Abstract
The objective of this study was to develop a suitable formulation for baicalein (a poorly water-soluble drug exhibiting high melting point) to prepare solid dispersions using hot melt extrusion (HME). Proper carriers and plasticizers were selected by calculating the Hansen solubility parameters, evaluating melting processing condition, and measuring the solubility of obtained melts. The characteristic of solid dispersions prepared by HME was evaluated. The dissolution performance of the extrudates was compared to the pure drug and the physical mixtures. Physicochemical properties of the extrudates were characterized by differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier transform infrared spectroscopy (FTIR). Relative bioavailability after oral administration in beagle dogs was assessed. As a result, Kollidon VA64 and Eudragit EPO were selected as two carriers; Cremophor RH was used as the plasticizer. The dissolution of all the extrudates was significantly improved. DSC and PXRD results suggested that baicalein in the extrudates was amorphous. FTIR spectroscopy revealed the interaction between drug and polymers. After oral administration, the relative bioavailability of solid dispersions with VA64 and EPO was comparative, about 2.4- and 2.9-fold greater compared to the pure drug, respectively.
Figure.
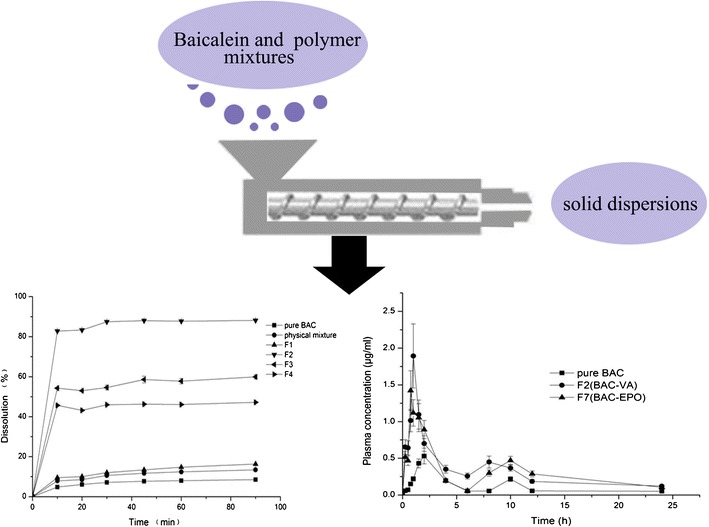
ᅟ
KEY WORDS: baicalein, carrier, high melting point, hot melt extrusion, plasticizer, solid dispersion
INTRODUCTION
Improving the solubility and oral bioavailability of insoluble drug has been one of the greatest challenges among researchers in the pharmaceutical industry. Various methods are developed; the traditional technologies include salt formulation, particle size reduction, solubilization, and solid dispersion. The emerging approach is using nanotechnology. Solid dispersion technology may be the most commonly used pharmaceutical approach among these methods (1).
Solid dispersion technology was developed by Higuchi early in 1960s; the principle was the drug highly dispersed in hydrophilic carrier. Numerous studies proved it was a very effective method to increase the solubility and oral bioavailability, but only few products are in clinical use due to some obvious obstacles, such as aging, use of organic solvent, and no proper manufacturing method. But in the past decade, solid dispersion method regained vigor because of the development of hot melt extrusion (HME).
As a novel processing technology in solid dispersions, HME is a continuous process of converting raw materials and carriers into a uniform product with a screw under high temperature and shear (2,3). The HME process comprises the melting, mixing, and shaping. The high temperature and shear may cause the drug to be melted completely and dispersed in the polymer at molecular level to obtain maximum benefit (4). Compared with traditional techniques, such as solvent evaporation and spray drying, HME may offer numerous advantages, shorter time to produce the final product, environmental advantages due to elimination of solvents in processing, and easing of aging phenomenon because of the special operating condition. Several research groups have proved that HME is a promising method to enhance the solubility of insoluble drugs, such as nimodipine (5), oxeglitazar (6), fenofibrate (7), indomethacin, and famotidine (8). In addition, a number of products prepared by HME have already entered the market, including Kaletra®, Norvir®, and Cesamet®.
Baicalein (BAC) is a type of flavonoid to inhibit certain types of lipoxygenase and acts as an anti-inflammatory agent. It is a drug belonging to Biopharmaceutics Classification System (BCS) Class II with very low solubility. The poor solubility results in very low bioavailability and limited use in the pharmaceutical field (9). Some strategies have been utilized to improve its oral absorption, such as nanocrystal (10), HP-β-CD inclusion (11), and self-microemulsifying (12). The main aim of this study is to apply the new HME technology to improve the solubility and oral bioavailability of BAC.
