Abstract
It has been advocated that biopharmaceutic risk assessment should be conducted early in pediatric product development and synchronized with the adult product development program. However, we are unaware of efforts to classify drugs into a Biopharmaceutics Classification System (BCS) framework for pediatric patients. The objective was to classify five drugs into a potential BCS. These five drugs were selected since both oral and intravenous pharmacokinetic data were available for each drug, and covered the four BCS classes in adults. Literature searches for each drug were conducted using Medline and applied to classify drugs with respect to solubility and permeability in pediatric subpopulations. Four pediatric subpopulations were considered: neonates, infants, children, and adolescents. Regarding solubility, dose numbers were calculated using a volume for each subpopulation based on body surface area (BSA) relative to 250 ml for a 1.73 m2 adult. Dose numbers spanned a range of values, depending upon the pediatric dose formula and subpopulation. Regarding permeability, pharmacokinetic literature data required assumptions and decisions about data collection. Using a devised pediatric BCS framework, there was agreement in adult and pediatric BCS class for two drugs, azithromycin (class 3) and ciprofloxacin (class 4). There was discordance for the three drugs that have high adult permeability since all pediatric permeabilities were low: dolasetron (class 3 in pediatric), ketoprofen (class 4 in pediatric), and voriconazole (class 4 in pediatric). A main contribution of this work is the identification of critical factors required for a pediatric BCS.
KEY WORDS: absorption, bioavailability, bioequivalence, biopharmaceutics classification system, pediatric
INTRODUCTION
The Biopharmaceutics Classification System (BCS) is employed to provide biowaivers for new and generic drugs that contain BCS class 1 drug (1). The BCS considers factors such as drug solubility, drug permeability, and product dissolution rate (2). BCS-based biowaivers eliminate unnecessary human drug exposures, reduce regulatory burden, and provide economic relief, while maintaining the high public health standard for therapeutic equivalence (3). A BCS-based biowaiver has become an important and cost-saving tool in approval of new and generic drugs (4).
The BCS is implicitly a framework for the regulation of drug products for adults. However, these same biopharmaceutic considerations would appear to be applicable to products for pediatric patients as well, although such biopharmaceutic considerations have not been well elucidated in the scientific community. The development of pediatric drug products is difficult, including the assessment of biopharmaceutic risk in the pediatric population (5). It has been recognized that further research is needed to facilitate the development of a pediatric-specific BCS system (6). Of course, adult and pediatric patients differ in many aspects (7,8). For example, pediatric patients have developing and significantly different absorption, distribution, metabolism, and excretion processes, in comparison to adults (7,8). These differences, along with other changes during growth and development, indicate the need for a BCS for pediatric medications.
The Pediatric Biopharmaceutics Classification System Working Group has outlined considerations for a possible pediatric BCS (8). Work in this present study extends findings of the Pediatric Biopharmaceutics Classification System Working Group. The objective was to classify five drugs into a potential pediatric BCS. Dolasetron, ketoprofen, voriconzole, azithromycin, and ciprofloxacin were selected since both oral and intravenous pharmacokinetic data was available for each drug. Additionally, these five drugs cover all four of the adult BCS classes, as discussed below. Most drugs suffer from insufficient pharmacokinetic data and lack of information on calculated pediatric dosage. Dolasetron was selected since it is FDA approved in pediatrics for preventing postoperative nausea and vomiting (PONV) (9). Ketoprofen has been reported to be safe and effective in children for post-surgical pain and the relief of pain and fever in inflammatory conditions, although not labeled for this indication in children (10). Voriconazole, a triazole antifungal drug, accounted for about 10% of prescribed systemic antifungal therapy in nearly 63,000 US pediatric inpatients in 2006 (11). However, no efficacy and safety studies have been conducted in patients younger than 12 years of age. Similarly, azithromycin is used for several types of gram-positive and gram-negative bacterial infections in children with no safety and efficacy studies in infants under 6 months of age (12). Ciprofloxacin HCl was selected since it has pediatric indications for treatment of anthrax and urinary tract infections (13). Relative to a vast majority of other FDA-approved drugs, these five drugs represent relatively favorable scenarios to attempt to classify these drugs into a pediatric BCS since some bioavailability data is available in adults and pediatrics. As discussed below, several challenges persist in classifying these five drugs.
MATERIALS AND METHODS
Pharmacokinetic Data
Dolasetron, ketoprofen, voriconzole, azithromycin, and ciprofloxacin were identified from Medline literature searches to be drugs with both oral and intravenous pharmacokinetic data in a pediatric population. Pharmacokinetic data for each drug used several key words, including the drug name, ‘pharmacokinetics’, and ‘pediatric’. Identified studies were further evaluated for availability of pharmacokinetic exposure parameters (e.g., AUC) for both oral and intravenous routes in patients between ages 0 and 16 years.
Drug Solubility
Drug solubility (milligrams per milliliter) of ketoprofen, voriconazole, and ciprofloxacin were obtained from Benet et al. (14). Azithromycin solubility was obtained from a study by Curatolo (15). Dolasetron’s “freely soluble” solubility was obtained from the package insert and interpreted relative to the USP solubility table per Takagi et al. (16).
