot-melt Extrusion (HME) technology. Miconazole incorporated into these matrix systems were found to be stable for 8 months by X-ray diffraction (XRD). The addition of miconazole increased area under the curve (AUC) at contact time intervals of 30 and 60 sec, while the bioadhesion decreased with an increase in processing temperatures. The release profiles suggest that a sustained release of miconazole was observed from all of the tested HME film formulations for approximately 10 h. The release from the optimal HME film extruded at 205°C was found to be significantly different than that extruded at 190°C. Therefore, this matrix system may address the present shortcomings of currently available therapy for oral and pharyngeal candidiasis.
KEY WORDS: bioadhesive properties, formulation factors, processing parameters, release studies, stability properties
INTRODUCTION
Oral candidiasis is an infectious process of yeast fungi of the genus Candida on mouth mucous membranes, which affects many populations all over the world. Miconazole is approved for use in oropharyngeal candidiasis in humans. Miconazole (1-2- ((2, 4 dichlorophenyl)-2-(2, 4-dichlorophenyl)-methoxy) ethyl) -1-imidazole) is the first generation of azole antifungal agents, which has been use in medicine since the early 1970s. It has received considerable attention in medicine and built a reputation as an antifungal drug due to being widely used in the local treatment of vaginal, skin and nail infections by yeasts and dermatophytes (1). Miconazole has been proved equally effective in both Candida and dermatophyte infections of the skin and oral mucosal. Miconazole inhibits cytochrome P-450, suppresses steroidogenesis and disrupts steroidogenic acute regulatory protein expression, inhibits Ca2+-activated K+ channel, reduces agonist-evoked protein-tyrosine phosphorylation and evokes membrane depolarization in human platelets (2). Miconazole is available as topical creams (3), slow-release tablets (4), oral gels (5), as well as other dosage forms. However, one of the most common problems with miconazole is that patients often cease using it before the infection is completely eradicated, leading to reinfection (6). Additionally, it presents poor water solubility and should be protected from light. The properties of miconazole show a major challenge in the development of a suitable dosage form. Therefore, an attempt has been made to deliver miconazole through the oral transmucosal route in the form of an Antifungal Denture Adhesive Film (ADA) system for oral candidiasis produced by hot-melt extrusion. The mucoadhesive film may increase residence time and improve contact at the absorption site, provide localization to specified oral mucosa regions to enhance bioavailability, which will address an unmet need in the market.
Bioadhesion is defined as the ability of a biological or synthetic material to stick to the human epidermis or a mucous membrane and was shown to be a necessary consideration (7). Numerous mechanisms of bioadhesion have been studied and proposed, such as surface energy and contact angle considerations, polymer chain inter-penetration, and the swelling rate of a polymer interacting with skin or mucin (8). Numerous factors will affect the bioadhesive property, for example, drug content (9), wetting time, bioadhesive polymer type, bioadhesive polymers concentration, and contact times (10). Moreover, Repka and McGinity concluded that methods developed using a Chatillon testing apparatus may be employed as a novel technique to determine bioadhesion of hot-melt extruded films (11). Bioadhesive characteristics are essential parameters in the successful product development of films for the transmucosal route of administration and have received increasing attention in the scientific literature. Consequently, the objective of this study was also to determine the influence of processing parameters and formulation factors on the bioadhesive profiles of miconazole in hot-melt extruded films. In addition, the effect of drug loading concentration and contact time was also assessed.
In the present study, the feasibility of a novel technology hot-melt extrusion to produce a miconazole-containing ADA system was investigated. The influence of different processing temperatures, different extruder screw speeds, and various formulation factors on the bioadhesive, temperature stability and drug release properties of miconazole in PolyOx® and Klucel® matrix systems was examined.