In this study, we tried using HME technology to improve the dissolution and bioavailability of BAC. But the high melting point of BAC hindered the research. As well known, due to the limitation of processing temperatures in HME, it remains a challenge to prepare solid dispersion by HME for compounds with high melting point (13). Meloxicam, melting point at 247°C, has been prepared for single phase solid dispersion using KinetiSol Dispersing technology rather than HME (14). Ghosh et al. converted a drug with high melting point to an amorphous form before preparing solid dispersion by HME (13). Liu X et al. applied cocrystal technology to reduce the processing temperature before HME (15). Our present work found that using proper plasticizer in carrier would be an effective approach for drugs with high melting point in HME. In our study, after adding Cremophor RH in the carrier, BAC melted at an acceptable temperature, thus ensuring the operational feasibility of HME. Cremophor RH behaves like a plasticizer because it is capable of softening carriers to make them more flexible. It also can increase the free volume among carrier chains, and at the same time, the Tg and the melt viscosity of a carrier decrease (16); all of these are beneficial for the melting of BAC.
MATERIALS AND METHODS
Materials
Baicalein (purity > 95%) was supplied by China Pharmaceutical University, Nanjing, China. Kollidon VA64, Soluplus, Cremophor RH, and Poloxamer 188 were friendly supplied by Basf Auxiliary Chemicals Co., Ltd., Shanghai, China. Eudragit EPO was a gift from Evonik Degussa Special Chemicals Co., Ltd., Shanghai, China. Hydroxypropyl methycellulose (HPMC) 2910 was kindly provided by Colorcon Co., Ltd., Shanghai, China. Polyethylene glycol (PEG) 2000 was purchased from Wanqing Chemical Development Co., Ltd, Nanjing, China.
Preliminary Formulation Study
Some common carriers were selected to prepare BAC solid dispersion by HME, including Kollidon VA64, Eudragit EPO, HPMC, and Soluplus. But in operating process, we found the solid dispersion was unsatisfied due to no enhanced solubility. The phenomenon had no improvement even at very low drug loading and very high operation temperature. Further investigation showed that the BAC had very high melting point at about 265°C, so it was difficult to melt in carriers, which would affect the dispersion state of drug in carrier and subsequent dissolution. We consider adding some plasticizer to help BAC melt in the carrier.
Screening Proper Mixtures of Carrier and Plasticizer
Hansen Solubility Parameters
It is very important to choose the miscible drug and carrier in HME, so the first step was to screen the proper carriers and plasticizer for BAC. Kollidon VA64, Eudragit EPO, HPMC, and Soluplus were common carriers; PEG 2000, Poloxamer 188, and Cremophor RH were chosen as plasticizers. Hansen solubility parameter was used to predict the miscibility of BAC and these polymers, which can be calculated using the group contribution method of Hoftyzer and Van Krevelen (17).
Melting Process
BAC, carriers, and plasticizers were mixed according to Table I. The ratio of carrier to plasticizer was fixed at 5.67 (w/w), while the content of drug in the mixture was 20%. Each mixture was heated slowly to observe the melting temperature. Once determined, the mixture was heated at the given temperature for 5 min, then cooled in an ice bath for 10 min to form the film of solid dispersion. The film transparency was observed to assess the miscibility of drug and carrier.
Table I.
Formulation of Solid Dispersions Using Melting Method
| Formulation | Components (% w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| BAC | Carrier | Plasticizer | ||||||
| Kollidon VA64 | Eudragit EPO | Soluplus | HPMC | Cremophor RH | PEG 2000 | Poloxamer 188 | ||
| SD1 | 20 | 68 | – | – | – | 12 | – | – |
| SD2 | 20 | – | 68 | – | – | 12 | – | – |
| SD3 | 20 | – | – | 68 | – | 12 | – | – |
| SD4 | 20 | – | – | – | 68 | 12 | – | – |
| SD5 | 20 | 68 | – | – | – | – | 12 | – |
| SD6 | 20 | – | 68 | – | – | – | 12 | – |
| SD7 | 20 | – | – | 68 | – | – | 12 | – |
| SD8 | 20 | 68 | – | – | – | – | – | 12 |
| SD9 | 20 | – | 68 | – | – | – | – | 12 |
| SD10 | 20 | – | – | 68 | – | – | – | 12 |
BAC baicalein, HPMC hydroxypropyl methycellulose, PEG polyethylene glycol
Solubility Test
The above solid dispersions were pulverized and sieved through an 80-mesh sieve to determine the solubility. The corresponding physical mixtures were set as the contrast. The solubility test was carried out by taking an excess amount of solid dispersion or physical mixture in 25 mL of 0.1% sodium dodecyl sulfate. The samples were shaken at least 72 h at 37°C. Samples were taken from the saturated solutions and then filtered through a 0.45-μm micropore filter. The drug concentration was analyzed by Shimadzu LC-20AT HPLC system (Shimadzu Corporation, Japan) at the wavelength of 276 nm. The analyses were performed using a mobile phase of methanol and 0.1% acetic acid (65:35, v/v) at a flow rate of 1.0 mL/min. The method has demonstrated with good linearity from 0.1 to 100.0 μg/mL with r = 0.9996, the limit of detection for BAC was 20 ng/mL, the recovery was 98.6~100.6%, and the intra- and inter-batch variability values were less than 2.0% (relative standard deviation, RSD). Each study was repeated three times.
