Abstract
Nanoemulsion dosage form serves as a vehicle for the delivery of active pharmaceutical ingredients and has attracted great attention in drug delivery and pharmacotherapy. In particular, nanoemulsions act as an excellent vehicle for poorly aqueous soluble drugs, which are otherwise difficult to formulate in conventional dosage forms. Nanoemulsions are submicron emulsions composed of generally regarded as safe grade excipients. Particle size at the nanoscale and larger surface area lead to some very interesting physical properties that can be exploited to overcome anatomical and physiological barriers associated in drug delivery to the complex diseases such as cancer. Along these lines, nanoemulsions have been engineered with specific attributes such as size, surface charge, prolonged blood circulation, target specific binding ability, and imaging capability. These attributes can be tuned to assist in delivering drug/imaging agents to the specific site of interest, based on active and passive targeting mechanisms. This review focuses on the current state of nanoemulsions in the translational research and its role in targeted cancer therapy. In addition, the production, physico-chemical characterization, and regulatory aspects of nanoemulsion are addressed.
KEY WORDS: cancer, homogenization, microenvironment, microfluidization, nanoemulsion, size distribution, targeted delivery
INTRODUCTION—APPLICATIONS OF NANOEMULSIONS IN DRUG DELIVERY
The advent of combinatorial chemistry and high-throughput screening methods based on molecular understanding of diseases have allowed for rational design of novel potent therapeutic agents. Despite of rational design and rapid screening process, the number of drugs reaching the market has not increased dramatically. Many of these highly promising agents are dropped from the development pipeline because of their poor aqueous solubility. About 40% of newly discovered drugs are highly hydrophobic and fail to reach market due to their poor aqueous solubility (1). In recent years, nanoemulsions, a heterogeneous liquid dispersion of nanoscale droplets of one liquid within another, have started evolving as a feasible carrier for the delivery of hydrophobic drugs. Their high solubilization capacity of hydrophobic drugs, ease of production, and long-term stability make nanoemulsions as promising drug delivery systems (2–9). Nanoemulsion dosage forms have found wide applications in oral drug delivery to enhance the solubility and bioavailability of the hydrophobic drugs (4,10,11). Recently, there has been a surge in the exploration of nanoemulsions for parenteral drug delivery for the treatment of complex diseases such as cancer therapy (2,3,5–9,12). The capacity of nanoemulsion to solubilize large amounts of hydrophobic drugs and their ability to protect the drugs from enzymatic degradation and hydrolysis make them ideal platform for the purpose of parenteral drug delivery. Furthermore, they can be exploited for image-guided drug delivery by utilizing targeting and imaging components (7,13). They are also being investigated widely for potential applications in transdermal delivery (11), ophthalmic (14), and pulmonary (15) drug delivery.
Nanoemulsion is a heterogeneous system in which the oil phase is dispersed as droplets in an aqueous phase and stabilized by emulsifying agents (10,16). Emulsifying agents are amphiphilic surface-active molecules or surfactants that can reduce interfacial tension between two immiscible liquid phases of oil and water by preferentially adsorbing at their interfaces (Fig. 1). Emulsifying agents have non-polar hydrocarbon tails that prefer to be in non-polar liquids, such as oils, and polar or charged head groups that prefer to reside in polar liquids, such as water (Fig. 1). Their droplet size is usually between 50 and 200 nm basing on the composition and production methods. The system can exist as oil-in-water and water-in-oil form, where the dispersed phase is either oil or water, respectively. Nanoemulsions are commonly prepared from Generally Recognized as Safe (GRAS) grade excipients approved by the United States Food and Drug Administration (FDA). Nanoemulsions are easily produced in large quantities by high shear stress or mechanical extrusion process that is available worldwide.
Fig. 1.
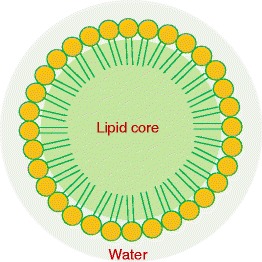
A schematic showing an internal arrangement of oil-in-water nanoemulsion delivery system
A number of drug-containing nanoemulsion dosage forms have been introduced in the pharmaceutical market and several others are under preclinical and clinical stage of development (Table I). Nanoemulsions not only serve as excellent vehicles for drug encapsulation, they also mitigate the toxicities associated with surfactant and ethoxylated castor oil (Cremophor®EL)-based formulations. Cremophor®EL is associated with nephrotoxicity, hypotension, and bronchospasms and can produce anaphylactic reaction. For example, propofol injectable solution was prepared in Cremophor®EL by Imperial Chemical Industries (ICI) was gone into clinical usage but later withdrawn due to toxicity of Cremophor®. Propofol was reformulated in oil-in-water emulsion using GRAS-grade excipients such as soybean oil, glycerol, egg lecithin, and disodium edetate and launched with the trade name Diprivan® by ICI (now Astra Zeneca) (17). Diprivan® is used in intensive care medicine as a short-acting, intravenous sedative and known to have low toxicity, controlled sedation effect, rapid onset, and quick recovery despite prolonged usage (17). Soybean oil- and safflower oil-based emulsions have been widely used in the clinic as parenteral nutrition (e.g., Intralipid®). These lipids provide a rich source of essential fatty acids such as omega-3 and omega-6 PUFA, non-glucose-based calories, and vitamins E and K. Similarly, a Cremophor® EL-free formulation of paclitaxel was prepared using TOCOSOL nanoemulsion to overcome anaphylactic reactions associated with Cremophor® formulation. TOCOSOL paclitaxel formulation was approved by FDA in 2003 for the treatment of nonsuperficial urothelial cancer. In another example, a Cremophor®EL/ethanol-based cyclosporine (Sandimmune® Injection) was reformulated into nanoemulsion using soybean oil and egg lecithin composition. The studies indicated that a change in the vehicle may reduce the acute nephrotoxic side effects associated with cyclosporine in the Cremophor®EL formulation (18). Alprostadil palmitate, amphotericin B, dexamethasone, flurbiprofen axetil, and vitamins A, D, E, and K are some other examples of therapeutically relevant compounds that have been formulated in nanoemulsions for clinical applications.
Table I.
Commercially Available Nanoemulsion (Sub-micron Emulsion) Drug Delivery Systems
| Drug | Marketed name | Manufacturer | Indication |
|---|---|---|---|
| Alprostadil palmitate | Liple® | Mitsubishi Pharmaceuticals | Vasodilator, platelet inhibitor |
| Clevidipine | Cleviprex | The Medicines Company | Calcium channel blocker |
| Dexamethasone palmitate | Limethasone | Mitsubishi Pharmaceuticals | Steroid |
| Diazepam | Diazemuls | Kabipharmacia | Sedative |
| Flurbiprofen axetil | Lipfen | Green Cross | Nonsteroidal analgesic |
| Flurbiprofen axetil | Ropion | Kaken Pharmaceuticals | Nonsteroidal analgesic |
| Propofol | Diprivan | Astra Zeneca | Anaesthetic |
| Vitamins A, D, E, K | Vitalipid® | Fresenius Kabi | Parenteral nutrition |
Another interesting and active development is the use of nanoemulsion for the targeted drug delivery to cancer. Because of the nanometer oil droplet size, they can easily be targeted to the tumor tissue using targeting moieties on the surface of nanoemulsion. However, it is important that the nanoemulsions do not change size after administration during the blood circulation. Recent studies indicate that they have received wide attention as colloidal carriers for targeted delivery of several anticancer drugs (2,5,7,12) and diagnostic agents (13,19). Multifunctional nanoparticles, as envisioned by Ferrari (20) and other researchers (21,22), combine the following structural components: (1) core material for drug encapsulation (2), and surface modification for passive or active targeting and (3) imaging component for disease visualization. Along these lines, we have conceptualized nanoemulsions as a multimodal platform (Fig. 2) for target-specific and image-guided drug delivery for the cancer therapy (7,13,23).
Fig. 2.
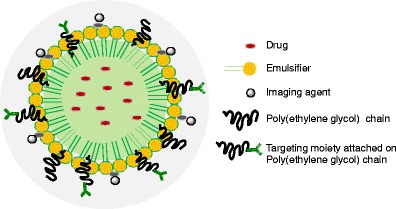
A schematic representation of multifunctional nanoemulsion system for image-guided drug delivery to the cancer
NANOEMULSIONS IN CANCER THERAPY—OPPORTUNITIES AND CHALLENGES
Exploiting Tumor Microenvironment
The tumor microenvironment is composed of the extracellular matrix, activated fibroblasts, immune cells, pericytes, adipocytes, epithelial cells, glial cells, vascular cells, endothelial cells, and proteins (24). These components of the tumor microenvironment support the physiology, structure, and function of the tumor nurturing an environment that promotes the tumor to progress into a malignant phenotype. As the tumor microenvironment supports cancer growth, there are significant differences such as angiogenesis, vascular abnormalities, oxygenation, perfusion, pH, and metabolic states which can be exploited for the delivery of therapeutic agents to tumor tissue (Fig. 3).
Fig. 3.