MATERIALS AND METHODS
Materials
Hydroxypropylcellulose (HPC JF) (Klucel JF, MW140 000 D) was kindly donated by Aqualon Division, Hercules Inc (Wilmington, DE). Poly (ethylene oxide) (PEO 750) (PolyOx WSR N-750 [PEO 750 N–750], MW 300 000 Da) was kindly donated by Dow Chemical Co (Midland, MI). Miconazole was purchased from Fisher BioReagents (Fair Lawn, NJ). Dibasic sodium phosphate and Monobasic potassium phosphate were obtained from Spectrum Chemical (Gardena, CA). Sodium chloride was gotten from Fisher Scientific (Fair Lawn, NJ). Ammonium acetate, acetonitrile and methanol (highperformance liquid chromatography [HPLC] grade) were purchased from Fisher Chemicals (Fair Lawn, NJ). Double distilled and deionized water was used throughout the experiment and all other materials used were of analytical or pharmaceutical grade.
Preparation of the Polymeric Films by HME
Based on previous studies (12), hydroxypropyl cellulose (HPC JF) and poly (ethylene oxide) (PEO 750) were chosen as polymeric carriers for the matrix film formulations. The polymers containing the model antifungal drug miconazole at 0%, 2.5%, 5%, 10% and 15% were geometrically diluted and dried in an oven (Isotemp Incubator 625D, Fisher Scientific, Rockville, MD) at 40°C to 50°C for 24 hours and then blended for content uniformity in a V-blender at 100 rpm for 15 minutes. The resultant blend was fed into a twin-screw hot-melt extruder (Prism 16 mm EuroLab, Thermo Fisher Scientific) equipped with a 6-inch flex-lip die. Four different processing temperatures (160, 175, 190, and 205°C) and extruder screw speed (30, 50 and 70 rpm) were evaluated. The die opening was adjusted to 0.005 inches (0.127 mm). The residence time of the materials within the extruder was ~2 to 3 minutes. The extruded films were cooled to room temperature by passing over a chill roll, which is shown in Fig. 1. The films were measured for thickness using an electronic digital caliper. Films were then rolled, labeled, and stored in 5-mil foil-lined polypropylene bags (1 mil = 0.001 inches).
Fig. 1.

TF 207 Pharma Chill Roll 24 mm (Courtesy of ThermoFisher Scientific)
Content Uniformity Determinations
Content uniformity determinations were produced on hot-melt extruded films containing miconazole. Random portions (n = 3) of each film was weighed and dissolved in a known volume of methanol by sonicating it for 10 min. The resulting solutions was filtered and analyzed for the amount of drug remaining utilizing the high performance liquid chromatography (HPLC) method developed. The purpose of this experiment was to determine whether the drug content is uniform in polymeric matrix systems.
Thermo Gravimetric Analysis
Thermo gravimetric analysis (TGA) was recorded using a Perkin-Elmer Pyris-1 TGA instrument. To assess the thermal stability, the pure polymer, miconazole, physical mixtures (with and without miconazole), and miconazole in hot-melt extruded films prepared at different temperatures (160, 175, 190, 205°C) were used as samples to determine the stability of the formulation as a function of weight loss. Samples of 2–3 mg were loaded into the pan and were monitored from 25°C to 220°C at 10°C /min. Volatiles were removed from samples by storing powders under vacuum with desiccants at 25°C for 72 h prior to thermal studies. The test was terminated when no significant loss of weight (< 0.1%/hr) was observed.
X-Ray diffraction Analysis
The crystallinity of the drug and excipients in the hot-melt extruded films and corresponding physical mixtures was analyzed using X-Ray diffraction (XRD). The studies were performed on an X-Ray Diffractometer (Rigaku D/Max 2000, Japan) equipped with Cu Kα radiation and the Jade 5.0 software program. The generator voltage and current were 40 kVand 30 mA, respectively. The 2-theta scanning range was from 5 to 50° at a rate of 5°/min. The step size was 0.02°, and the dwell time at each step was 1 second. The extrudates were ground into fine powder before analysis.