Hot Melt Extrusion
According to the above screening result, two groups of formulations (Table II) were used to prepare BAC solid dispersions by HME. The solid dispersion using Kollidon VA-64 as carrier was named BAC-VA system, and another group using Eudragit EPO as carrier was named BAC-EPO system. In these formulations, the content of BAC varied from 10% to 33%, the ratio of carrier to plasticizer was fixed at 5.67 (w/w), and the exceptions were F1 and F5; both formulations had no plasticizer for comparison.
Table II.
Formulations of Solid Dispersion Using HME
| System | Formulation | Components (% w/w) | |||
|---|---|---|---|---|---|
| BAC | Kollidon VA64 | Eudragit EPO | Cremophor RH | ||
| BAC-VA | F1 | 20 | 80 | – | – |
| F2 | 10 | 76.5 | – | 13.5 | |
| F3 | 20 | 68 | – | 12 | |
| F4 | 33 | 57 | – | 10 | |
| BAC-EPO | F5 | 20 | – | 80 | – |
| F6 | 10 | – | 76.5 | 13.5 | |
| F7 | 20 | – | 68 | 12 | |
| F8 | 33 | – | 57 | 10 | |
BAC baicalein, BAC-VA baicalein with Kollidon VA-64, BAC-EPO baicalein with Eudragit EPO
For each formulation, the carrier and plasticizer were premixed in a clean mortar, then adding BAC for further blend. The blends were manually fed into the hopper of Haake MiniLab II twin-screw extruder (Thermo Fisher Scientific, Germany), the temperature and screw speed were fixed at 160°C and 100 rpm, respectively, and the residence time of the materials was about 2 min. The obtained extrudates were collected by aluminum plate, allowed to cool, and then milled and sieved through an 80-mesh sieve. The powders were stored in a desiccator for further investigation.
The Physicochemical Characterization
In Vitro Dissolution Study
The dissolution of BAC solid dispersion shown in Table II was determined using a ZRS-8G dissolution apparatus (Tianfa Technology, China), and at the same time, a pure BAC powder and a physical mixture with 20% BAC were also assessed for comparison.
Samples containing approximately 100 mg BAC were added to 900 mL 0.1% sodium dodecyl sulfate at 37°C with a paddle rotation speed of 100 rpm. Five-milliliter test solution was withdrawn at 10, 20, 30, 45, 60, and 90 min. Dissolution samples were passed through a 0.45-μm Millipore filter and then 5 mL fresh medium was utilized to replace sample volume. The concentration of drug in the filtrate was analyzed by HPLC under the same condition mentioned previously in solubility test. Each study was performed in triplicate.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was performed to characterize the state of drug in solid dispersion using STA449-C instrument (NETZSCH, Germany). About 10 mg of the sample was placed in aluminum pans. The measurement was conducted with a heating rate of 10°C/min from 40°C to 300°C.
Powder X-ray Diffraction
Powder X-ray diffraction (PXRD) was carried out on a D/Max-2400 X-ray Fluorescence Spectrometer (Rigaku, Japan) using Cu Kα radiation at 40 kV and 40 mA. Samples were scanned over a 2θ range of 3–40° at a rate of 0.02°/min; the residence time was 0.3 s.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) was generated with a Bruker IFS-55 interferometer (Bruker, Germany). Samples were dispersed in KBr and then compressed into pellets. The scanning range was from 4,000 to 400 cm−1 and the resolution was 1 cm−1.
The Primary Stability Study
To study the stability of F2 and F7, the powder of F2 and F7 without package was respectively stored at stress testing conditions, including high temperature (60 ± 2°C), high humidity (75 ± 5% relative humidity), or light (4,500 ± 500 lx) for 10 days. The content and dissolution were detected by HPLC under the same condition mentioned previously in solubility test.
We also evaluated the long-term stability of F2 and F7. The powder of F2 and F7 packaged in an airtight container was stored at 25 ± 2°C and 60 ± 5% relative humidity. After 6 months, the content and dissolution were determined and compared with the original state.