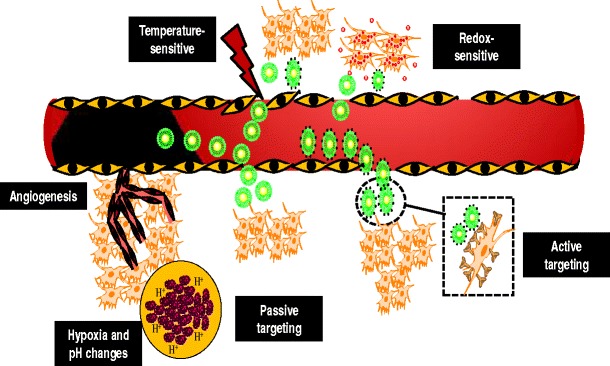
An illustration showing nanoemulsion-based drug delivery exploiting tumor microenvironment and passive and active targeting mechanisms
Angiogenesis is a physiological mechanism which promotes formation of new blood vessels from existing ones. In small-sized solid tumors, oxygen and nutrients reach the tumor cells by simple diffusion; however, once the tumor size reaches over 2 mm3, the core of the tumor is exposed to hypoxic conditions (25). In order to survive in these conditions, cancer cells stimulate angiogenesis to maintain presence of oxygen and nutrients for further growth. In 1971, an American medical scientist and biomedical pioneer, Judah Folkman hypothesized that, as cancer cells are dependent on the process of angiogenesis for the supply of oxygen and nutrients, if this process was inhibited, there was a potential for treating cancer (26). Following his observation and subsequent work, several natural and synthetic angiogenesis inhibitors were identified, and many were clinically utilized for cancer therapy including bevacizumab (Avastin®), sorafenib (Nexavar®), sunitinib (Sutent®), pazopanib (Votrient®), and everolimus (Afinitor®). Several of these angiogenesis inhibitors have been reported to have toxicity or delivery problems (27). Nanoemulsion delivery systems can be used to encapsulate these angiogenesis inhibitors providing reduced toxicity and improved therapeutic efficacy. Betulinic acid is a pentacyclic triterpene which has the ability to target tumor cells and also vascular endothelial cells, thus functioning as an angiogenesis inhibitor. Dehelean et al. recently assessed the in vivo anti-angiogenic and anti-inflammatory effects of betulinic nanoemulsion (8). Based on this work, it was identified that betulinic acid could be formulated in a nanoemulsion delivery system to attain potent anti-inflammatory activity with reduced exposure of the betulinic acid to vital organs. Similarly, fumagillin is a mycotoxin which has demonstrated antiangiogenic properties; however, its use has been associated with neurotoxicity at systemic doses. Winter et al. utilized αvβ3-targeted perfluorocarbon fumagillin-loaded nanoemulsion to suppress neovasculature, reduce exposure to clearance organs, and prevent neurocognitive dysfunction (28). Therapeutic efficacy studies indicated that following encapsulation in nanoemulsions the systemic dose of fumagillin was 1,000-fold lower in animal studies and 60-fold lower in relation to clinically tested related anti-angiogenic compounds such as TNP-470. Thus, indicating that nanoemulsion based delivery systems can be used to overcome some of the shortcomings of potential angiogenesis inhibitors exploiting a phenomenon which is highly exhibited in the tumor microenvironment compared with normal tissues.
The pH of the tumor microenvironment is another unique characteristic which can be exploited for tumor targeted delivery. The extracellular tumor pH is between 6 to 7 in comparison to normal tissue and blood with a pH of 7.4. The dependence of tumor cells on glycolysis as a primary mechanism of generating cellular energy leads to the production of lactate as a by-product. This by-product is eliminated from tumor cells via the monocarboxylate transporter system using a proton which leads to acidification of the tumor interstitium. Likewise in hypoxia, overexpression of carbonic anhydrase IX leads to conversion of CO2 to bicarbonate which leads to subsequent uptake of the weak base into the intracellular environment. This net effect of the H+ and bicarbonate flux creates pH gradients between the intracellular and extracellular tumor microenvironments, providing a unique feature in tumor cells which can be exploited for delivery of therapeutic agents. Shen et al. recently reported dual-drug delivery by conjugating camptothecin to short polyethylene glycol (PEG) chains via an ester bond forming liposome-like nanocapsules which were encapsulated with doxorubicin. At a pH < 5 or following addition of esterases, a rapid release of doxorubicin and camptothecin was observed which translated into increased anti-tumor efficacy in vivo (29). Apart from the pH in the microenvironment, following intracellular uptake nanocarriers are exposed to intracellular pH of 5.9–6.2 in early endosomes and 5.0–5.5 in late endosomes and lysosomes. In literature, several authors have reported development of pH-sensitive tumor-targeted nanoparticles which have shown improved anti-tumor efficacy and reduced chemotherapy related toxicity profile (30–32). Nanoemulsions can also be formulated with lipid components that respond to tumor microenvironment and release its payload. The lipid components with pH-sensitive and thermo-sensitive properties are discussed elsewhere in this review.
Passive Targeting
The dependence of tumor tissue on angiogenesis for survival and metastasis leads to development of poorly developed tumor vasculature with pericyte deficiency, an aberrant basement membrane and fenestrations which induce increased vascular permeability at the tumor site (Fig. 3). Likewise, tumor tissues often have absent or non-functional lymphatic vessels which lead to poor lymphatic drainage from the tumor site. The combination of the enhanced vascular permeability and poor lymphatic drainage is a phenomenon which has been coined as the enhanced permeability and retention (EPR) effect and has been a gold standard for delivery of several nano-therapeutic agents to tumor tissues (33). Majority of the solid tumor exhibit a vascular pore size between 380 and 780 nm; however, this depends on the type of tumor, growth rate, and microenvironment. An optimal size between 10 and 100 nm is critical to achieve maximal therapeutic accumulation in the tumor microenvironment using the EPR effect (25). Nanoparticles with size less than 400 nm easily extravasate into the tumor tissue; however, particles below 10 nm undergo rapid renal filtration, and particles larger than 100 nm get easily recognized by the cells of the mononuclear phagocyte system (MPS) (34). The surface charge of the nanoparticles is also a critical component of the nano-delivery system and should be neutral or anionic to evade clearance.
In literature, several authors have explored improved therapeutic activity and minimization of adverse effects by formulating therapeutic agents in nanoparticles which take advantage of the EPR effect. Some systems have already been successfully translated into clinical practice (35,36). In relation to nanoemulsions, researchers typically report a size of less than 200 nm (37) and a negative surface charge (38,39). A higher negative charge on a nanoemulsion is also known to prevent droplet coalescence during formulation development, and in vivo, it is known to affect blood–brain barrier (BBB) permeability (38,39). Apart from size and surface charge, the circulation time also plays a crucial role to determine the fate of the nanoemulsion delivery systems. Nanoemulsions usually have a short circulating half-life due to rapid opsonization by MPS cells. Hence, the surface of nanoemulsion is often coated with hydrophilic polymers such as PEG, poloxamers, or poloxamine to suppress blood protein adsorption and recognition by MPS cells which leads to an increase in circulation half-life and improved therapeutic efficacy (36). Incorporating longer PEG chains and post-insertion of distearoylphosphatidylethanolamine (DSPE)-PEG 2000 or 5000 provided nanoemulsions with longer residence time and improved tumor accumulation (40,41). Thus, indicating a combination of nanoemulsion size, surface charge and surface hydrophilicity can be modified to achieve nanoemulsion efficacy by passive targeting of nanoemulsion to the tumor sites.
Active Targeting
Active targeting of nanoemulsions is achieved by attaching a component to the nanoemulsion surface that recognizes a target within the tumor-affected organ, tissue, cell, or intracellular organelle, leading to preferential accumulation of the nanoemulsion. In comparison to passive targeting, active targeting of nanoemulsion not only exploits the physiological benefits offered by the tumor microenvironment, but it also enables delivery specifically to the tumor tissue (Fig. 3). This is particularly valuable for therapeutic agents that are not internalized efficiently, require facilitation by fusion, endocytosis, or other process. Active targeting of the therapeutic agent also enables larger payloads to be delivered intra-cellularly by receptor-mediated uptake of the nanoemulsion systems. By conjugating ligands to the carrier, the drug remains unmodified enabling the drug to exhibit its therapeutic activity following intracellular uptake.
A variety of targeting ligands can be attached to the surface of nanoemulsions to enable recognition by appropriate receptors expressed at target site. It is critical to select receptors which are overexpressed on tumor cells but not on normal cells to enable selectivity for tumor tissue over normal cells. In literature, several authors have reported targeting receptors which are overexpressed on cancer cells including transferrin, folate, and epidermal growth factor receptor (EGFR) (7,42,43).
The EGFR family is composed of an extracellular ligand binding domain, a hydrophobic trans-membrane domain, and a cytoplasmic tyrosine kinase containing domain. EGFR is often abnormally activated in many epithelial tumors with up to 60% expression in ovarian cancer with this aberrant expression being associated with poor outcome (44,45). The concept of EGFR-based targeting of nano-carriers has been explored to improve cellular cytotoxicity, and in vivo, it has shown greater anti-proliferative, anti-angiogenic, and pro-apoptotic activity (43,46). Similarly, folic acid (FA) a low-molecular-weight vitamin is required by eukaryotic cells in the biosynthesis of nucleotide bases (purines and pyrimidines). The glycoprotein-based FA transport system is expressed at high levels on the surface of many cancer cells (especially tumor cells of the ovaries, mammary gland, colon, lung, prostate, nose, throat, and brain), which makes it a rational target for drug delivery to tumor tissues. A range of polymers with an improved biocompatibility have been used for the development of folate-targeted nano-carriers (47–50). Our group recently investigated EGFR- and FR-mediated targeting of PIK75, a phosphotidyl-inositol-3-kinase inhibitor in ovarian adenocarcinoma cell line, and observed that the targeted nanoemulsions enhanced intracellular uptake by receptor mediated uptake and improved cellular cytotoxicity compared with non-targeted nanoemulsions (7).