Bioadhesion Studies
Bioadhesive parameters including peak adhesive force (PAF), area under the curve (AUC) were recorded utilizing a TA.XT2i Texture Analyzer™ (Texture Technologies Corp., Scarsdale, NY/Stable Micro Systems, Godalming, Surrey, UK) equipped with Texture Expert™ software. Elongation at adhesive failure and post-peak area under the curve were also calculated to compare the stringiness and cohesiveness of the films, respectively. Porcine buccal mucosa was used as a biological substrate. The polymeric matrices incorporated with miconazole (n = 5) were wetted with simulated artificial saliva (2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl per liter of nanopure water adjusted with phosphoric acid to pH 6.75). Different contact times on the optimal formulation from the above F1, F2, and F3 were all studied and placed on the lower base of the instrument. The mucosal substrate was attached to the probe with a cyano-acrylate adhesive and equilibrated with the artificial saliva before the bioadhesion testing. The probe lined with mucosa was set to approach the sample with a predetermined speed of 1 mm/s and applied a force of 3.5 N. The test speed was 0.1 mm/s. These parameters were chosen based on previous studies(10). The influence of the following variables on bioadhesion was studied:
- Different formulation
Formulation F1 F2 F3 F4 HPC JF (% wt/wt) 75 55 35 55 PEO 750 N–750 (% wt/wt) 15 35 55 45 Miconazole (% wt/wt) 10 10 10 0 Different processing temperatures: 160, 175, 190, and 205°C
Different extruder screw speed: 30,50, and 70 rpm
Different contact times: 15, 30, 60, and 120 s
In Vitro Release Studies
In vitro release studies (n = 3) were tested using a Hanson SR8-Plus dissolution test system according to the US Pharmacopeia 31 apparatus 5, paddle-over-disk method. The effect of different processing temperatures, different extruder screw speed, and formulation factors on the release of miconazole was investigated. 900 mL of 1% wt/vol sodium lauryl sulfate (SLS) aqueous solution at 37°C was used as the dissolution medium and the paddle rotation speed was 50 rpm. Samples were collected at predetermined time intervals (0.5, 1, 2, 3, 4, 6, 8, and 10 h) and replaced with an equal volume of the fresh dissolution medium. The samples were filtered using a 0.45-micron nylon syringe filter to remove insoluble excipients, and analyzed by HPLC. In addition, the effect of different drug content (0%, 2.5%, 5%, 10% and 15%) was investigated.
HPLC Analysis
Samples were analyzed for drug content using a Waters 600 pump and a dual wavelength Waters 2,487 UV detector (Waters Corp, Milford, MA). The column was a Luna 5 μ C-18 (2), 150 × 4.60 mm column (Phenomenex, Torrance, CA). The temperature of the column was maintained at 25°C. The mobile phase contained a mixture of 0.5% Ammonium acetate: acetonitrile: methanol = 10%: 45%: 45%. The flow rate was maintained at 1.0 mL/min. The injection volume was 20 uL and the detection wavelength was 230 nm. The data were collected and integrated using Empower® Version 5.0 software. Linearity was demonstrated from 25 to 500 mg/mL (r2 ≥ 0.9993) and the relative standard deviation of six injections was less than 1%.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel® and a Student’s t-test was used to analyze the results. A p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Content Uniformity Determinations
The amount of miconazole remaining in hot-melt extruded films could be improved with formulation strategies (Fig. 2). Miconazole incorporated into the film formulations F2 & F3 was not significantly decreased after 3 months.
Fig. 2.
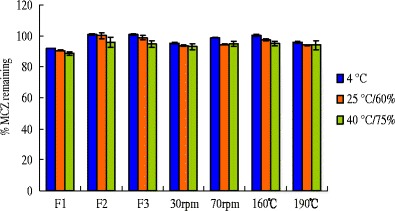
Amount of MCZ in HME films stored under different conditions for 3 months
Thermo Gravimetric Analysis
Thermo Gravimetric Analysis (TGA) was utilized to determine the maximum processing temperatures since this method indicates thermal stability by measuring weight loss due to decomposition as a function of temperature. The thermo gravimetric profiles of the pure polymer, miconazole, physical mixtures with miconazole, and miconazole in hot-melt extruded films prepared at different temperatures are illustrated in Fig. 3. It was observed that formulation components did not demonstrate weight loss at the processing temperatures used in the extrusion experiments which ranged from 160 to 205°C. Miconazole exhibited a little weight loss in the thermogram after 200°C. This indicated that the formulation components are likely to be thermally stable under the conditions of high temperatures used during hot-melt extrusion for incorporation of MCZ into polymeric matrix systems. TGA can provide a working temperature range, so that the stability of MCZ may be greatly improved during processing.