Pharmacokinetic Study
Animal Study
The pharmacokinetic characteristic of BAC solid dispersion by HME was studied in beagle dog. All studies were conducted in accordance with the principles of Laboratory Animal Care and were approved by the Department of Laboratory Animal Research at China Pharmaceutical University. The preparations included F2 and F7, pure BAC as a comparison. Each preparation was a capsule containing 100 mg BAC.
Six male beagle dogs were used in the present study. The study design was a single dose with three treatments. Each dog was administered with three preparations separately by at least 1 week washout between each treatment. The dogs were starved for 24 h prior to experiment and continued fasting until 4 h post-dose, but free access to water was allowed.
The preparations containing 100 mg of BAC were administered. Blood was withdrawn via needle from the front legs. About 3 mL of blood was collected in heparinized tubes from the cephalic vein of the hind leg at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after the administration. Blood was centrifuged for 5 min and plasma was stored at −20°C until analyzed.
Analysis of Plasma Samples
Plasma (100 μL) was mixed with 50 μL β-glucuronidase/sulfatase (180 U/mL in pH 5.0 acetate buffers) and 20 μL solution of sodium thiosulfate (200 mg/mL). The mixture was incubated at 37°C for 8 h. Methanol solution (10 μL) of propyl paraben (50 μg/mL) was added as an internal standard. Then, the mixture was acidified with 10 μL hydrochloric acid solution (0.1 mol/L), followed by liquid–liquid extraction with 1.5 mL of ethyl acetate.
The organic layer was separated and reduced to dryness under nitrogen at 45°C. The residue was reconstituted in 100 μL of the mobile phase. Twenty microliters of this solution was injected into the HPLC for analysis under the same condition as mentioned previously. The method has validated that the linearity ranged from 0.05 to 15.0 μg/mL with r = 0.9995, the limit of quantification for BAC in plasma was 30 ng/mL with good accuracy and precision, the mean plasma extraction recovery was beyond 95%, and the intra- and inter-batch variability values were less than 10.81% (RSD).
Data Statistical Analysis
The results were analyzed by one-way ANOVA with Bonferroni post t tests using GraphPad InStat Software 1.13 (GraphPad Software, USA), and p < 0.05 was considered statistically significant. The pharmacokinetic parameters were determined using non-compartmental pharmacokinetics data analysis software (Thermo Electron Corporation, USA).
RESULTS AND DISCUSSION
Screening Proper Mixtures of Carrier and Plasticizer
Hansen Solubility Parameters
The solubility parameter (δ) of BAC and each polymer was listed in Table III. The difference (Δδ) of each polymer to BAC was calculated. Greenhalgh indicated that materials with Δδ < 7.0 were miscible, while systems with Δδ > 7.0 were likely to be immiscible (18). The differences between BAC and each polymer were less than 7.0, which meant these polymers would be miscible with BAC theoretically.
Table III.
Calculated Solubility Parameters of Drug and Polymers
| Compound | δ (Mpa0.5) | Δδ (Mpa0.5) | Compound | δ (Mpa0.5) | Δδ (Mpa0.5) |
|---|---|---|---|---|---|
| BAC | 17.2 | – | Soluplus | 23.4 | 6.2 |
| Kollidon VA64 | 21.1 | 3.9 | Cremophor RH | 19.0 | 1.8 |
| Eudragit EPO | 18.9 | 1.7 | Poloxamer 188 | 19.6 | 2.4 |
| HPMC | 22.4 | 5.2 | PEG 2000 | 20.1 | 2.9 |
BAC baicalein, HPMC hydroxypropyl methycellulose, PEG polyethylene glycol
Melting Process
Table V showed the melting temperature and film transparency of solid dispersions. Compared with Cremophor RH (SD1–3), the solid dispersions using PEG 2000 (SD5–7) and Poloxamer 188 (SD8–10) as plasticizer needed higher operating temperature to melt. Moreover, the film of solid dispersions was slightly turbid. It seemed Cremophor RH was a better plasticizer.
Table V.