Pediatric Solubility Classification: General Approach
Pediatric drug solubility classification was determined via pediatric dose number calculation, following common clinical dose equations (7). A pediatric dose number required pediatric dose, as well as pediatric reference volume. Complicating the consideration for a pediatric BCS is that subpopulations exist within the pediatric population. A pediatric reference volume (Vp) was calculated for each of four subpopulations: neonates (0–1 months), infant (1–24 months), children (2–12 years), and adolescents (12–16 years). For each subpopulation, the volume was calculated based on body surface area (BSA), relative to the adult volume of 250 ml and adult BSA of 1.73 m2. Sub-population BSA was calculated from:
where height and weight were taken to be the 50 percentile of boy values in the CDC growth charts for ages 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). These BSA-derived volumes yield 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. Of note, there is very little drug information in pediatrics younger than 2 years old.
Pediatric dose (Mp) was calculated using several different formulas, including Young’s Rule, Clark’s Rule, Modified Weight Rule, and Body Surface Area Method (7). These various methods were applied since no single definitive rule exists in practice. The formulas for Young’s Rule, Clark’s Rule, Modified Weight Rule, and Body Surface Area Method are:
| 1 |
| 2 |
| 3 |
| 4 |
Regarding a pediatric solubility classification, a pediatric dose number was taken to be
where Dp is pediatric dose number, Mp is pediatric dose, Vp is pediatric reference volume, and Cp is pediatric drug solubility. Pediatric dose number follows the “adult BCS” dose number in terms of considering dose, volume, and drug solubility, although clearly needs to consider the pediatric application (1). Pediatric dose numbers greater than 1 were classified as low solubility. Pediatric dose numbers that are one or less were classified as high solubility.
Pediatric Solubility Classification: Doses from Literature
In addition to the general approach above, pediatric drug solubility classification was also determined from doses that were administered in the literature used here for permeability classification. For dolasetron, the mean weight was used to determine oral dose, which was provided in terms of 1.8 mg/kg (19). Mean weight and height were used to calculate BSA-derived pediatric reference volume using mean age of 10.8 years. For ketoprofen, the manuscript indicated target oral doses of either 12 or 25 mg (10). Individual patient data was available and applied to consider dose, which was provided in terms of 0.74 to 1.39 mg/kg. Individual weight and height were used to calculate BSA-derived pediatric reference volume. For voriconazole, the oral dose was 200 mg (20). Mean weight and height were determined from mean age of 5.4 years via the CDC growth chart for boys, which were then used to calculate BSA-derived pediatric reference volume. For azithromycin, the oral dose was 60 or 80 mg (21). Individual ages were given, such that individual weight and height were determined from the CDC growth chart for boys, which were then used to calculate BSA-derived pediatric reference volume. For ciprofloxacin, the oral dose was 300, 450, or 600 mg (22). Individual ages and weights were given, such that individual heights were determined from the CDC growth chart for boys, which were then used to calculate BSA-derived pediatric reference volume.
Pediatric Permeability Classification
Permeability classification applied the “adult BCS” definition, but was based on absolute bioavailability in pediatrics. High permeability required a fraction dose absorbed of 90% or more (1). Hence, permeability classifications of dolasetron, ketoprofen, voriconzole, azithromycin, and ciprofloxacin were based on literature oral and intravenous AUC data (i.e., absolute bioavailability in pediatrics). Absolute bioavailability was calculated by
where Fp is the absolute bioavailability, AUCpo is the area under the curve of the oral dose, dosepo is the dose administered by mouth, AUCIV is the area under the curve of the intravenous dose, and doseIV is the intravenous dose administered.
RESULTS
Dolasetron Pediatric Classification
Two approaches were employed to identity pediatric drug solubility class for dolasetron and the four other drugs. Firstly, a general approach was taken, where common dose formulas were used to calculate pediatric dose from adult dose (i.e., Eqs. 1–4). Additionally, for each drug, the dose that was used in the oral pharmacokinetic study for permeability classification was used to classify solubility.
Dolasetron is “freely soluble” per its package insert and interpreted to be 100 mg/ml (16). In Table I, for all dose formulas and subpopulations using the general approach (i.e., Eqs. 1–4), dolasetron was estimated to be highly soluble (i.e., dose number less than one). Interestingly, dose and dose number varied with pediatric age groups. For each dose formula except BSA, dose and dose number generally increased with age. For example, using Clark’s Rule, the dose number was 0.00169, 0.00227, 0.00264, and 0.00340 for neonates, infants, children, and adolescents, respectively. However, since pediatric reference volume was based on BSA, the BSA-based dose number was always 0.004 across all four pediatric subpopulations.
Table I.