Imaging
Imaging is an important aspect of cancer care which provides real-time monitoring of cancer with minimal invasiveness and tissue destruction. Biomedical imaging is often used for prediction, screening, biopsy guidance, staging, prognosis, therapy planning, and guidance. Computed X-ray tomography, magnetic resonance imaging, and ultrasound have been traditional techniques of anatomical imaging. Ultrasound is commonly used as an external stimulus due to its accessibility, cost-effectiveness, and ability to be used in conjunction with multi-modal systems which can incorporate ultrasound contrast agents such as microbubbles. These microbubbles can be developed as perfluorocarbon (PFC) nanoemulsions. PFCs are synthetic organic compounds in which all or most of the hydrogen atoms have been replaced with fluorine atoms. The 19 F isotope of PFC’s is biologically and chemically inert and thus provides excellent sensitivity in vivo. Further to this, a wide variety of contrast agents or therapeutic agents can be encapsulated in or conjugated on PFCs due to which multi-modal imaging or delivery is a possibility with PFC nanoemulsions (51,52). When microbubbles are formulated as PFC nanoemulsions, upon intravenous administration, the nanoemulsions accumulate in the tumor by the EPR effect. Upon accumulation in the tumor, under the action of ultrasound, the nanodroplets can be converted to bubbles. When cytotoxic agents are incorporated in these nanoemulsions, post-droplet-to-bubble transition, the encapsulated drug is released with enhanced intracellular uptake and effective tumor regression (51,53,54). These nanoemulsions systems can also be actively targeted to the tumor tissue, achieving greater tumor accumulation. Bae et al. recently reported the development of bimodal imaging contrast agent, PFC/rhodamine nanoemulsions with MRI, and optical imaging capabilities (55). The nanoemulsions were also infused with folate which allowed localization into FR expressing tumors improving tumor detection and providing excellent signal sensitivity and high specificity. It was observed that folate–PFC/rhodamine nanoemulsions could be utilized for early detection of disease, accurate diagnosis, and targeted tumor therapy for clinical applications.
Apart from perfluorocarbon nanoemulsion use for image-guided therapy, Gianella et al. recently reported the development of multi-functional nanoemulsion with iron oxide nanocrystals for MRI, Cy7 for near-infra red fluorescence imaging, and prednisolone acetate valerate for therapeutic purposes. The multi-functional delivery system provided unique theranostic properties which could be applied for image-guided therapy in cancer (19), thus indicating the capability of multimode nanoemulsions system (Fig. 2) for therapeutic delivery and tissue imaging.
NANOEMULSION COMPOSITION
Composition of nanoemulsion is a key parameter governing the physico-chemical properties of the system and can be precisely tailored to design a delivery vehicle with desired characteristics. Most importantly, choice of material gives leverage in customizing the properties of the nanoemulsions such as imparting them with stimuli-responsive behavior or tethering ligands on their surface to develop target-specific delivery systems. The following section will focus on the composition of nanoemulsions and their effect on the physico-chemical properties.
General Components
Emulsions by definition are dispersion of two immiscible liquids where one liquid is dispersed as droplets in the continuous phase of other liquid, and when the droplet size reaches sub-micron scale, such emulsions are called as nanoemulsions (4,9,23). Oil and water are two most commonly used immiscible liquids for emulsion formation in general, but, from the perspective of drug delivery system, a nanoemulsion could be a core of non-polar material suspended in a polar environment. The non-polar lipophilic core could be comprised of mono-, di-, or triglycerol oils. Choice of oil is an important criterion to consider, since it impacts the loading of the therapeutic payload, size of droplets, chemical properties, and most importantly the stability of the nanoemulsion system itself (56). Nanoemulsions prepared using long-chain triglycerides (LCT) are found to be larger (D = 120 nm) in droplet size than those formed from shorter-chain triglycerides (SCT) (D = 40 nm) (57). Compared with larger droplet size, the smaller droplet size increases the nanoemulsion stability against gravitational separation, flocculation, or creaming owing to increased Brownian motion. However, the higher solubility of SCT oils in water renders the nanoemulsion prone to Ostwald ripening (Fig. 4) (58). Since Ostwald ripening process involves the movement of oil molecules from small droplets to larger droplets (Fig. 4), thus, Ostwald ripening occurs whereby large droplets grow at the expense of small droplets because the solubility of a material within a droplet increases as the interfacial curvature increases. Long-chain oils on the other hand show considerably low continuous-phase solubility due to their high molar volume (Vm) resulting in low tendency to undergo Ostwald ripening. A systematic study employing SCT and LCT oils shows that that the rate of Ostwald ripening decreases with increasing Vm (57). Size of the nanoemulsion also influences its optical property, such that the smaller the size of the nanoemulsion (r < < λ), higher is the optical transparency of the nanoemulsion system (59). Property of oil therefore is a trade-off between two contrasting stability-influencing factors that should be taken into account when designing nanoemulsion composition.
Fig. 4.
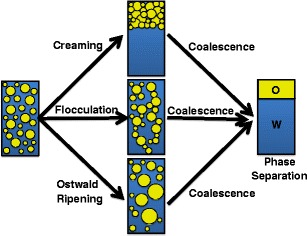
The main mechanism of nanoemulsion breakdown through Ostwald ripening
Emulsifiers or surface-active agents that stabilize the oil/water interface of an emulsion (Fig. 1) are yet another important component, which have tremendous impact on nanoemulsion stability. An ideal emulsifier should show three properties: (1) rapidly adsorb at the oil/water interface, (2) reduce the interfacial tension, and (3) stabilize nanoemulsion surface by stearic or electrostatic interactions. An emulsifier could be any amphiphilic molecule such as surfactants (e.g., Tweens, sodium dodecyl sulfate, etc.), phospholipids (e.g., egg, or soy lecithin, etc.), amphiphilic proteins (e.g., WPI, caseinate, etc.), polysaccharides (e.g., gum Arabic, modified starch, etc.), or polymer (e.g., PEG) that can adsorb at the interface, thereby contributing to nanoemulsion stability via steric stabilization (59). The choice of emulsifier not only impacts the stability of nanoemulsion but can also present with a possibility of tailoring their size and functional properties (60). PEG-modified nanoemulsion for example can be used for linking ligands exposed on nanoemulsion surface resulting in higher cell-specific targeting efficiency (7), as well as providing long circulating properties for in vivo drug delivery (61).
Other components that could be used for making nanoemulsions could be texture modifiers, weighting agents, or ripening retarders, which can impart important properties to the nanoemulsion. A texture modifier is any substance such as sugars, polyols, polysaccharides, and proteins that can improve the viscosity of the aqueous phase by crosslinking, giving it gel-like property and in the process prevents the likelihood of gravitational separation as well. A weighting agent on the other hand is a lipophilic entity that can be mixed to the oil phase to reduce the density contrast between oil and continuous phase and thus prevents gravitation separation (62). Finally, a ripening retarder is a highly lipophilic material that can be added to the oil phase to prevent or impede the process of Ostwald’s ripening (Fig. 4) (63).
Thermo-sensitive Lipids
Stimuli-responsive materials, which show change in their physicochemical properties in response to an external stimulus, are of great interest in drug delivery applications due to the versatility of drug loading and ability to trigger release the payload at will (64). A number of lipid-based thermo-responsive materials have been designed and applied as drug carriers in the preclinical studies for treatment of cancer (65). Thermo-sensitive nanoemulsion can also be made using thermo-sensitive lipids which can respond to external stimuli. Such systems undergo temperature-dependent phase transitions such as gel-to-liquid or lamellar-to-hexagonal states, thereby rendering the delivery systems leaky and resulting in release of the payload. A typical example is dipalmitoylphosphatidylcholine (DPPC), a commonly used thermo-sensitive lipid that undergoes phase transition at 41°C. Most importantly as observed in case of liposomal formulations (66), DPPC phase transition temperature can be readily modified by addition of co-lipids such as distearoylphosphatidylcholine (DSPC) or monopalmitoylphosphatidylcholine (MPPC).
Once the thermo-sensitive delivery system is administered into the body, its loaded content can be released at the site of action by an external induction of hyperthermia that could be mediated by ultrasound (67), light (68), heating coil (69), or magnetic field (70). Such triggered discharge of the payload enables a control over temporal dose of the drug and has proven to significantly improve the drug accumulation and therapeutic benefits. Given the advantages, thermo-responsive lipid-based delivery systems have been extensively researched and a plethora of work has been published using them not only in stand-alone drug delivery systems but also in multi-functional theranostic system with targeting, imaging, and therapeutic capabilities (71–75).
pH-Responsive Lipids
Desired properties of a delivery system are driven by the physiological milieu of their target site of action to achieve an efficient drug release. Low pH environment within tumors is a well-established fact and has been exploited to design internally controlled drug delivery systems. Delivery systems comprising of pH-responsive lipid are stable at the normal physiological pH of 7.4 but rapidly destabilized in the acidic environment in the tumor or in the endosomal compartment, resulting in the release of the loaded therapeutic moiety. Liposomes certainly are the most studied lipid-based systems for a variety of delivery applications and thus have been extensively explored as pH-responsive lipid nanocarriers as well. In this context, dioleoyl phosphatidylethanolamine (DOPE) is the most used lipid for preparing pH-responsive liposomes especially because of their inverted cone shape. Chloramphenicol acetyltransferase encoding gene loaded pH-sensitive immune-liposomes formulated using DOPE, cholesterol, and oleic acid with H-2Kk antibody as targeting ligand demonstrated significant enzyme activity in vivo in ascites–tumor-bearing mice (76). The same group also demonstrated that the immune-liposomal delivery system can be used for efficient targeted delivery of small-molecule antitumor drug to cells (77). A comprehensive review on pH-sensitive liposomes containing DOPE-based preparation has been published and is recommended to the readers for further insight. In a very recent work, a pH-responsive lyotropic liquid crystal delivery system composed of monolinolein and linoleic acid (97:3 wt% ratio) was reported that undergoes phase change from lm3m reverse bicontinuous cubic at pH 7 to HII reverse columnar hexagonal at pH 2 (78). The system shows a four times greater release kinetics at pH 7 than the HII phase, making it a suitable nanocarrier for oral delivery of drugs.