The Stability Results of F2 and F7 at the Stress Testing Conditions (Mean ± SD, n = 3)
| 5 days | 10 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation | 0 day | 60 ± 2°C | 75 ± 5% RH | 4,500 ± 500 lx | 60 ± 2°C | 75 ± 5% RH | 4,500 ± 500 lx | |
| Content (%) | F2 | 100.0 ± 0.1 | 101.5 ± 0.2 | 97.3 ± 0.2 | 98.2 ± 0.2 | 98.7 ± 0.3 | 97.7 ± 0.1 | 99.7 ± 0.3 |
| F7 | 100.0 ± 0.3 | 99.6 ± 0.4 | 98.7 ± 0.2 | 98.6 ± 0.3 | 98.3 ± 0.1 | 98.1 ± 0.3 | 102.0 ± 0.1 | |
| Dissolution (%) | F2 | 86.7 ± 2.2 | 70.1 ± 2.8 | 65.1 ± 1.9 | 83.4 ± 2.5 | 60.7 ± 4.2 | 58.7 ± 3.9 | 80.7 ± 2.8 |
| F7 | 88.9 ± 3.5 | 74.7 ± 2.1 | 69.7 ± 3.7 | 86.2 ± 3.2 | 66.1 ± 3.7 | 56.9 ± 1.5 | 83.1 ± 2.0 | |
The exception of Cremophor RH group was SD4 with HPMC as carrier. The operating temperature was highest and the film was turbid with obviously crystalline shape. This may be attributed to the characteristic of HPMC. Compared with the lower glass transition temperature (Tg) of other carriers from 44°C to 101°C, the Tg of HPMC reaches 170°C. This made HPMC lack the capacity to reduce the melting temperature of the whole system in operating process. So, HPMC was not a good choice for BAC in HME.
Solubility Test
The solubility of solid dispersions and corresponding physical mixtures was given in Table V. The solubility of BAC was similar in all physical mixtures, which was much lower than the solid dispersions. In solid dispersions, when using the same plasticizer and different carrier, such as SD1, SD2, and SD3, Eudragit EPO was the best in improving the solubility, followed by Kollidon VA64, and Soluplus had obvious gap with Eudragit EPO and Kollidon VA64; when using the same carrier and different plasticizer, such as SD1, SD5, and SD8, Cremophor RH showed much better effect compared with PEG 2000 and Poloxamer 188.
In summary, all chosen polymers were miscible with BAC according to Hansen solubility parameter, but further assessment based on melting process and solubility optimized these polymers. In the above-mentioned study, Cremophor RH was the best plasticizer, and at the same time, its function as an excellent solubilizer should not be ignored. Eudragit EPO and Kollidon VA64 seemed decent carriers; so, these polymers were chosen to prepare BAC solid dispersions by HME.
The Physicochemical Characterization
In Vitro Dissolution Study
Dissolution profiles of BAC-VA64 and BAC-EPO systems in Table IV were shown in Figs. 1 and 2, respectively.
Table IV.
The Result of Solid Dispersions Using Melting Method
| Formulation | Component | Melting temperature (°C) | Film transparency | Solubility (μg/mL) | ||
|---|---|---|---|---|---|---|
| Carrier | Plasticizer | Physical mixtures | Solid dispersions | |||
| SD1 | Kollidon VA64 | Cremophor RH | 160 | Transparent | 23.4 | 107.6 |
| SD2 | Eudragit EPO | Cremophor RH | 160 | Transparent | 26.6 | 124.1 |
| SD3 | Soluplus | Cremophor RH | 160 | Transparent | 17.3 | 72.4 |
| SD4 | HPMC | Cremophor RH | 190 | Turbid, crystalline | ND | ND |
| SD5 | Kollidon VA64 | PEG 2000 | 165 | Slightly turbid | 16.3 | 69.1 |
| SD6 | Eudragit EPO | PEG 2000 | 165 | Slightly turbid | 18.9 | 77.5 |
| SD7 | Soluplus | PEG 2000 | 165 | Slightly turbid | 13.5 | 49.6 |
| SD8 | Kollidon VA64 | Poloxamer 188 | 170 | Slightly turbid | 15.8 | 59.9 |
| SD9 | Eudragit EPO | Poloxamer 188 | 170 | Slightly turbid | 16.5 | 68.2 |
| SD10 | Soluplus | Poloxamer 188 | 170 | Slightly turbid | 12.7 | 48.8 |
The solubility was an average from the three determined values
ND not detected, PEG polyethylene glycol
Fig. 1.
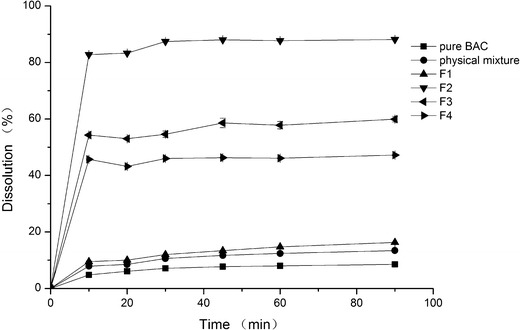
Dissolution profiles of BAC-VA group (the component of physical mixture is correspondent to F3)
Fig. 2.