Dolasetron Pediatric Dose Number for Various Pediatric Subpopulations
| Dose formula | Neonates (0–1 months) |
Infant (1–24 months) |
Children (2–12 years) |
Adolescents (12–16 years) |
|---|---|---|---|---|
| Young’s Rulea, b | 0.0000996 | 0.00119 | 0.00289 | 0.00244 |
| Clark’s Rulea, c | 0.00169 | 0.00227 | 0.00264 | 0.00340 |
| Modified Weight Rulea, d | 0.00230 | 0.00310 | 0.00361 | 0.00463 |
| Body surface areaa, e | 0.00400 | 0.00400 | 0.00400 | 0.00400 |
| FDA-approved labeled dosef | Not indicated | Not indicated | 0.00324 | 0.00417 |
Age was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). Conversion from dose to dose number employed a BSA-derived volume of 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. BSA employed height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). Drug solubility was taken to be 100 mg/ml
aIn calculating pediatric dose for each subpopulation and from each dose formula, the adult dose was taken to be 100 mg
bFrom Young’s Rule, the dose was calculated to be 0.346, 7.99, 36.8, and 53.8 mg for neonates, infants, children, and adolescents, respectively
cClark’s Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Clark’s Rule, the dose was calculated to be 5.87, 15.3, 33.7, and 74.8 mg for neonates, infants, children, and adolescents, respectively
dModified Weight Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Modified Weight Rule, the dose was calculated to be 8.00, 20.9, 46, and 102 mg for neonates, infants, children, and adolescents, respectively
eBody surface area employs height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From body surface area, the dose was calculated to be 13.9, 27.0, 51.0, and 88.1 mg for neonates, infants, children, and adolescents, respectively
fThe FDA-approved labeled dose for prevention of chemotherapy induced nausea and vomiting from the package insert is 1.8 mg/kg for the pediatric population >2 years old (9). From FDA-approved label dose, the dose was calculated to be 41.4 and 91.8 mg for children and adolescents, respectively. FDA-approved labeled dose employs weight, which were taken to be the 50 percentile boy value in the CDC growth charts for children and adolescents (17,18)
For any one age group, the differing dose formulas yielded different doses and dose numbers. Young’s Rule tended to provide the lowest doses and dose numbers. BSA tended to provide the highest dose and dose numbers. Clark’s Rule yielded the lowest values for children. The modified Weight Rule calculated the highest values for adolescents; interestingly, the calculated dose of 102 mg was higher than the adult dose of 100 mg. Of note, the four dose formulas yielded an over 40-fold range in doses and dose numbers for neonates. For example, for neonates, Young’s Rule provided a dose number of 0.0001, while BSA calculated a dose number of 0.004. For infants, the range spanned over threefold. For children and adolescents, the ranges were about twofold.
Additionally, the dose number from the literature study, which concerned children, was 0.00369 (19). This low solubility classification agreed with the result from Young’s Rule, Clark’s Rule, Modified Weight Rule, and Body Surface Area Method in Table I.
The pediatric absolute bioavailability of dolasetron was calculated to be 60.9%, from two separate studies conducted in a similar fashion by the same investigators (19,23). From an oral dose of 1.2 mg/kg, the AUCpo was 0.578 μg h/ml. From an intravenous dose of 1.2 mg/kg, the AUCIV was 0.949 μg h/ml. Since the absolute bioavailability of dolasetron was less than 90%, the drug was classified as a low permeability drug in children. Overall, these considerations indicate dolasetron is class 3 in a pediatric BCS, compared to class 1 in adults.
Ketoprofen Pediatric Classification
Ketoprofen’s solubility is 0.18 mg/ml (14). In Table II, ketoprofen was usually low solubility (dose number > 1), although was high solubility (dose number < 1) in five occasions: neonates using Young’s rule, Clark’s rule, and Modified Weight Rule (dose numbers of 0.0415, 0.703, and 0.960, respectively); and infants using Young’s rule and Clark’s rule (dose numbers of 0.494 and 0.948, respectively).
Table II.
Ketoprofen Pediatric Dose Number for Various Pediatric Subpopulations
| Dose formula | Neonates (0–1 months) |
Infant (1–24 months) |
Children (2–12 years) |
Adolescents (12–16 years) |
|---|---|---|---|---|
| Young’s Rulea | 0.0415 | 0.494 | 1.20 | 1.02 |
| Clark’s Ruleb | 0.703 | 0.948 | 1.10 | 1.41 |
| Modified Weight Rulec | 0.960 | 1.29 | 1.50 | 1.93 |
| Body surface aread | 1.67 | 1.67 | 1.67 | 1.67 |
In calculating pediatric dose for each subpopulation and from each dose formula, the adult dose was taken to be 75 mg. Age was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). Conversion from dose to dose number employed a BSA-derived volume of 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. BSA employed height and weight, which were taken to be the 50 percentile of boy values in the CDC growth charts for ages 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). Drug solubility was taken to be 0.180 mg/ml (14). Ketoprofen is not indicated for use in the pediatric population (24)
aYoung’s Rule employs age, which was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively) (17,18). From Young’s Rule, the dose was calculated to be 0.260, 5.99, 27.6, and 40.4 mg for neonates, infants, children, and adolescents, respectively
bClark’s Rule employs weight, which was taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From Clark’s Rule, the dose was calculated to be 4.40, 11.5, 25.3, and 56.1 mg for neonates, infants, children, and adolescents, respectively
cModified Weight Rule employs weight, which was taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From Modified Weight Rule, the dose was calculated to be 6.00, 15.7, 34.5, and 76.5 mg for neonates, infants, children, and adolescents, respectively
dBody surface area employs height and weight, which were taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From body surface area, the dose was calculated to be 10.4, 20.2, 38.3, and 66.1 mg for neonates, infants, children, and adolescents, respectively
Like for dolasetron, dose and dose number for ketoprofen varied with pediatric age groups. For example, for each dose formula except BSA, ketoprofen dose and dose number generally increased with age. Like for dolasetron, the four dose formulas yielded an over 40-fold range in ketoprofen doses and dose numbers for neonates. For neonates, Young’s Rule provided a dose number of 0.0415, while BSA calculated a dose number of 1.67.