Acid-labile zwitterionic peptide lipid derivatives are another class of pH-responsive lipids that have been employed to devising fusogenic liposomes that facilitates uptake as well as drug release (79). Obata et al. used 1,5-hexadecyl N-glutamyl-l-glutamate and 1,5-hexadecyl N,N-diglutamyl-lysyl-l-glutamate as pH-responsive component of doxorubicin-loaded liposomes that could effectively deliver the drug at acidic endosomal pH in in vitro conditions and significant drug efficacy in treatment of human breast cancer tumor xenograft in BALB/c mice (80). Mo et al. have similarly used zwitterionic oligopeptide HHG2C(18)-L conjugate of 1,5-dioctadecyl-l-glutamyl 2-histidyl-hexahydobenzoic acid as multistage pH-responsive liposomes for targeting mitochondria of tumor cells (79).
NANOEMULSIONS PRODUCTION
The key unit operations involved in the production of typical nanoemulsion formulation is shown in the Fig. 5. Oil- and water-soluble ingredients are generally dissolved in the oil and aqueous phases, respectively. Emulsifier, such as phospholipids, can be dispersed in either oil or aqueous phase. Oil and aqueous phases are adequately heated and then mixed under controlled temperature and agitation to form a homogenously dispersed coarse emulsion. The coarse emulsion is then homogenized using external shear force to decrease the droplet size to desired range. The nanoscaled droplets which are formed as a result of external shear force display a lipophilic core separated from the surrounding aqueous phase by a monomolecular layer of emulsifiers (Fig. 1). The monomolecular layer or interfacial layer provides a mechanical barrier and offers repulsive forces to stabilize the nanoemulsion system. The repulsive forces can be electrostatic (e.g., phsopholipids such as lecithins), steric (e.g., block copolymers), or electrosteric (e.g., combination of both phospholipids and block copolymers) depending on the nature of emulsifier used in the formulation.
Fig. 5.
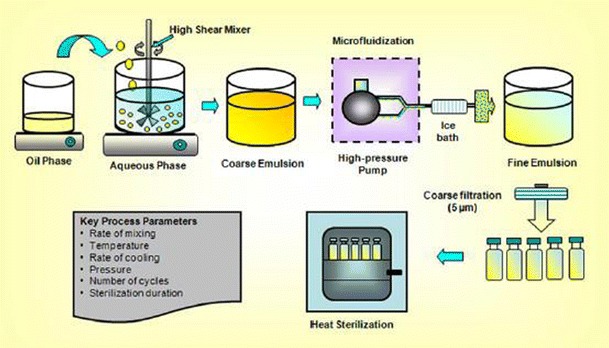
Key unit operations involved in the production of nanoemulsion formulations (adapted from 16, with permission from Springer)
The high shear methods producing intensely disruptive forces to form nanoemulsions can be achieved with high pressure homogenizer, microfluidizers, and ultrasonicators (5,9,81). The formation of nanoscaled droplets will depend on the type of instruments employed and their processing conditions like energy, number of cycles, time, and temperature, along with formulations composition. High-pressure homogenization techniques involve the use of high-pressure homogenizer or piston gap homogenizers to produce low particle size. During high-pressure homogenization process, several forces such as hydraulic shear, intense turbulence, and cavitation forces act together to yield nanoemulsions with extremely small droplet size. During this process, coarse emulsion is passed through the high pressure homogenizer until nanoemulsion with desired droplet size and narrow distribution (polydispersity index) is obtained. In case of microfluidization, a high-pressure positive displace pump is used in the process, which forces the product through the interaction chamber, consisting of small channels called “microchannels.” The product passes through the microchannels on to an impingement area resulting in very fine droplets of nanosize range. High-energy ultra-sonication technique is also routinely employed in the preparation of nanoemulsion. Ultra-sonication energy produces cavitation forces on the samples resulting in disruption of dispersion phase, leading to small droplet formation. High-pressure homogenization and microfluidization methods are used in the production of nanoemulsion at laboratory and industrial scale, whereas ultrasonic emulsification is limited to laboratory scale.
Despite that high-energy methods produce nanoemulsions with desired properties and have scale-up manufacturing, they may not be suitable for thermo-labile drugs such as nucleic acids, proteins, enzymes, and retinoids (82). In such cases, mild conditions involving low energy can be employed. Low-energy emulsification methods used to produce nanoemulsions according to the phase behavior and properties of the constituents that promote the formation of nanoscaled droplets (83). These methods include self-emulsification, phase transition, and phase inversion temperature methods (82). These low-energy methods can be brought about by altering the parameters which affect the hydrophilic lipophilic balance of the system-like compositions and temperature (82,83).
DRUG INCORPORATION METHODS
Nanoemulsions are ideal vehicles for the poorly aqueous soluble drug delivery, and the drugs with or without the aid of co-solvents can be incorporated into these formulations by de novo or extemporaneous addition. In de novo method, the water-insoluble drug is solubilized in the oil phase prior to nanoemulsion preparation, whereas in extemporaneous method, the drug is added to the pre-formed nanoemulsion. Drugs that are highly oil-soluble can be dissolved directly into the phase. However, drugs that are slightly soluble in oil can be incorporated into the nanoemulsion formulation with the aid of co-solvents. In this case, water-insoluble drug is first dissolved in the organic solvent and added to oil phase, and then the solvent is removed during the manufacturing process (2–5,9). In another approach, drug and phospholipids first dissolved in organic solvent and the solvent is removed by vacuum evaporation. The resulting lipid film is hydrated with aqueous phase and sonicated to form liposome like dispersion to which oil phase was added and emulsified to form nanoemulsion (84). The use of organic solvents requires careful assessment of drug precipitation upon organic solvent removal, physical and chemical stability of emulsions, and drug partitioning in the nanoemulsion. In addition, scale-up costs could be higher due to additional step involving removal of organic solvents.
SolEmuls Technology, a solvent-free method to incorporate drugs into nanoemulsion that localizes the drug at the interface, has been introduced recently. In this technique, ultra-fine powder/nanocrystal form of the water-insoluble drug is added to pre-formed nanoemulsion, and the mixture is then homogenized until the drug powder/crystals are dissolved, resulting in localization of drug at the interface. For example, amphotericin B emulsion formulated using this method has been shown to be more effective and less toxic than the commercially available formulations (85). With some water-insoluble drugs, elevation of temperature and use of fatty acids as lipophilic counter-ions can aid in the solubilization process (86). Drugs like propofol and halothane which are highly oil-soluble and liquids at room temperature can be extemporaneously added to pre-formed emulsions such as Intralipid®, whereon the drug preferentially partitions into the oil phase of emulsion.
PHYSICO-CHEMICAL CHARACTERIZATION
Accurate characterization of any delivery system is of utmost importance to gauge its performance in preclinical and clinical settings. Physical parameters such as shape, charge, and size, as well as chemical composition influences the interaction, uptake, drug release kinetics, clearance from body, and toxicity of any nanocarrier. Size, for example, is an extremely important parameter in EPR effect, where particles below the threshold size are eliminated from circulation by renal clearance while particles above the size limit fail to extravasate from systemic circulation and are eventually removed by MPS. The following section is devoted to the methods that are routinely employed in physico-chemical characterization of lipid-based delivery systems in general and nanoemulsions in particular.
Size Distribution
The size and polydispersity (PDI) index of a nanoemulsion is routinely characterized by photon correlation spectroscopy (aka dynamic light scattering). This method has been advanced based on classical light scattering theory for particles smaller than the wavelength of light (Rayleigh scattering) and subsequently for larger particles (Mie Theory). The underlying theory of size measurement relies on scattering of light from colloid particles in random Brownian motion, which is detected by photo multiplier tube as in-phase or out-of-phase based on constructive or destructive interference of light. The analysis of the frequency distribution of the intensity fluctuation of scattered light is used for calculation of translational and rotation diffusion coefficients of the particles. The average hydrodynamic radius (Stokes radius) can therefore be calculated by intensity-weighted basis using the Stokes–Einstein equation and is often reported as Z-average along with PDI as a measure of width of the particle size distribution. PCS remains the most popular method for average size analysis of the nanoemulsions due to the simplicity of the methods, minimal sample preparation, and fast result output. It is however extremely important to understand the sample preparation and experimental parameters to be accounted for, to get an accurate measurement of size and PDI. Factors such as high particle concentration, size range (0.002–2 μm recommended), changes in sample properties during measurement (aggregation, sedimentation, etc.), or presence of dust could lead to erroneous size estimation. Besides, due to the measurable size constraint, PCS is unable to account for the presence of small population of large-size oil droplets in the nanoemulsion. Despite the limitations, PCS is a versatile tool for nanoemulsion stability assessment, which is directly related to the size and PDI of the droplets.