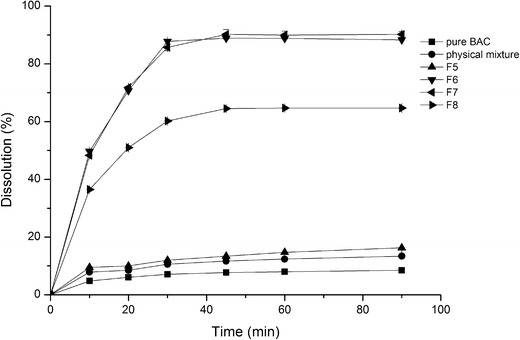
Dissolution profiles of BAC-EPO group (the component of physical mixture is correspondent to F7)
In Fig. 1, the dissolution of pure drug was very low; only 8% of the drug was dissolved at 90 min. The physical mixture showed little effect in improving dissolution, and the similar result was F1, but the other three formulations (F2–4) greatly improved dissolution. As the solid dispersion was prepared by HME, the main difference of F1 to F2–4 was no Cremophor in formulation. Based on the dissolution results of pure drug and the physical mixture, we found that the solubilization effect of Cremophor was limited in the present formulations; it behaved more like a plasticizer to improve the plasticity of the formulation. Just as no Cremophor in F1, the drug melted incompletely in carrier which resulted to the low dissolution. The present results indicated that the plasticizer may be playing an important role in HME for the drug with high melting point.
In Fig. 1, the difference of F2–4 was the drug content, from 10% to 33%; it was obvious the solid dispersion with lower drug content had the higher dissolution. The dissolution of F2 with 10% drug content reached to 90% at 90 min, 11.2 times than that of the pure drug.
The same tendency in BAC-EPO system was shown in Fig. 2. But there was some variance. We noticed both dissolution of F6 and F7 which reached 90% at 90 min, although the drug content of F7 was 20%, two times of F6. It showed that Eudragit EPO had better miscibility with BAC than Kollidon VA64, which can accommodate more drugs in solid dispersion. The previous screening study also proved the result.
Another noteworthy phenomenon was the dissolution rate of BAC-VA group was quicker than BAC-EPO group, which may be related with the wettability of polymer. Kollidon VA64 is a copolymer of vinylpyrrolidone and vinyl acetate. The ratio of both monomers is balanced in such a way that the polymer is still freely water soluble. While Eudragit EPO is a cationic copolymer which solubility is influenced by the pH, its wettability is inferior to Kollidon VA64 in water.
Based on the dissolution results, the F2 of BAC-VA system (the drug content was 10%) and F7 of BAC-EPO system (the content of drug was 20%) were used for further analyses.
Differential Scanning Calorimetry
Figure 3 was the DSC profiles of F2 (BAC-VA system) and F7 (BAC-EPO system). The single BAC showed a sharp endothermic peak around 265°C, a very high melting point. To the BAC-VA physical mixtures, the endothermic peak of BAC still can be observed, but disappeared in BAC-EPO physical mixture, which may be attributed to drug dissolved in Eudragit EPO following the increasing temperature. In both solid dispersions, the DSC profiles were similar with the carrier, no observation in the endothermic peak of BAC. The peak absence of BAC in solid dispersion may be attributed to the change of BAC from crystalline to amorphous form after HME powder X-ray diffraction.
Fig. 3.
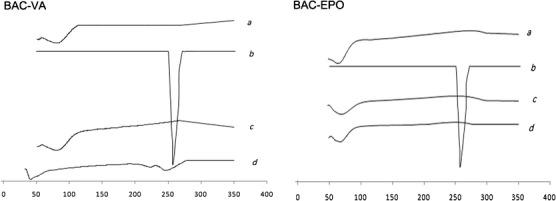
DSC profiles of (a) mixture of carrier and plasticizer, (b) BAC, (c) solid dispersion, and (d) physical mixture (the ratio was correspondent to the solid dispersion)
Figure 4 was the PXRD patterns of F2 (BAC-VA system) and F7 (BAC-EPO system). BAC showed obvious crystalline peaks, while Kollidon VA64 and Eudragit EPO were amorphous. The physical mixtures still exhibited BAC crystalline peaks, but weakened due to mixing some polymers. To the solid dispersions, the absence of drug peaks indicated that most of BAC dispersed in the amorphous state, which was in agreement with the DSC results.
Fig. 4.