Fig. 3.
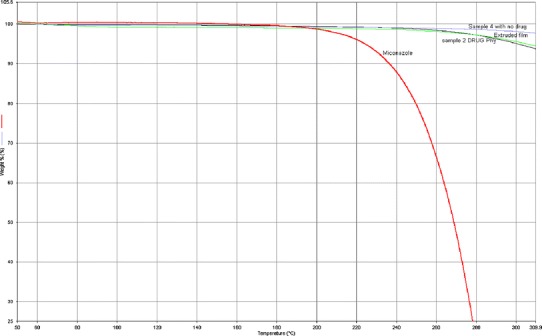
Thermo gravimetric analysis of formulation components. a pure polymer (HPC JF and PEO 750). b Pure miconazole. c Physical mixtures with miconazole. d Miconazole in hot-melt extruded films prepared at 160, 175, 190, and 205°C
X-Ray diffraction Analysis
As shown in the Fig. 4, X-Ray diffraction (XRD) studies were performed on the pure polymer PEO 750 and HPC JF, miconazole, physical mixtures, and miconazole in hot-melt extruded films stored for 8 months to further study the crystallinity of the drug at room temperature. Two peaks were observed in PEO 750 polymer as it is a kind of semi-crystalline polymer, while there is no peak from HPC JF poly as it is an amorphous polymer. There is a high degree of crystallinity found in the pure miconazole with primary peaks. These primary peaks were also observed in the physical mixture but no crystalline pattern corresponding to miconazole was found within HME films. It is probably that miconazole is in a molecular dispersed state within the extruded film. Miconazole incorporated into HPC JF films was found to be stable as no recrystallization was observed in 4, 6, and 8 months HME films.
Fig. 4.
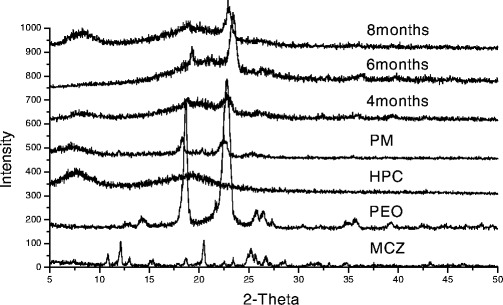
XRD patterns of HME films under different storage times
Bioadhesion Studies
Influence of Different Formulations
Two primary bioadhesive parameters, peak adhesive force (PAF) and the area under the curve (AUC), at different contact time intervals (15, 30, 60, and 120 sec) are depicted in Fig. 5. Miconazole was incorporated in HPC JF and PEO 750 matrices at 10% (w/w). Although these three formulations (F1, F2, F3) consisting of the same polymer, their chemical nature, molecular structure as well as their hydration status are the same, different ratios of polymers exhibited different bioadhesion properties. At contact time of 15 seconds, there was no significant effect in peak adhesive force and work of adhesion with different formulations.
Fig. 5.
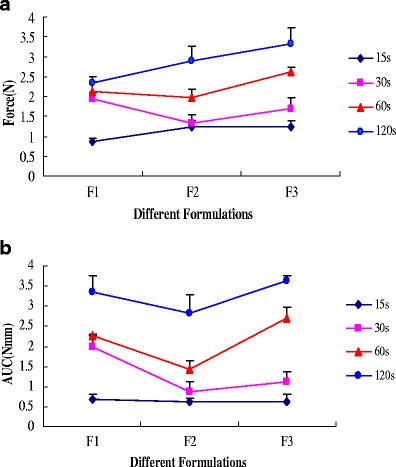
Influence of different formulations on a peak adhesive force and b work of adhesion of HME films containing miconazole
As compared to the control (F1), AUC and PAF increased when addition of PEO 750 in the matrix system (55%) at contact time intervals of 30, 60, and 120 sec. As the PEO 750 polymer concentration was increased, the number of penetrating polymer chains per unit volume of mucus increased, which resulted in the formation of a stronger adhesive bond. The linear flexible chains of the PEO 750 molecule have extremely high segmental mobility due to the ether linkages, which make for a very flexible backbone, and hence penetration into the substrate networks is deep and relatively rapid. This inter-penetration results in an intimate contact and hence enhances bioadhesion. However, AUC decreased with a corresponding increase in PEO 750 concentration (35%) at contact time intervals of 30, 60, and 120 sec. This can be explained by the fact that bioadhesion properties are not only due to the polymer, but also some other reasons. For example, bond formation between the polymers and mucus glycoproteins, secondary chemical bonds formed by electrostatic and vander Waals attraction, as well as hydrogen and hydrophobic bonds, are potential factors.