Electrophoretic Mobility and Zeta Potential
Charge of any drug delivery system is an important property that governs its stability in suspension due to electrostatic interactions as well as its performance in vivo. Electrophoretic mobility measures the net charge/potential on a particle surface (zeta potential) based on its movement in an externally applied electric field. The suspended colloidal particles are subjected to an electric field, and their movement in response to the field gives their electrophoretic mobility, which is governed by the Henry equation,
where ζ is the zeta potential, μ is the electrophoretic mobility, ε is the dielectric constant, η is the viscosity, and f (ka) is the Henry’s function. Two values are generally used for Henry’s function, i.e., 1.5 or 1. Electrophoretic mobility measurements of nanoemulsions are generally performed in aqueous suspension with minimum to moderate electrolyte concentration for which Henry’s function value is 1.5 and is commonly referred to as Smoluchowski’s formula,
Thus, with all the other parameters known, calculation of zeta potential from the electrophoretic mobility becomes straightforward if the measurements conditions meet Smoluchowski approximation, i.e., particle size larger than 0.2 μm and the dispersion system with salt concentration more than 10−3 M. Smoluchowski formula is valid for particles with dimension greater than the double-layer thickness and based on the assumption that the application of the electric field does not cause distortion in the double layer.
Oil Droplet Morphology Assessment
Electron microscopy (EM) is the most popular method for morphology and size analysis of the oil droplets in the nanoemulsions. Where PCS gives the hydrodynamic radius of the suspended oil droplets, electron microscopy aids direct visualization of the sample morphology, accurate measurement of the size of the oil droplets, and presence of any other type of contaminants in the formulation. EM, for e.g., shows the presence of a small population of large-size oil droplets that could not be detected by PCS measurement (87). Transmission electron microscopy (TEM) is largely the most exploited technique for imaging of nanoemulsions where the sample is coated onto a formavar-coated copper grid and imaged under an electron beam. A typical nanoemulsion is incapable of generating sufficient contrast, and thus, a negative staining method is typically used where the sample on the copper grid is further exposed to phosphotungstic acid or its salt or uranyl acetate (88). Cryo-TEM is a more suitable method for nanoemulsion imaging where the samples are quickly frozen and then transferred on to a low-temperature stage for visualization. Scanning electron microscopy (SEM) and environmental-SEM (e-SEM) are some other electron microscopic methods that have been used for nanoemulsion visualization, but a detailed account of these methods is out of the scope of this article. Readers are strongly encouraged to follow some recent very comprehensive reviews published on the application of EM (88) and cryo-TEM (89) in imaging of drug delivery systems including nanoemulsions.
Assessment of Encapsulation Efficiency
Nanoemulsions are popular choice as delivery system due to their effectiveness in encapsulating hydrophobic therapeutic drug in the oil phase. The drug loading, however, depends on its solubility in the choice of lipid as well as their tendency to partition into the aqueous phase. It is therefore important to monitor the efficiency of encapsulation of a drug into the nanoemulsion. The drug encapsulation efficiency of the nanoemulsions is estimated by using ultrafiltration method using centrifugal filter devices (3). A known volume of nanoemulsion sample is loaded into the upper donor chamber and centrifuged at high speed to separate the aqueous phase into the sample recovery chamber while the drug along with the oil remains in to donor chamber. The aqueous phase from the sample recovery chamber is assessed for any drug partitioning, and the drug encapsulation efficiency is determined indirectly by mass balance (90). The choice of drug analysis method largely depends on the drug properties, but the most common methods used include ultraviolet-visible spectroscopy, fluorescence spectroscopy, high-pressure liquid chromatography, and liquid chromatography coupled with mass spectroscopy.
Stability Analysis
The FDA has laid down strict guidelines for stability testing of new drugs and products, since it is an important criterion that defines the shelf-life of a formulation with respect to its excipients, different phases, and the therapeutic moiety loaded. In this context, stability studies should be performed to assess the integrity of the nanoemulsion system itself, as well as its excipients and loaded drug. The most simplistic approach to stability testing is to centrifuge the nanoemulsions at 3,000–3,500 rpm for 30 min to accelerate creaming. The height of oil layer on the top, emulsion phase in the middle, and aqueous phase at the bottom is usually reported as a measure of the stability of the nanoemulsion. In regular laboratory setup, long-term formulation stability is usually monitored by incubating the nanoemulsions at 4 and 25°C under sealed conditions for 3–6 months, and a sample is withdrawn periodically for size, charge, and drug encapsulation efficiency analysis. No significant change in these parameters over time indicates that the nanoemulsions are stable at both the storage conditions. However, as per the FDA guidelines, there are three levels of drug/drug product stability testing namely long-term, intermediate, and accelerated; approved conditions for each type vary based on the storage conditions. Heating and cooling and freeze–thaw cycles are also often used to assess the thermodynamic stability of the nanoemulsion. Shakeel et al. for example subjected nanoemulsions to six heating and cooling incubation cycles at 5 and 45°C, respectively, for <48 h at each temperature, followed by analysis of their size and charge. Nanoemulsions found to be stable under those conditions were further subjected to three freeze–thaw cycles at −21 and 25°C. This study demonstrated that nanoemulsions were stable at all stress conditions (91).
Stability of the oil phase of the nanoemulsion is another factor that contributes to the overall stability of the system. Oil usually undergoes oxidation, which is a series of reaction that leads to oil rancidity accompanied with a series of by-products that are used to check the oil stability. There are series of parameters that are measured to evaluate the overall oxidation state of oil. Peroxide value (PV) is the most common analysis where the primary oxidized product of oil, hydroperoxides, is estimated by titration of iodine in the presence sodium thiosulphate in acidic medium. PV value however may not provide an accurate measurement of oil stability, since it decreases when hydroperoxides decompose to form carbonyls and other products. The byproducts at this level of oil rancidity are measured by the anisidine value (AV) where oil is dissolved in iso-octane, reacted with para-anisidine in acidic medium, and measured spectroscopically. Total oxidation value (TOTOX) gives a wholesome oxidation estimation and is defined as
Other measures of the oil rancidity include acid value, thiobarbituric acid value, and iodine value. Temperature, presence of oxygen, light, moisture, or presence of transition metals such as copper promotes the oxidation of oil, thereby affecting the stability of the nanoemulsion while presence of antioxidants retards the oxidation process.
pH
The change in pH or presence of ions in a nanoemulsion formulation can have a very complex impact on the delivery system and thus is of paramount importance in formulation design as well as subsequent stability studies. The pH of the nanoemulsion at the preparation step can affect the stability of the therapeutic drug and other excipients. Shafiq et al. for example reported that Ramipril, a hypertensive drug, is sensitive to moisture and alkaline pH, and nanoemulsion formulation therefore has to be prepared at an acidic pH (5.0) to ensure drug encapsulation in its effective form (92). Similarly, post-formulation pH changes due to oxidation of oil can significantly change the physical parameters of the system. Qian et al. studied the color change and mean particle size variation of nanoemulsion as a function of pH over time, and their results indicate that no significant color change is observed in the pH range of 3–5, though the rate of color change was appreciably faster at pH 3 (93). However, the mean particle size of these protein-stabilized nanoemulsions increased dramatically around pH 4–5, which could be explained in the variation in the electrostatic repulsion between nanoemulsions due to effect of pH. These results confirm that pH is an important physical parameter to be monitored carefully to ensure a stable nanoemulsion formulation.
Drug Release
The drug release profile is the most important consideration for a delivery system, since it controls in vivo bioavailability, absorption, and clearance kinetics and most importantly the therapeutic efficacy. It is therefore imperative to study the in vitro drug release kinetics of nanoemulsions to ascertain its delivery capability and performance. The most popular and common method to study in vitro drug release is called dialysis bag (dynamic dialysis) method where the nanoemulsion sample is placed in a dialysis bag and dialyzed against a buffer solution (“sink” receiver compartment) under stirring conditions, typically at human body temperature (37°C). A known volume of buffer is withdrawn from the receiver compartment at pre-determined time intervals, and an equivalent volume of fresh buffer is replaced to maintain a constant volume. The collected buffer volume is processed to extract the drug out, which is quantified by using an analytical method suitable for the drug. The result of drug release is often presented as cumulative percent drug released from the nanoparticles at different time intervals.
Several models such as zero-order, first-order, Higuchi, Weibull model, etc., are used to simulate and understand the drug release kinetics from a formulation (94). Barzegar-Jalali et al. have critically summarized a comparative analysis of various models, their prediction accuracy and a detailed literature survey of reports on drug release kinetics from different types of nanoparticle systems including nanoemulsions (95). Recent reports also suggest that the data interpretation from a dynamic dialysis method may not be very accurate and simplistic. The drug released from a nanoparticle system has to diffuse through the dialysis chamber to reach the dialysis membrane to escape to the receiver compartment, presenting a double-barrier condition. Modi et al. recently studied the drug release kinetics of a lipophilic drug from a liposomal formulation and demonstrated that several parameters should be considered to accurately and reliably predict the release kinetics of a drug (96).