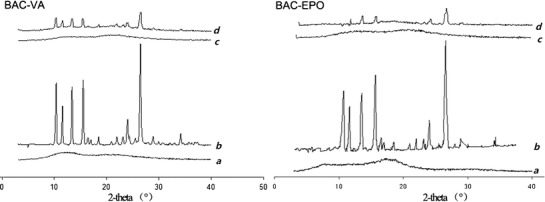
PXRD patterns of (a) mixture of carrier and plasticizer, (b) BAC, (c) solid dispersion, and (d) physical mixture (the ratio was correspondent to the solid dispersion)
Fourier Transform Infrared Spectroscopy
FTIR was used to study the possible interactions of BAC with polymer. The results were shown in Fig. 5. The structure of the carriers and BAC was shown in Fig. 6. The absorption peak at 3,410.2 cm−1 for BAC was free hydroxyl group, 3,090.5 and 3,054.4 cm−1 for the binding peaks were derived from intermolecular hydroxyl group, and the peaks of 1,657.8, 1,618.1, and 1,585.3 cm−1 were due to the absorption of benzene ring.
Fig. 5.
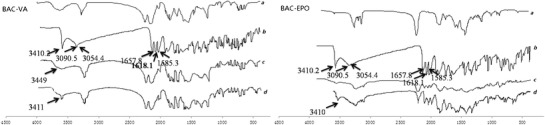
FTIR spectra of (a) mixture of carrier and plasticizer, (b) BAC, (c) solid dispersion, and (d) physical mixture (the ratio was correspondent to the solid dispersion)
Fig. 6.
The structure of the carriers and BAC
In the physical mixture, the peak at 3,411 cm−1 that originated from BAC is still observed, which indicated that simple physical mixing had no interaction between BAC and polymer, while a broadened peak at 3,449.4 cm−1 in the solid dispersion meant the intermolecular hydrogen bonds between drug and carrier after HME. In addition, the peaks of BAC-EPO solid dispersion from 1,500 to 1,600 cm−1 changed obviously, broaden and lower intensity, which meant there was stronger interaction between BAC and Eudragit EPO. This may explain why BAC and Eudragit EPO had better miscibility than Kollidon VA64.
The Primary Stability Study
The stability results of F2 and F7 without package at the stress testing conditions were listed in Table V. The content of F2 and F7 kept uniform, but the dissolution decreased evidently at high temperature and high humidity, which meant F2 and F7 were sensitive to the temperature and humidity. The long-term stability results of F2 and F7 with package were shown in Table VI. After storage at 25 ± 2°C and 60 ± 10% relative humidity for 6 months, no content was changed, and the dissolution slightly decreased. These results indicated that the suitable package to prevent the moisture and the suitable temperature were very important for BAC solid dispersion.
Table VI.
The Stability Results of F2 and F7 at the Long-Time Stability Testing Condition (Mean ± SD, n = 3)
| Formulation | 0 month | 1 month | 3 months | 6 months | |
|---|---|---|---|---|---|
| Content (%) | F2 | 100.0 ± 0.5 | 98.5 ± 0.1 | 97.3 ± 0.2 | 97.6 ± 0.3 |
| F7 | 100.0 ± 0.3 | 98.9 ± 0.3 | 97.1 ± 0.1 | 98.2 ± 0.2 | |
| Dissolution (%) | F2 | 86.7 ± 1.3 | 86.5 ± 2.1 | 83.3 ± 2.4 | 81.6 ± 4.3 |
| F7 | 88.9 ± 0.9 | 87.4 ± 2.4 | 85.1 ± 3.1 | 84.9 ± 3.6 |
Pharmacokinetic Study
The plasma concentration versus time curves was shown in Fig. 7. The Tmax of F2 and F7 shortened to about 1 h compared with 2 h of pure drug, which can be due to the fast dissolution of the solid dispersion. The Cmax of F2 and F7 was significantly higher than that of pure drug (p < 0.05), about 3.56 and 2.68 times, respectively. The area under concentration-time curve (AUC) of F2 and F7 were about 2.89- and 2.41-fold of pure drug, respectively (p < 0.05). Compared with the pure drug, BAC showed better bioavailability after HME; it was obvious that the amorphous state of BAC in carrier resulted in the faster and higher dissolution. As BAC belongs to BCS Class II, so the improved dissolution was very important to the oral bioavailability of BAC.
Fig. 7.
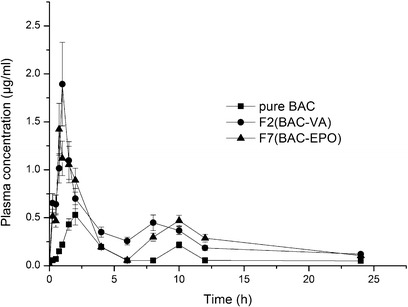
Mean plasma concentration–time profiles of BAC in beagle dog. Each symbol shows the mean ± SD (n = 6)
The Cmax and AUC of F2 (BAC-VA) were slightly higher than F7 (BAC-EPO), which may be attributed to the higher hydrophilic nature of Kollidon VA64 than Eudragit EPO, but there was no significant difference between F2 and F7 (p > 0.05). Moreover, as previously mentioned, Eudragit EPO can accommodate more BAC, which would be attractive in formulation design.