Effect of Drug Loading
Bioadhesion strength can be increased or decreased by incorporation of a hydrophobic drug into polymeric matrices since hydrophobic drug can alter the chain mobility and swelling rate of the bioadhesive polymer, thus retard or enhance the formation of intimate contact between the mucosal and the adhesive surfaces. The influence of miconazole on peak adhesive force and work of adhesion of the films at different contact time intervals is illustrated in Fig. 6. The addition of miconazole increased AUC at contact time intervals of 30 and 60 sec as compared to the control group (0% miconazole). Miconazole may exist in between the polymer chains allowing each chain to hydrate freely, which may result in weak hydrogen bonding areas around the miconazole molecules. These areas may increase the strength of the swollen layer followed by an obvious increase in the amount of penetrated water. Miconazole may increase the polymer chain mobility in bioadhesive matrices, resulting in increased interpenetration and entanglement of the polymeric chains with the mucin molecules.
Fig. 6.
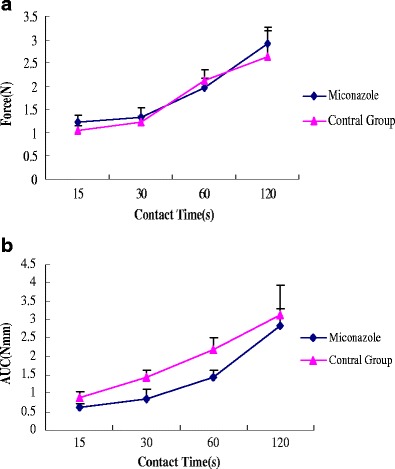
Influence of drug loading on a peak adhesive force and b work of adhesion of HME films containing miconazole
For the films tested at contact time intervals of 15, 30, 60, and 120 sec, drug loading had no significant effect on work of adhesion of HME films containing miconazole and the films without miconazole. This can be explained by the fact that at 10% drug concentration was insufficient to impact adhesion and therefore the work of adhesion remained unchanged. It was observed that the bioadhesion increased with an increase in contact time. These results are consistent with our lab previous studies (12). These findings indicated that the contact time and the drug loading can influence the bioadhesion of polymeric matrices.
Different Processing Temperatures
Various instrument and formulation parameters such as extrusion temperature, extrusion screw speed and moisture content in the formulation are important factors that need to be optimized based on the polymers and drug that are being extruded. A temperature range depends on the molecular structure of the polymer and is associated with the onset of a thermal degradation. It can lead to poor film properties, poor content uniformity, and cause stability issues to the drug and polymer. The temperatures used for extrusion of the drug were essentially below the melting point of the actives and above the softening/melting point of polymer.
As depicted in Fig. 7, the bioadhesion decreased with an increase in processing temperatures. At the processing temperatures of 205°C, there was no significant effect in peak adhesive force and work of adhesion at different contact time. This result can be explained by the fact that PEO 750 transitioned from extended chain crystallites to the folded chain crystallites from upon hot-melt extrusion and then slowly transitioned back into the extended chain crystallites.
Fig. 7.
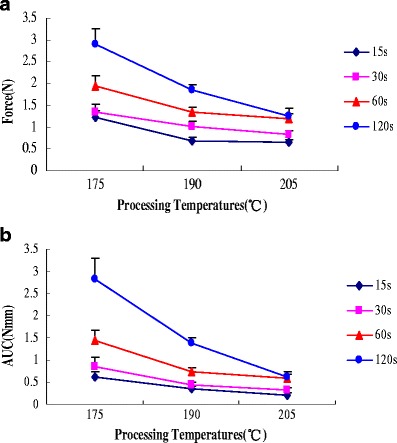
Influence of processing temperatures on a peak adhesive force and b work of adhesion of HME films containing miconazole
Different Extruder Screw Speed
Screw speed is a critical extrusion process variable that often dictates the energy, the degree of mixing, the melt temperature and sometimes the melt pressure of the extrusion process. Screw speed is one of the parameters that controls the residence time of the material inside the heated barrel and thus affects the chemical stability of thermolabile drugs, as well as homogeneity of the extrudate.