From the literature pharmacokinetic study where ketoprofen was orally administered as either 12 mg or 25 mg doses to pediatric patients, Table III shows that the 12 mg dose yielded high solubility and 25 mg yielded low solubility (10). In Table III, both infants and half of the eight children afforded ketoprofen to be high solubility since each received 12 mg dose. The other half of the children received the 25 mg dose and afforded ketoprofen to be low solubility. Overall, solubility classification results from dosing equations (Table II) and this literature study (Table III) are similar and provided high and low solubilities under various conditions.
Table III.
Calculation of Ketoprofen Dose Number for Individual Patients Reported in Kokki et al. (10)
| Subject | Age (month) |
Weight (kg) |
Height (cm) | BSA-derived volume (ml) | Dose (mg) |
Dose number |
|---|---|---|---|---|---|---|
| 11 | 16 | 12 | 84 | 76.5 | 12.5 | 0.907 |
| 12 | 52 | 17 | 100 | 99.3 | 12.6 | 0.704 |
| 13 | 22 | 15 | 89 | 88.0 | 12.5 | 0.786 |
| 14 | 35 | 15 | 95 | 90.9 | 12.5 | 0.761 |
| 15 | 61 | 20 | 111 | 113.5 | 25.0 | 1.22 |
| 16 | 54 | 19 | 110 | 110 | 25.1 | 1.27 |
| 17 | 59 | 23 | 115 | 124 | 25.1 | 1.12 |
| 18 | 25 | 12 | 88 | 78.3 | 12.5 | 0.886 |
| 19 | 69 | 17 | 111 | 104 | 12.6 | 0.668 |
| 20 | 69 | 18 | 114 | 109 | 25.0 | 1.27 |
Subject age, weight, height and dose were obtained from the article to calculate BSA-derived pediatric reference volume. Dose numbers for each infant and child reflected high solubility when dose was low (i.e., 12 mg) and low solubility when the dose was high (i.e., 25 mg). Dose number values here were approximately one, in agreement with the general approach (Table II)
Using oral and intravenous data from two separate studies, the absolute bioavailability of ketoprofen was calculated to be 46.9% (10,25,26). From an oral dose of 25 mg, the AUCpo was 11.4 mg h/L (10,26). From an intravenous dose of 16.2 mg, the AUCIV was 15.4 mg h/L (25,26). Overall, these considerations indicate ketoprofen would be class 4 in the two older pediatric subpopulations: children and adolescents. This classification differs from ketoprofen being a class 2 drug in adults (14). However, for neonates and infants, differing dose formulas yield either class 3 or class 4.
Voriconazole Pediatric Classification
Voriconazole’s solubility is 0.39 mg/ml (14). In Table IV, voriconazole was generally found to be lowly soluble. However, the drug was highly soluble in three occasions: neonates using Young’s rule and Clark’s rule (dose numbers of 0.0511 and 0.866 respectively); and infants using Young’s rule (dose number of 0.608).
Table IV.
Voriconazole Pediatric Dose Number for Various Pediatric Subpopulations
| Dose formula | Neonates (0–1 months) |
Infant (1–24 months) |
Children (2–12 years) |
Adolescents (12–16 years) |
|---|---|---|---|---|
| Young’s Rulea, b | 0.0511 | 0.608 | 1.48 | 1.25 |
| Clark’s Rulea, c | 0.866 | 1.17 | 1.36 | 1.74 |
| Modified Weight Rulea, d | 1.18 | 1.59 | 1.85 | 2.37 |
| Body surface areaa, e | 2.05 | 2.05 | 2.05 | 2.05 |
| FDA-approved labeled dosef | Not indicated | Not indicated | Not indicated | 2.33 |
Age was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). Conversion from dose to dose number employed a BSA-derived volume of 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. BSA employed height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). Drug solubility was taken to be 0.39 mg/ml (14)
aIn calculating pediatric dose for each subpopulation and from each dose formula, the adult dose was taken to be 200 mg
bYoung’s Rule employs age, which was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). From Young’s Rule, the dose was calculated to be 0.692, 16.0, 73.7, and 107.7 mg for neonates, infants, children, and adolescents, respectively
cClark’s Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Clark’s Rule, the dose was calculated to be 11.7, 30.7, 67.5, and 149 mg for neonates, infants, children, and adolescents, respectively
dModified Weight Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Modified Weight Rule, the dose was calculated to be 16.0, 41.8, 92.0, and 204 mg for neonates, infants, children, and adolescents, respectively
eBody surface area employs height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From body surface area, the dose was calculated to be 27.8, 35.9, 102, and 176 mg for neonates, infants, children, and adolescents, respectively
fThe FDA-approved labeled dose for candidiasis from the package insert is 200 mg for the pediatric population > 12 years old (27)
This propensity for low solubility was also reflected in the literature study of children, where the dose number was 4.22 (20). This low solubility classification agreed with the result from Young’s Rule, Clark’s Rule, Modified Weight Rule, and Body Surface Area Method in Table IV.