Sterility
The ultimate aim of development of any drug delivery system is to use it for therapeutic benefit against serious human ailments such as cancer. Sterility of the formulation is therefore pertinent to warrant its safe application for treatment and should be taken into account at the early stages of formulation development. Routinely practiced methods for sterilization such as autoclaving are not suitable for nanoemulsion as they involve high temperature and pressure while maintaining aseptic conditions could be usually expensive. Thus, suitable care is required in the choice of method adopted for accomplishing sterility of the nanoemulsions without altering its physico-chemical properties. Due to constraint with stability of the nanoemulsion, they are usually filter-sterilized using 0.22-μm filters. The sterilization of the nanoemulsion is validated by direct spectroscopic method to assess any potential microbial contamination and by plating method to check for microbial growth (15).
IN VIVO FATE OF NANOEMULSIONS
Interactions with Blood Components
When nanoemulsions are administered, upon entering the bloodstream, they primarily interact with erythrocytes, plasma proteins (opsonins), immune cells (monocytes, platelets, leukocytes, and dendritic cells), and tissue resident macrophages (Kupffer cells in liver, dendritic cells in the lymph nodes, macrophages, and B cells in the spleen). Erythrocytes occupy the largest fraction of blood, and hence, nanoemulsions are very likely to interact with them upon entering systemic circulation. If the nanoemulsions induce hemolysis and adsorb to hemoglobin or cell debris, they are rapidly cleared by macrophages (97). Likewise, due to longer plasma half-life of nanoemulsions, there is a greater tendency of them to interact with blood components which increases the likelihood of inducing thrombogenicity which can lead to partial or complete occlusion of blood vessels (98,99). Further to this, the uptake of nanoemulsions by immune cells and macrophages can be facilitated by the adsorption of opsonins to the surface of the nanoemulsions which can redirect the delivery systems to endogenous clearance mechanisms reducing drug delivery and efficacy at the target site (100). Apart from reducing in vivo efficacy, complement activation can also induce undesirable consequences including life-threatening allergic, anaphylactic, and hypersensitivity reactions (101).
The size, charge, and surface functionality of the nanoemulsions can play a crucial role in their interaction with blood components. Large-sized particles with cationic or anionic surface charge often show greater degree of phagocytosis compared with small-sized or neutrally charged particles (102). Cationic surfaces also increase the likelihood of erythrocyte damage and hemolytic potency of nanoparticles (97). Opsonization of nanoparticles is often found to be reduced by surface functionalization of the nanoparticles with PEG (97), poloxamer (103), or poloxamine (104). PEG has been evaluated for use in a range of nanoemulsion-based delivery systems as it is hypothesized that PEG coating on the surface of nanoemulsions creates a steric shield around the nanoemulsions, preventing plasma proteins from adhering to the surface of the nanoemulsions, avoiding uptake by the MPS cells. Thus, during nanoemulsion formulation development, the compatibility of the nanoparticle size, surface charge, and surface functionalities with blood components should be critically evaluated.
The unavoidable interaction of nanoemulsions with blood components has recently been exploited to develop targeted nanoemulsion systems (105–107). For example, cholesterol-rich nanoemulsions (LDE) upon contact with plasma can acquire apo E and other apolipproteins from native lipoproteins and bind to low-density lipoprotein (LDL) or LDL-related protein receptors (108). These receptors are highly expressed on cancer cells, and inclusion of cytotoxic agent can promote cancer cell-specific delivery (109). Maranhao et al. investigated the delivery of several cytotoxic agents including etoposide, carmustine, and paclitaxel in these nanoemulsion systems (106,107,110). Along with issues of etoposide lipophilicity, upon its dilution in plasma it can be converted into its inactive cis-lactone form. Inclusion of etoposide in these LDE delivery systems isolated the drug from the plasma until delivery to the cancer cells intracellular compartments (107). In patients with advanced cancer, these LDEs have also been shown to minimize inadvertent toxicity, proving the promise of this targeted delivery system for cancer therapeutics (110).
Interactions with Membrane Barriers
Once the nanoemulsion overcome the barriers in the blood, its interaction with membrane barriers is crucial for the therapeutic payload to be delivered at the desired site. At the cellular level, uptake of nanoparticles can occur via phagocytosis, macropinocytosis, or endocytosis, and they can accumulate in lysosomes, intracellular vacuoles, or cytoplasm. Although targeted nanoemulsions are being investigated to enable site specific delivery and overcome membrane barriers, the BBB poses a significant hindrance to the delivery of therapeutic agents to the brain (23). Beduneau et al. designed OX26 MAb-conjugated nanoemulsions which were actively targeted for transferrin receptors that are overexpressed on the cerebral endothelium (111). The targeted nanoemulsions showed significant accumulation in the brain 24 h after administration compared with the non-targeted nanoemulsions indicating that nanoemulsion-based delivery systems can be developed to overcome a highly regulated and efficient barrier to drug delivery.
Apart from the physiological membrane barriers which hinder drug delivery, overexpression of trans-membrane drug efflux pumps (such as P-glycoprotein (Pgp), breast cancer resistance protein (ABCG2), and multi-drug resistance-associated protein (MRP-1)) reduce drug uptake and thus contribute to the development of multi-drug resistance (MDR). Our group recently investigated co-administration of paclitaxel and curcumin as nanoemulsions to overcome MDR in tumor cells. It was observed that curcumin nanoemulsions could inhibit NFkB activity and downregulate P-gp expression in resistant cells lines, promoting successful intracellular delivery of PTX (3). Oral administration of these nanoemulsions showed that mice pre-treated with curcumin nanoemulsions showed increased bioavailability of paclitaxel and enhanced anti-tumor activity without induction of acute toxicity (4). Although the increase in oral bioavailability of PTX would be attributed to inhibition of Pgp, the ability of nanoemulsions to readily disperse in gut fluids was also thought to improve oral bioavailability (4). Thus, nanoemulsion-based delivery systems can be developed to overcome membrane barriers to achieve successful intracellular delivery of therapeutic agents.
In Vivo Metabolism
Metabolism is the biochemical modification of xenobiotics via specialized enzymatic mechanisms that usually convert lipid-soluble components to generate water-soluble products which can be eliminated from the body (112). Hepatic clearance is the primary route for circulating nanoparticle metabolism with the nanoparticles being exposed to parenchymal cells (hepatocytes) or non-parenchymal cells (including sinusoidal hepatic endothelial cells, Kupffer cells, and hepatic stellate cells) (113). Nanoparticles, which are catabolized by the hepatocytes, are excreted into the biliary system while those that are phagocytosed by the Kupffer cells are endocytosed, degraded, and eliminated. Apart from hepatic clearance, renal excretion also plays a critical role in the elimination of water soluble nanoparticles or their metabolites. Glomerular filtration of nanoparticles in the kidney is highly dependent on the size of the nanoparticles with nanoparticles less than 6 nm being filtered into the kidney and larger nanoparticles being returned to the systemic circulation (114).
KEY CONSIDERATIONS—REGULATORY REQUIREMENTS
Safety Considerations
The toxicities associated with any drug delivery carrier pose a limitation in drug delivery. Ideal carrier would efficiently encapsulate and deliver the payload at desired site while being mostly inert to immune systems and show predictable clearance from the body. Nanoemulsion formulations are generally composed of GRAS-grade excipients. However, to exploit tumor microenvironment, novel nanoemulsion formulations are being developed to accommodate, controlled release, pH-responsive, and thermo-responsive properties. In addition, targeting moieties and imaging components are incorporated for targeted cancer therapy and imaging. In such situations, novel materials are incorporated in the nanoemulsion formulations. The safety and long-term biological effects of these novel materials need to be evaluated. The in vitro cell viability tests that are used to assess the toxicity profiles of novel materials frequently used in the laboratories, but these methods are often questionable in determining the safety of the system. These results cannot be translated because of lack of true dynamic conditions and active immunizing system. Also, care must be taken when translating the results obtained from in vivo animal models to clinical setting, because there is always a risk of intra- and inter-species difference.
Large-Scale Production and Quality Control
Quality-by-design (QbD) is a new frame work currently being implemented by the FDA for the manufacturing and quality control of pharmaceuticals. As part of QbD principles, the design of experiments (DOE) and process analytical technology are routinely employed start from the raw material procurement stage to finished product. To fulfill QbD requirements, it is essential to develop robust and documented process knowledge for the manufacturing of nanoemulsion delivery systems. The better understanding of key process variables such as raw materials, equipment, and protocols at an early stage of product development will lead to easy translation of dosage forms from lab-scale to industrial-scale. Successful procurement of process knowledge will allow manufacturers to bring nanoemulsion delivery systems to the clinic with unique multifunctional abilities and novel therapeutic applications.
Qbd is officially defined as “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” (115). The adoption of QbD in nanoemulsion-based product manufacturing will include defining a target product profile, development of the manufacturing process based on good DOE, identification of critical product quality attributes, process parameters, and sources of variabilities of the manufacturing process. The FDA has come out with guidance that covers Pharmaceutical Development, Quality Risk Management, and Quality System with a predisposition that the future state of biopharmaceutical manufacturing, of which nano-products will be a part, will be an environment directed by QbD (115–117).
CONCLUSIONS AND FUTURE DIRECTIONS
Nanoemulsions are versatile drug delivery vehicles in the delivery of small and macromolecular therapeutics to treat complex diseases. One of the major problems of using nanoemulsion as parenteral drug delivery system is its rapid uptake by MPS. For drug delivery purposes, it is important to control and modify the uptake of the droplets. Adding PEG to stabilize nanoemulsion droplets can prevent MPS uptake and prolong the circulation time. Nanoemulsions also can be engineered with specific attributes such as targeting and imaging functionalities, barrier permeability enhancement, and combination therapeutic delivery. Depending on the choice of lipid core composition, phospholipids, and surface modifier concentration, different drug loadings may be achieved and exploited for drug delivery in cancer chemotherapy.