Figure 8 presents the influence of extruder screw speed on peak adhesive force and work of adhesion of HME films containing MCZ at four different contact times (15, 30, 60, and 120 sec). It was observed that bioadhesion increased with an increase in contact time. The influence of extruder screw speed on bioadhesion was less than that of contact time. These results may be explained by the following: contact time between the bioadhesive and mucus layer determines the extent of swelling and interpenetration of the bioadhesive polymer chains, and a longer contact time permits sufficient time for the polymers to uncoil and penetrate into the mucus network to a greater extent, thus resulting in the formation of a stronger bond between the two.
Fig. 8.
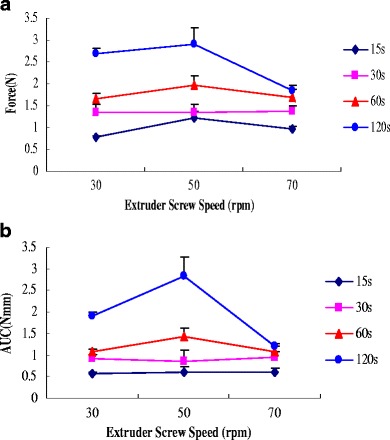
Influence of extruder screw speed on a peak adhesive force and b work of adhesion of HME films containing miconazole
In Vitro Release Studies
The release profiles suggest that a sustained release of miconazole was observed from all of the tested HME film formulations into the SLS dissolution media for up to 10 h. Drug load is an important factor, which can influence drug release profiles. The influence of miconazole content on the release rate of miconazole from HME film is presented in Fig. 9. The release characteristics of miconazole with different concentrations did not exhibit dose-dependent behavior. In summary, there are no significant differences in the miconazole release among these formulations.
Fig. 9.
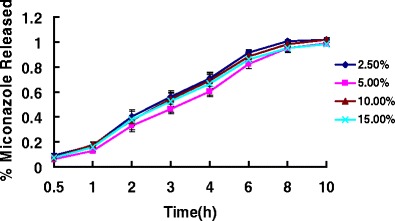
Release profiles of miconazole-containing HME films with different drug loadings
To determine the influence of processing parameters and formulation factors, different processing temperatures and different extruder screw speeds were investigated in these extrudates on the mechanisms and kinetics of miconazole release (Figs. 10 and 11). Processing temperatures had a significant impact on the drug release properties of HME films containing miconazole. The release from HME films produced at 205°C was significantly different from those extruded at 190°C. This can be explained in terms of the transition of PEO 750 from extended chain crystallites to the folded chain crystallites from upon hot-melt extrusion techniques. Thus, miconazole demonstrated somewhat faster release at the processing temperatures of 205°C than 190°C due to higher permeation of the films produced at the higher temperature.
Fig. 10.
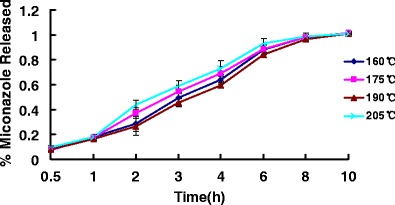
Release profiles of miconazole-containing HME films with various processing temperatures
Fig. 11.
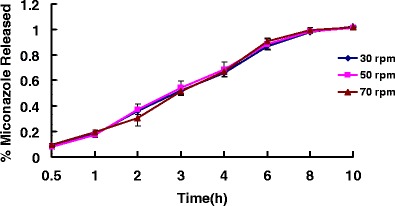
Release profiles of miconazole-containing HME films with different extruder screw speeds
The influence of extruder screw speed on the drug release properties was not shown to be significant. Screw speed is a critical extrusion process variable that often dictates the energy, the degree of mixing, the melt temperature and sometimes the melt pressure of the extrusion process. Screw speed is also one of the parameters that controls the residence time of the material inside the heated barrel and thus affects the chemical stability of thermolabile drugs, as well as homogeneity of the extrudate. However, in the same formulation prepared at the same temperature, the same polymer molecular weight and ratio may be a more important factor in determining drug release rates.