CONCLUSION
In our study, four carriers including Kollidon VA64, Eudragit EPO, HPMC, and Soluplus were screened; the results proved Kollidon VA64 and Eudragit EPO were more suitable carriers for BAC, due to their operability and solubilization effect. But there are still some differences between them. Compared with hydrophobic Eudragit EPO, hydrophilic Kollidon VA64 helps BAC faster dissolution in vitro and in vivo, but Eudragit EPO has stronger interaction with BAC which can accommodate more BAC in preparation; this would be favorable for BAC of high dose.
Our study also indicated the importance of the plasticizer for the drug with high melting point in HME. Using the plasticizer in HME would help to soften the carrier and the drug with high melting point, which decrease the melting temperature to meet the requirement of the machine. At the same time, the drug can more easily disperse in the carrier to achieve the amorphous state, which is the main cause of improved dissolution and bioavailability.
In summary, we adopted the proper carrier and plasticizer to obtain BAC solid dispersions by HME. The dissolution of the solid dispersions was improved than that of pure drug and physical mixtures. Furthermore, the solid dispersions also exhibited a significantly enhanced bioavailability.
ACKNOWLEDGMENTS
This study was financially supported by the Program for New Century Excellent Talents (NCET-11-0736) and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (no. JKGP201102). We would like to thank the BASF and Evonik Corporations for providing carriers.
REFERENCES
- 1.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 2.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 3.Van den Mooter G. The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Tech. 2012;9(2):e79–e85. doi: 10.1016/j.ddtec.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Sarraf A, Tissot H, Tissot P, Alfonso D, Gurny R, Doelker E. Influence of hot-melt extrusion and compression molding on polymer structure organization, investigated by differential scanning calorimetry. J Appl Polym Sci. 2001;81(13):3124–3132. doi: 10.1002/app.1764. [DOI] [Google Scholar]
- 5.Zheng X, Yang R, Tang X, Zheng L. Part I: characterization of solid dispersions of nimodipine prepared by hot-melt extrusion. Drug Dev Ind Pharm. 2007;33(7):791–802. doi: 10.1080/03639040601050213. [DOI] [PubMed] [Google Scholar]
- 6.Kalivoda A, Fischbach M, Kleinebudde P. Application of mixtures of polymeric carriers for dissolution enhancement of oxeglitazar using hot-melt extrusion. Int J Pharm. 2012;439:145–156. doi: 10.1016/j.ijpharm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 7.He H, Yang R, Tang X. In vitro and in vivo evaluation of fenofibrate solid dispersion prepared by hot-melt extrusion. Drug Dev Ind Pharm. 2010;36(6):681–687. doi: 10.3109/03639040903449720. [DOI] [PubMed] [Google Scholar]
- 8.Maniruzzaman M, Rana M, Boateng J, Mitchell J, Douroumis D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev Ind Pharm. 2013;39(2):218–227. doi: 10.3109/03639045.2012.670642. [DOI] [PubMed] [Google Scholar]
- 9.Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PDL. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol. 2003;55(2):205–209. doi: 10.1211/002235702522. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420(1):180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Qiu L, Gao J, Jin Y. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Int J Pharm. 2006;312(1):137–143. doi: 10.1016/j.ijpharm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Tian R, Hu W, Jia Y, Jiang H, Zhang J, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83:1532–1539. doi: 10.1016/j.fitote.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh I, Snyder J, Vippagunta R, Alvine M, Vakil R, Tong W-QT, et al. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int J Pharm. 2011;419(1):12–19. doi: 10.1016/j.ijpharm.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 14.Hughey JR, Keen JM, Brough C, Saeger S, McGinity JW. Thermal processing of a poorly water-soluble drug substance exhibiting a high melting point: the utility of KinetiSol® Dispersing. Int J Pharm. 2011;419(1):222–230. doi: 10.1016/j.ijpharm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm Res. 2012;29(3):806–817. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- 16.Aharoni SM. Increased glass transition temperature in motionally constrained semicrystalline polymers. Polym Adv Technol. 1998;9(3):169–201. doi: 10.1002/(SICI)1099-1581(199803)9:3<169::AID-PAT740>3.0.CO;2-Z. [DOI] [Google Scholar]
- 17.Mohammad MA, Alhalaweh A, Velaga SP. Hansen solubility parameter as a tool to predict cocrystal formation. Int J Pharm. 2011;407(1):63–71. doi: 10.1016/j.ijpharm.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh DJ, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999;88(11):1182–1190. doi: 10.1021/js9900856. [DOI] [PubMed] [Google Scholar]