The pediatric absolute bioavailability of voriconazole was calculated to be 69.4% from a single pharmacokinetic study evaluating an intravenous to oral switch (20). From an oral dose of 200 mg, the AUCpo was 18.6 μg h/ml. From an intravenous dose of 7 mg/kg, the AUCIV was 21.4 μg h/ml. The drug was classified as a low permeability drug in children. Overall, these considerations indicate voriconazole would be a BCS class 4 in most pediatric groups, except class 3 using Young’s rule for neonates and infants, and Clark’s rule for neonates. These results do not correspond with adults in whom voriconazole is class 2.
Azithromycin Pediatric Classification
Azithromycin’s solubility is 5 mg/ml (15). In Table V, azithromycin was highly soluble in all cases. High solubility is also calculated from the literature study (21). Table VI lists the calculated dose number for each individual infant and child from the literature study, which were always less than one. This high solubility classification agreed with results from Young’s Rule, Clark’s Rule, Modified Weight Rule, and Body Surface Area Method in Table V.
Table V.
Azithromycin Pediatric Dose Number for Various Pediatric Subpopulations
| Dose formula | Neonates (0–1 months) |
Infant (1–24 months) |
Children (2–12 years) |
Adolescents (12–16 years) |
|---|---|---|---|---|
| Young’s Rulea, b | 0.0119 | 0.142 | 0.346 | 0.293 |
| Clark’s Rulea, c | 0.203 | 0.273 | 0.317 | 0.408 |
| Modified Weight Rulea, d | 0.276 | 0.372 | 0.433 | 0.556 |
| Body surface areaa, f | 0.480 | 0.480 | 0.480 | 0.480 |
| FDA-approved labeled dosef | Not indicated | 0.310 | 0.360 | 0.460 |
Age was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). Conversion from dose to dose number employed a BSA-derived volume of 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. BSA employed height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). Drug solubility was taken to be 0.06871 mg/ml (14)
aIn calculating pediatric dose for each subpopulation and from each dose formula, the adult dose was taken to be 600 mg
bYoung’s Rule employs age, which was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). From Young’s Rule, the dose was calculated to be 2.08, 47.9, 221, and 323 mg for neonates, infants, children, and adolescents, respectively
cClark’s Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Clark’s Rule, the dose was calculated to be 35.2, 92.0, 202, and 449 mg for neonates, infants, children, and adolescents, respectively
dModified Weight Rule employs weight, which was taken to be the 50 percentile boy weight in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From Modified Weight Rule, the dose was calculated to be 48.0, 126, 276, and 612 mg for neonates, infants, children, and adolescents, respectively
eBody surface area employs height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for neonates, infants, children, and adolescents (17,18). From body surface area, the dose was calculated to be 83.4, 162, 307, and 529 mg for neonates, infants, children, and adolescents, respectively
fThe FDA-approved labeled dose for community-acquired pneumonia from the package insert is 10 mg/kg for the pediatric population > 6 months old (12). From FDA-approved label dose, the dose was calculated to be 104.5, 230, and 510 mg for infants, children, and adolescents, respectively (12). FDA-approved labeled dose employs weight, which were taken to be the 50 percentile boy value in the CDC growth charts for infants, children and, adolescents (17,18)
Table VI.