CONCLUSION
The hot-melt extruded films containing miconazole demonstrated sustained release. The extrusion temperature and polymer molecular weight were the critical variables influencing the drug release rate of miconazole from the HME films. Additionally, the bioadhesive properties of hot-melt extruded films containing miconazole were influenced by formulation factors such as polymer blend ratio, different processing variables such as extrusion temperature, extruder screw speed and contact time with the mucosal tissue. Based on the results obtained, the prepared hot-melt extruded films containing miconazole proved promising for further development of an ADA System for oral candidiasis.
Acknowledgments
This work was supported by the project of Research Center of Drug-Delivery Systems in Universities of Guangdong Province (No.: GCZX-A0802). In addition, this research was supported by Grant Number P20GM104931 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH.
References
- 1.Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS. Miconazole: a review of its antifungal activity and therapeutic efficacy. Drugs. 1975;9(6):406–23. doi: 10.2165/00003495-197509060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Chang HT, Chen WC, Chen JS, Lu YC, Hsu SS, Wang JL, et al. Effect of miconazole on intracellular Ca2+ levels and proliferation in human osteosarcoma cells. Life Sci. 2005;76(18):2091–101. doi: 10.1016/j.lfs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Devaraj A, O’Beirne JP, Veasey R, Dunk AA. Interaction between warfarin and topical miconazole cream. Bmj. 2002;325(7355):77. doi: 10.1136/bmj.325.7355.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammed FA, Khedr H. Preparation and in vitro/in vivo evaluation of the buccal bioadhesive properties of slow-release tablets containing miconazole nitrate. Drug Dev Ind Pharm. 2003;29(3):321–37. doi: 10.1081/DDC-120018206. [DOI] [PubMed] [Google Scholar]
- 5.Hynninen VV, Olkkola KT, Neuvonen PJ, Laine K. Oral voriconazole and miconazole oral gel produce comparable effects on the pharmacokinetics and pharmacodynamics of etoricoxib. Eur J Clin Pharmacol. 2009;65(1):89–95. doi: 10.1007/s00228-008-0556-9. [DOI] [PubMed] [Google Scholar]
- 6.Mandal TK. Swelling-controlled release system for the vaginal delivery of miconazole. Eur J Pharm Biopharm. 2000;50:7. doi: 10.1016/S0939-6411(00)00124-7. [DOI] [PubMed] [Google Scholar]
- 7.Bioadhesion of Carbopol polymers, BF Goodrich Specialty Chemicals, Cleveland, Ohio. 1994: 7.
- 8.Repka MA, McGinity JW. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J Control Release. 2001;70(3):341–51. doi: 10.1016/S0168-3659(00)00365-5. [DOI] [PubMed] [Google Scholar]
- 9.Repka MA, ElSohly MA, Munjal M, Ross SA. Temperature stability and bioadhesive properties of delta9-tetrahydrocannabinol incorporated hydroxypropylcellulose polymer matrix systems. Drug Dev Ind Pharm. 2006;32(1):21–32. doi: 10.1080/03639040500387914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thumma S, Majumdar S, ElSohly MA, Gul W, Repka MA. Chemical stability and bioadhesive properties of an ester prodrug of 9-tetrahydrocannabinol in poly(ethylene oxide) matrices: Effect of formulation additives. Int J Pharm. 2008;362:7. doi: 10.1016/j.ijpharm.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repka MA, McGinity JW. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials. 2000;21(14):1509–17. doi: 10.1016/S0142-9612(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 12.Prodduturi, S., K.L. Urman, J.U. Otaigbe and M.A. Repka, Stabilization of hot-melt extrusion formulations containing solid solutions using polymer blends. AAPS PharmSciTech, 2007. 8(2): Article 50. [DOI] [PMC free article] [PubMed]