To fully realize the potential of nanoemulsion dosage forms for delivery of contemporary anticancer therapeutics in clinical setting, it is essential that researchers also address the material safety, scale-up, and quality control issues. Material characterization and scale-up becomes extremely challenging especially when dealing with multifunctional nanoemulsion systems designed to carry targeting ligands, imaging agents, and combination therapeutics. Moreover, in vivo distribution and metabolism of nanoemulsions engineered using novel materials are need to be fully assessed before being used in clinical application.
ACKNOWLEDGEMENTS
The authors are thankful to the support by the National Cancer Institute of the National Institutes of Health grant U54-CA151881.
REFERENCES
- 1.Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2(1):12. doi: 10.4103/2230-973X.96921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganta S, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in parenteral emulsion. Int J Pharm. 2008;360(1–2):115–21. doi: 10.1016/j.ijpharm.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–39. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 4.Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci. 2010;99(11):4630–41. doi: 10.1002/jps.22157. [DOI] [PubMed] [Google Scholar]
- 5.Ganta S, Sharma P, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J Drug Target. 2010;18(2):125–33. doi: 10.3109/10611860903244199. [DOI] [PubMed] [Google Scholar]
- 6.Dehelean CA, Feflea S, Ganta S, Amiji M. Anti-angiogenic effects of betulinic acid administered in nanoemulsion formulation using chorioallantoic membrane assay. J Biomed Nanotechnol. 2011;7(2):317–24. doi: 10.1166/jbn.2011.1297. [DOI] [PubMed] [Google Scholar]
- 7.Talekar M, Ganta S, Singh A, Amiji M, Kendall J, Denny WA, et al. Phosphatidylinositol 3-kinase inhibitor (PIK75) containing surface functionalized nanoemulsion for enhanced drug delivery, cytotoxicity and pro-apoptotic activity in ovarian cancer cells. Pharm Res. 2012;29(10):2874–86. doi: 10.1007/s11095-012-0793-6. [DOI] [PubMed] [Google Scholar]
- 8.Dehelean CA, Feflea S, Gheorgheosu D, Ganta S, Cimpean AM, Muntean D, et al. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J Biomed Nanotechnol. 2013;9(4):577–89. doi: 10.1166/jbn.2013.1563. [DOI] [PubMed] [Google Scholar]
- 9.Ganta S, Devalapally H, Baguley BC, Garg S, Amiji M. Microfluidic preparation of chlorambucil nanoemulsion formulations and evaluation of cytotoxicity and pro-apoptotic activity in tumor cells. J Biomed Nanotechnol. 2008;4(2):165–73. [Google Scholar]
- 10.Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2007;66(2):227–43. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Investig Dermatol. 2007;127(1):154–62. doi: 10.1038/sj.jid.5700485. [DOI] [PubMed] [Google Scholar]
- 12.Patlolla RR, Vobalaboina V. Pharmacokinetics and tissue distribution of etoposide delivered in parenteral emulsion. J Pharm Sci. 2005;94(2):437–45. doi: 10.1002/jps.20249. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari S, Tan Y-M, Amiji M. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J Biomed Nanotechnol. 2006;2(3–4):3–4. [Google Scholar]
- 14.Gallarate M, Chirio D, Bussano R, Peira E, Battaglia L, Baratta F, et al. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm. 2013;440(2):126–34. doi: 10.1016/j.ijpharm.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Nesamony J, Shah IS, Kalra A, Jung R. Nebulized oil-in-water nanoemulsion mists for pulmonary delivery: development, physico-chemical characterization and in vitro evaluation. Drug Dev Ind Pharm. 2013. doi:10.3109/03639045.2013.814065. [DOI] [PubMed]
- 16.Hippalgaonkar K, Majumdar S, Kansara V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS PharmSciTech. 2010;11(4):1526–40. doi: 10.1208/s12249-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockshott ID. Propofol ('Diprivan') pharmacokinetics and metabolism—an overview. Postgrad Med J. 1985;61(Suppl 3):45–50. [PubMed] [Google Scholar]
- 18.Tibell A, Larsson M, Alvestrand A. Dissolving intravenous cyclosporin A in a fat emulsion carrier prevents acute renal side effects in the rat. Transplant Int Off J Eur Soc Organ Transplant. 1993;6(2):69–72. doi: 10.1007/BF00336647. [DOI] [PubMed] [Google Scholar]
- 19.Gianella A, Jarzyna PA, Mani V, Ramachandran S, Calcagno C, Tang J, et al. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5(6):4422–33. doi: 10.1021/nn103336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 21.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim KY. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine Nanotechnol Biol Med. 2007;3(2):103–10. doi: 10.1016/j.nano.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ganta S, Deshpande D, Korde A, Amiji M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol Membr Biol. 2010;27(7):260–73. doi: 10.3109/09687688.2010.497971. [DOI] [PubMed] [Google Scholar]
- 24.Lawler J. Editorial foreword: introduction to the tumour microenvironment review series. J Cell Mol Med. 2009;13(8 A):1403–4. doi: 10.1111/j.1582-4934.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 27.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter PM, Schmieder, Anne H, Caruthers, Shelton D, Keene, et al. Minute dosages of ανβ3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22(8):2758–67. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, et al. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132(12):4259–65. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 30.Devalapally H, Shenoy, Dinesh, Little, Steven, Langer, et al. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;59(4):477–84. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 31.Shenoy D, Little, Steven, Langer, Robert, Amiji, et al. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm Res. 2005;22(12):2107–14. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na K, Bae YH. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: characterization, aggregation, and adriamycin release in vitro. Pharm Res. 2002;19(5):681–8. doi: 10.1023/a:1015370532543. [DOI] [PubMed] [Google Scholar]
- 33.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 34.Caron WP, Lay JC, Fong AM, La-Beck NM, Kumar P, Newman SE, et al. Translational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacology. J Pharm Exp Ther. 2013. doi:10.1124/jpet.113.208801. [DOI] [PMC free article] [PubMed]
- 35.Gabizon A, Shmeeda, Hilary, Barenholz, Yechezkel Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42(5):419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. Semin Oncol. 2004;31(Supplement 13(0)):5–15. doi: 10.1053/j.seminoncol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wang Y-W, Huang Q. Enhancing stability and oral bioavailability of polyphenols using nanoemulsions. Micro/Nanoencapsulation Act Food Ingredients: Am Chem Soc. 2009. p 198–212.