Calculation of Azithromycin Dose Number for Individual Patients Reported in Nahata et al. (21)
| Subject | Age (years) |
Weight (kg) |
Height (cm) | BSA-derived volume (ml) | Dose (mg) |
Dose number |
|---|---|---|---|---|---|---|
| 1 | 2.3 | 13.0 | 88.0 | 81.5 | 60 | 0.15 |
| 2 | 2.1 | 12.5 | 87.5 | 79.7 | 60 | 0.15 |
| 3 | 2.3 | 13.0 | 88.0 | 81.5 | 60 | 0.15 |
| 4 | 5.0 | 18.0 | 108 | 106 | 80 | 0.15 |
| 5 | 3.9 | 16.0 | 101 | 96.8 | 80 | 0.17 |
| 6 | 1.9 | 12.5 | 86.5 | 79.2 | 60 | 0.15 |
| 7 | 2.0 | 12.7 | 87.0 | 80.1 | 60 | 0.15 |
| 8 | 1.7 | 12.0 | 84.0 | 76.5 | 60 | 0.16 |
| 9 | 1.1 | 10.6 | 77.0 | 68.8 | 60 | 0.17 |
| 10 | 1.4 | 11.5 | 81.0 | 73.5 | 60 | 0.16 |
| 11 | 1.5 | 11.7 | 82.0 | 74.6 | 60 | 0.16 |
| 12 | 0.5 | 8.0 | 67.0 | 55.8 | 40 | 0.14 |
| 13 | 2.4 | 13.50 | 90.5 | 84.2 | 80 | 0.19 |
Subject age and dose were obtained from the article. Weight and height were determined from the CDC growth chart for boys, which were then used to calculate BSA-derived pediatric reference volume. Dose numbers for each infant and child indicates high solubility, in agreement with the more general approach (Table V)
The pediatric absolute bioavailability of azithromycin was calculated to be 61.6%, from two separate studies (21,28). From a mean oral dose of 4.9 mg/kg, the AUCpo was 1.84 μg h/ml. From an intravenous dose of 10 mg/kg, the calculated AUCIV for 24 h was 6.1 μg h/ml. Using these considerations, azithromycin is pediatric BCS class 3 in agreement with class 3 in adults.
Ciprofloxacin Pediatric Classification
Solubility of ciprofloxacin HCl is 0.15 mg/ml (14). In Table VII, in almost all cases, ciprofloxacin was estimated to be lowly soluble (i.e., dose number greater than one). However, one scenario where ciprofloxacin was highly soluble was in neonates using Young’s Rule; the dose number was 0.498. Dose and dose number varied with pediatric age groups. For each dose formula except BSA, dose and dose number generally increased with age.
Table VII.
Ciprofloxacin HCl Pediatric Dose Number for Various Pediatric Subpopulations
| Dose formula | Neonates (0–1 months) |
Infant (1–24 months) |
Children (2–12 years) |
Adolescents (12–16 years) |
|---|---|---|---|---|
| Young’s Rulea, b | 0.498 | 5.93 | 14.4 | 12.2 |
| Clark’s Rulea, c | 8.44 | 11.4 | 13.2 | 17.0 |
| Modified Weight Rulea, d | 11.5 | 15.5 | 18.0 | 23.2 |
| Body surface areaa, e | 20.0 | 20.0 | 20.0 | 20.0 |
| FDA-approved labeled dosef | Not indicated | 20.7 | 24.0 | 22.7 |
Age was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). Conversion from dose to dose number employed a BSA-derived volume of 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. BSA employed height and weight, which were taken to be the 50 percentile boy value in the CDC growth charts for 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively (17,18). Drug solubility was taken to be 0.150 mg/ml (14)
aIn calculating pediatric dose for each subpopulation and from each dose formula, the adult dose was taken to be 750 mg
bYoung’s Rule employs age, which was taken to be the mid-point of the subpopulation age (i.e., 0.5 months, 12.5 months, 7 years, and 14 years for neonates, infants, children, and adolescents, respectively). From Young’s Rule, the dose was calculated to be 2.60, 59.9, 276, and 404 mg for neonates, infants, children, and adolescents, respectively
cClark’s Rule employs weight, which was taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From Clark’s Rule, the dose was calculated to be 44.0, 115, 253, and 561 mg for neonates, infants, children, and adolescents, respectively
dModified Weight Rule employs weight, which was taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From Modified Weight Rule, the dose was calculated to be 60.0, 157, 345, and 765 mg for neonates, infants, children, and adolescents, respectively
eBody surface area employs height and weight, which were taken to be the 50 percentile of boy values in the CDC growth charts for ages neonates, infants, children, and adolescents (17,18). From body surface area, the dose was calculated to be 104, 202, 383, and 661 mg for neonates, infants, children, and adolescents, respectively
fThe FDA-approved labeled dose for community-acquired pneumonia from the package insert is 20 mg/kg for the pediatric population > 1 year old (13). From FDA-approved label dose, the dose was calculated to be 209, 460, and 750 mg for infants, children, and adolescents, respectively (13). FDA-approved labeled dose employs weight, which were taken to be the 50 percentile boy value in the CDC growth charts for infants, children, and adolescents (17,18)
A literature study dosed children and adolescents (22). Table VIII shows their calculated individual dose numbers, which were always greater than one. This low solubility classification agreed with the result from Young’s Rule (except neonates), Clark’s Rule, Modified Weight Rule, and Body Surface Area Method in Table VII.
Table VIII.