- 38.Tagne J-B, Kakumanu S, Ortiz D, Shea T, Nicolosi RJ. A nanoemulsion formulation of tamoxifen increases its efficacy in a breast cancer cell line. Mol Pharm. 2008;5(2):280–6. doi: 10.1021/mp700091j. [DOI] [PubMed] [Google Scholar]
- 39.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009;379(2):201–9. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Hoarau D, Delmas P, David S, phanie, Roux E, Leroux J-C. Novel long-circulating lipid nanocapsules. Pharm Res. 2004;21(10):1783–9. doi: 10.1023/b:pham.0000045229.87844.21. [DOI] [PubMed] [Google Scholar]
- 41.Khalid M, Simard P, Hoarau D, Dragomir A, Leroux J-C. Long circulating poly(ethylene glycol)-decorated lipid nanocapsules deliver docetaxel to solid tumors. Pharm Res. 2006;23(4):752–8. doi: 10.1007/s11095-006-9662-5. [DOI] [PubMed] [Google Scholar]
- 42.Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398(1–2):190–203. doi: 10.1016/j.ijpharm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011;8(1):185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimova I, Zaharieva B, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Tissue microarray analysis of EGFR and erbB2 copy number changes in ovarian tumors. Int J Gynecol Cancer. 2006;16(1):145–51. doi: 10.1111/j.1525-1438.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin CK, Chao TK, Yu CP, Yu MH, Jin JS. The expression of six biomarkers in the four most common ovarian cancers: correlation with clinicopathological parameters. APMIS. 2009;117(3):162–75. doi: 10.1111/j.1600-0463.2008.00003.x. [DOI] [PubMed] [Google Scholar]
- 46.Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30(29):5737–50. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Li J, Lang M, Tang X, Li L, Shen X. Folate-functionalized nanoparticles for controlled 5-fluorouracil delivery. J Colloid Interface Sci. 2011;354(1):202–9. doi: 10.1016/j.jcis.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Zhao LQ, Dong YF, Zhang XY, Lin J, Chen Z. Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur J Pharm Biopharm. 2010;76(1):10–6. doi: 10.1016/j.ejpb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Esmaeili FGM, Ostad S, Atyabi F, Seyedabadi M, Malekshahi M, Amini M, et al. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA–PEG–folate conjugate. J Drug Target. 2008;16(5):415–23. doi: 10.1080/10611860802088630. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HLX, Gao F, Liu L, Zhou Z, Zhang Q. Preparation of folate-modified pullulan acetate nanoparticles for tumor-targeted drug delivery. Drug Deliv. 2010;17(1):48–57. doi: 10.3109/10717540903508979. [DOI] [PubMed] [Google Scholar]
- 51.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99(14):1095–106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 52.Kaneda M, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37(10):1922–33. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138(3):268–76. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48(4):260–70. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae P, Jung J, Lim SJ, Kim D, Kim S-K, Chung BH. Bimodal perfluorocarbon nanoemulsions for nasopharyngeal carcinoma targeting. Mol Imaging Biol. 2013;15(4):401–10. doi: 10.1007/s11307-013-0622-2. [DOI] [PubMed] [Google Scholar]
- 56.Almeida CP, Vital CG, Contente TC, Maria DA, Maranhao RC. Modification of composition of a nanoemulsion with different cholesteryl ester molecular species: effects on stability, peroxidation, and cell uptake. Int J Nanomedicine. 2010;5:679–86. doi: 10.2147/ijn.s12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–65. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- 58.Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interface Sci. 2004;107(2–3):125–55. doi: 10.1016/S0001-8686(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 59.McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter. 2011;7(6):2297–316. [Google Scholar]
- 60.Donsi F, Annunziata M, Vincensi M, Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159(4):342–50. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Ohguchi Y, Kawano K, Hattori Y, Maitani Y. Selective delivery of folate-PEG-linked, nanoemulsion-loaded aclacinomycin A to KB nasopharyngeal cells and xenograft: effect of chain length and amount of folate-PEG linker. J Drug Target. 2008;16(9):660–7. doi: 10.1080/10611860802201464. [DOI] [PubMed] [Google Scholar]
- 62.Chanamai R, McClements DJ. Impact of weighting agents and sucrose on gravitational separation of beverage emulsions. J Agric Food Chem. 2000;48(11):5561–5. doi: 10.1021/jf0002903. [DOI] [PubMed] [Google Scholar]
- 63.McClements DJ. Emulsion design to improve the delivery of functional lipophilic components. Annu Rev Food Sci Technol. 2010;1:241–69. doi: 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- 64.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release Off J Control Release Soc. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: a new temperature-sensitive liposome for use with mild hyperthermia. J Liposome Res. 1999;9(4):491–506. [Google Scholar]
- 67.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13(9):2722–7. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. Gold nanoparticles enable selective light-induced contents release from liposomes. J Control Release. 2007;122(1):86–93. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–75. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Landon CD, Park JY, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia. 2010;26(5):485–98. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. 2006;12(13):4004–10. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 74.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–7. [PubMed] [Google Scholar]
- 75.Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, et al. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6(9):3748–55. [PubMed] [Google Scholar]
- 76.Wang CY, Huang L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci U S A. 1987;84(22):7851–5. doi: 10.1073/pnas.84.22.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Connor J, Huang L. pH-sensitive immunoliposomes as an efficient and target-specific carrier for antitumor drugs. Cancer Res. 1986;46(7):3431–5. [PubMed] [Google Scholar]
- 78.Negrini R, Mezzenga R. pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir. 2011;27(9):5296–303. doi: 10.1021/la200591u. [DOI] [PubMed] [Google Scholar]
- 79.Mo R, Sun Q, Xue J, Li N, Li W, Zhang C, et al. Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater. 2012;24(27):3659–65. doi: 10.1002/adma.201201498. [DOI] [PubMed] [Google Scholar]
- 80.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142(2):267–76. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 81.Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–8. [Google Scholar]
- 82.Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(5):626–39. [Google Scholar]
- 83.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–10. [Google Scholar]
- 84.Han J, Washington C. Partition of antimicrobial additives in an intravenous emulsion and their effect on emulsion physical stability. Int J Pharm. 2005;288(2):263–71. doi: 10.1016/j.ijpharm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 85.Junghanns J-U, Buttle I, Müller R, Araujo I, Silva A, Egito E, et al. SolEmuls® technology: a way to overcome the drawback of parenteral administration of insoluble drugs. Pharm Dev Technol. 2007;12(5):437–45. doi: 10.1080/10837450701555885. [DOI] [PubMed] [Google Scholar]
- 86.Singh M, Ravin LJ. Parenteral emulsions as drug carrier systems. J Parenter Sci Technol Publ Parenter Drug Assoc. 1986;40(1):34–41. [PubMed] [Google Scholar]
- 87.Hatanaka J, Chikamori H, Sato H, Uchida S, Debari K, Onoue S, et al. Physicochemical and pharmacological characterization of alpha-tocopherol-loaded nano-emulsion system. Int J Pharm. 2010;396(1–2):188–93. doi: 10.1016/j.ijpharm.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi: 10.1016/j.micron.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 89.Kuntsche J, Horst JC, Bunjes H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int J Pharm. 2011;417(1–2):120–37. doi: 10.1016/j.ijpharm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Jiang SP, He SN, Li YL, Feng DL, Lu XY, Du YZ, et al. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J Nanomedicine. 2013;8:3141–50. doi: 10.2147/IJN.S47708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shakeel F, Ramadan W, Ahmed MA. Investigation of true nanoemulsions for transdermal potential of indomethacin: characterization, rheological characteristics, and ex vivo skin permeation studies. J Drug Target. 2009;17(6):435–41. doi: 10.1080/10611860902963021. [DOI] [PubMed] [Google Scholar]
- 92.Shafiq S, Shakeel F. Enhanced stability of ramipril in nanoemulsion containing Cremophor-EL: a technical note. AAPS PharmSciTech. 2008;9(4):1097–101. doi: 10.1208/s12249-008-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of Œ≤-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132(3):1221–9. doi: 10.1016/j.foodchem.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 94.Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23. [PubMed] [Google Scholar]
- 95.Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y, et al. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci. 2008;11(1):167–77. doi: 10.18433/j3d59t. [DOI] [PubMed] [Google Scholar]
- 96.Modi S, Anderson BD. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol Pharm. 2013;10(8):3076–89. doi: 10.1021/mp400154a. [DOI] [PubMed] [Google Scholar]
- 97.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukui H, Koike T, Nakagawa T, Saheki A, Sonoke S, Tomii Y, et al. Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS), with commercial lipid-based formulations. Int J Pharm. 2003;267(1–2):101–12. doi: 10.1016/j.ijpharm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Wehrung D, Geldenhuys WJ, Oyewumi MO. Effects of gelucire content on stability, macrophage interaction and blood circulation of nanoparticles engineered from nanoemulsions. Colloids Surf B: Biointerfaces. 2012;94:259–65. doi: 10.1016/j.colsurfb.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–78. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 101.Szebeni J, Alving CR, Rosivall L, Bunger R, Baranyi L, Bedocs P, et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res. 2007;17(2):107–17. doi: 10.1080/08982100701375118. [DOI] [PubMed] [Google Scholar]
- 102.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir ACS J Surf Colloids. 2006;22(19):8178–85. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 103.Stolnik S, Daudali B, Arien A, Whetstone J, Heald CR, Garnett MC, et al. The effect of surface coverage and conformation of poly(ethylene oxide) (PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. Biochim et Biophys Acta. 2001;1514(2):261–79. doi: 10.1016/s0005-2736(01)00376-5. [DOI] [PubMed] [Google Scholar]
- 104.Alvarez-Lorenzo C, Rey-Rico A, Sosnik A, Taboada P, Concheiro A. Poloxamine-based nanomaterials for drug delivery. Frontiers Biosci (Elite edition) 2010;2:424–40. doi: 10.2741/e102. [DOI] [PubMed] [Google Scholar]
- 105.Maranhao RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197(2):959–66. doi: 10.1016/j.atherosclerosis.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 106.Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibañez OCM, et al. Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol. 2005;55(6):565–76. doi: 10.1007/s00280-004-0930-y. [DOI] [PubMed] [Google Scholar]
- 107.Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CHM, Rodrigues DG, Maranhão RC. Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol. 2003;55(12):1615–22. doi: 10.1211/0022357022232. [DOI] [PubMed] [Google Scholar]
- 108.Maranhao RC, Roland IA, Toffoletto O, Ramires JA, Goncalves RP, Mesquita CH, et al. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids. 1997;32(6):627–33. doi: 10.1007/s11745-997-0080-6. [DOI] [PubMed] [Google Scholar]
- 109.Pinheiro KV, Hungria VT, Ficker ES, Valduga CJ, Mesquita CH, Maranhao RC. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin's and non-Hodgkin's lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother Pharmacol. 2006;57(5):624–30. doi: 10.1007/s00280-005-0090-8. [DOI] [PubMed] [Google Scholar]
- 110.Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, et al. Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol. 2002;49(6):487–98. doi: 10.1007/s00280-002-0437-3. [DOI] [PubMed] [Google Scholar]
- 111.Béduneau A, Saulnier P, Hindré F, Clavreul A, Leroux J-C, Benoit J-P. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’ fragments. Biomaterials. 2007;28(33):4978–90. doi: 10.1016/j.biomaterials.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Remmer H. The role of the liver in drug metabolism. Am J Med. 1970;49(5):617–29. doi: 10.1016/s0002-9343(70)80129-2. [DOI] [PubMed] [Google Scholar]
- 113.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (London, England) 2008;3(5):703–17. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.FDA. Q8(R1) Pharmaceutical development revision 1. International Conference on Harmonisation (ICH) Q8 Guideline 2008.
- 116.FDA. Guidance for Industry Q9 Quality Risk management. International Conference on Harmonisation (ICH) Q9 Guideline. 2006. http://www.fda.gov/.
- 117.FDA. Guidance for Industry Q10 Pharmaceutical Quality System. International Conference on Harmonisation (ICH) Q10 Guideline 2009. http://www.fda.gov/.