Calculation of Ciprofloxacin Dose Number for Individual Patients Reported in Schaefer et al. (22)
| Subject | Age (years) |
Weight (kg) |
Height (cm) | BSA-derived volume (ml) | Dose (mg) |
Dose number |
|---|---|---|---|---|---|---|
| 1 | 9.3 | 29 | 135 | 151 | 551 | 24.4 |
| 2 | 12.0 | 42 | 149 | 191 | 714 | 25.0 |
| 3 | 10.3 | 28.8 | 140 | 153 | 547 | 24.0 |
| 4 | 10.5 | 26.9 | 141 | 148 | 538 | 24.1 |
| 5 | 9.30 | 18 | 135 | 119 | 432 | 24.3 |
| 6 | 15.7 | 37 | 173 | 193 | 629 | 21.8 |
| 8 | 14.7 | 41 | 169 | 201 | 697 | 23.2 |
| 10 | 6.8 | 19 | 120 | 115 | 456 | 26.4 |
Subject age, weight, and dose were obtained from the article. Height was determined from the CDC growth chart for boys, which was then used to calculate BSA-derived pediatric reference volume. Dose numbers for each child and adolescent indicates low solubility, in agreement with the more general approach (Table VII)
The pediatric absolute bioavailability of ciprofloxacin HCl was calculated to be 57.3%, and used oral and intravenous data from a single study that employed a parallel design. From an oral dose of 15 mg/kg, the AUCpo was 27 mg h/L. From an intravenous dose of 10 mg/kg, the AUCIV was 31.4 mg h/L (22,29). Since the absolute bioavailability of ciprofloxacin HCl was less than 90%, the drug was classified as low permeability drug in children. Overall, these considerations indicate ciprofloxacin would almost always be a class 4 in a pediatric BCS, except class 3 for neonates using Young’s Rule. This propensity for class 4 for pediatric patients coincides with the “adult BCS”, where the drug has been classified as class 4 (14).
DISCUSSION
The objective was to classify dolasetron, ketoprofen, voriconazole, azithromycin, and ciprofloxacin into a potential pediatric BCS, as a pediatric BCS has not been developed. Hence, the main contribution of this work is the identification of factors required for a pediatric BCS. These five drugs were selected since oral and intravenous pharmacokinetic data were available, which is infrequently the case for most drugs. Inspection of the literature reveals the vast majority of drugs do not have oral and intravenous pharmacokinetic data. In this regard, the five drugs represent relatively favorable scenarios to attempt to classify drugs into a pediatric BCS. Additionally, these five drugs cover the four adult BCS classes. Table IX lists the adult BCS classes, along with the pediatric class identified here.
Table IX.
Summary of Pediatric BCS Classification and Comparison to Adult BCS Classification
| Drug | Adult BCS Class | Pediatric BCS Classa |
|---|---|---|
| Dolasetron | 1 | 3 |
| Ketoprofen | 2 | 4b |
| Voriconazole | 2 | 4c |
| Azithromycin | 3 | 3 |
| Ciprofloxacin | 4 | 4d |
aIt should be noted that the permeability class contribution was elucidated from literature pharmacokinetic data that did not include all pediatric subpopulations (i.e., did not include neonates, infants, children, and adolescents)
bHowever, neonates are calculated to be high solubility (i.e., Class 3) using Young’s rule, Clark’s rule and Modified Weight Rule. Infants are calculated to be high solubility (i.e., Class 3) using Young’s rule and Clark’s rule
cHowever, neonates are calculated to be high solubility (i.e., Class 3) using Young’s rule and Clark’s rule. Infants are calculated to be high solubility (i.e., Class 3) using Young’s rule
dHowever,Young’s rule for neonates indicates high solubility (i.e., Class 3)
For two of the five drugs, azithromycin and ciprofloxacin, the pediatric BCS class agreed with the adult BCS class (Table IX). Meanwhile, there was discordance for dolasetron, ketoprofen, and voriconazole. A contributing factor for agreement or disagreement between adult and pediatric BCS classification was that pediatric permeability classification was always low. Azithromycin and ciprofloxacin have low permeability in adults and in pediatrics. Meanwhile, dolasetron, ketoprofen, voriconazole have high permeability in adults, but have low permeability in pediatrics.
In contrast to these differences in permeability classifications between adult and pediatric, there was agreement in all cases for solubility classifications. All three drugs that have low solubility in adults have low solubility in most pediatrics (i.e., ketoprofen, voriconazole, and ciprofloxacin). Both drugs that have high solubility in adults have high solubility in pediatrics (i.e., dolasetron and azithromycin). Hence, in Table IX, discordances were always due to permeability classification, with pediatric permeability always being low for the five evaluated drugs.
Use of These Drugs in Pediatrics
These five drugs were selected since each has its use in pediatrics. The incidence of postoperative vomiting (POV) in children varies from 5 to 80% and is the leading cause of morbidity in pediatric surgical patients and may be associated with wound dehiscence, pulmonary aspiration, bleeding, dehydration, and electrolyte disturbance (30–32). Dolasetron is a serotonin receptor antagonist recommended for PONV prophylaxis for ages 2 and older. Postoperative use of dolasetron is found to be as effective as its prototype, ondansetron, in preventing nausea and vomiting along with being less expensive (32). In addition, its tolerability profile is similar to other drugs in its class with mild adverse related events such as headache, dizziness, and mild ECG changes.
The NSAID ketoprofen is widely used in adults and children to treat a number of inflammatory and pain related conditions, as well as fever. In adults, it is indicated for acute and long-term treatment of rheumatoid arthritis, osteoarthritis, and dysmenorrhea, as well as fever and pain. In children, it crosses the blood brain barrier and exhibits central analgesic effects (33). Furthermore, studies in pediatric patients have also proved its equivalence in efficacy to NSAID’s such as ibuprofen. However, ketoprofen is not labeled for use in pediatrics.
Voriconazole is a broad-spectrum triazole antifungal agent with activity against a wide range of yeasts and filamentous fungi. Voriconazole is approved for the primary treatment of acute invasive aspergillosis (IA) and as a salvage therapy for serious fungal infections caused by rare molds such as Scedosporium and Fusarium species in adults, as well as for the treatment of Candida infections. Variable exposures have led to multiple dosing regimens being approved to treat these infections. Due to extensive use in adults, it has been a frequent option in the pediatric population as well (20).
Azithromycin is an azalide antibiotic with a broad spectrum of activity against a variety of gram-positive and gram-negative organisms, including Staphylococcus aureus, Haemophilus influenzae, Legionella pneumophila, and Klebsiella pneumonia. In adults, azithromycin has a greater distribution in tissues, a longer elimination half-life, and a lower incidence of adverse effects than erythromycin. These pharmacokinetic features allow once-daily dosing and a shorter duration of therapy (21). In the younger population, safety and efficacy for the treatment of otitis media, acute bacterial sinusitis, and community-acquired pneumonia are not established for patients under 6 months of age (34).
Ciprofloxacin is a fluoroquinolone. It is indicated in the pediatric population against anthrax and urinary tract infection. It is increasingly prescribed for pediatric patients for conditions such as cystic fibrosis. Cystic fibrosis is an autosomal recessive disease with an incidence of 1:2,000 in newborns and usually characterized by a dysfunction of the exocrine glands. Cystic fibrosis is further associated with chronic obstructive pulmonary diseases, chronic sinusitis, nasal polyposis, and nasal airway obstruction (35). In children, antibiotics are largely used to eradicate Pseudomonas aeruginosa related infections for which fluoroquinolones such as ciprofloxacin are largely used. Common drug databases (e.g., Micromedex) suggest dosage regimens for unlabeled use of ciprofloxacin HCl in pediatrics. A majority of the studies of ciprofloxacin in children focus on children with cystic fibrosis, although cystic fibrosis is not an indication of ciprofloxacin. However, short-term safety data from a single trial in pediatric cystic fibrosis patients is in the label.
In addition to these five selected drugs being used in pediatrics, these drugs also have oral and intravenous pediatric pharmacokinetic data in the literature. Of course, most drugs do not have such pediatric data. A potential future goal is the use of cell culture or animal data to classify drugs within a potential pediatric BCS based on validation of such approaches, like the adult BCS allows.
Challenges in Devising Pediatric BCS Class Boundaries: Solubility
While the drugs chosen in this paper represent relatively favorable scenarios to attempt to classify into a pediatric BCS and cover all four adult BCS classes, efforts here identify several challenges in devising a pediatric BCS. These challenges were most notable in identifying BCS solubility criteria. Adult BCS references a dose volume of 250 ml, which is approximately equal to 8 fluid ounces of water ingested with a dose. Here, a BSA-based volume was applied, and volumes were 34.7, 67.4, 127.6, and 220.3 ml for neonates, infants, children, and adolescents, respectively. We found no literature about typical fluid volumes ingested by pediatric patients when medications are administered. Pediatric drug solubility is taken to be equal to the adult drug solubility. The adult BCS considers the lowest drug solubility from three pH conditions: pH 1.2, 4.5, and 6.8 (36). These media are assumed to apply to a pediatric BCS, although the gastric acid output (per kilogram basis) is not reached until 2 years of age. The gastric pH in term newborns is 6–8, which lowers to 1–3 within 24 h. Very premature infants take as long as 3 weeks to achieve lower gastric pH.
Complicating the consideration for a pediatric BCS is that subpopulations exist within the pediatric population. Pediatrics refers to individuals 17 years of age or less. Adolescents are 12–16 years of age. Children are 2–12 years. An infant is 1 month to 2 years. A neonate is less than 1 month. Of note, there is very little drug information in pediatrics younger than 2 years old.
Challenges in Using Drug Literature Data: Permeability
In addition to challenges in devising a pediatric BCS, literature data of each dolasetron, ketoprofen, voriconazole, azithromycin, and ciprofloxacin required assumptions and decisions about data collection. These limitations impacted drug bioavailability and hence permeability classification. For example, the oral and intravenous data for azithromycin were obtained from two different studies performed for two separate indications (21,28). Similarly, for each ketoprofen and dolasetron, oral and intravenous data was obtained from two different studies, although conducted in a similar fashion by the same research group (10,19,23). Nevertheless, data was obtained from a separate groups of patients. A further limitation to ketoprofen was that oral and intraveneous groups were not age-matched. The absolute oral bioavailability of ketoprofen was calculated from four numbers from Kokki et al.: oral dose of 25 mg, AUCpo of 11.4 mg h/L, intravenous dose of 16.2 mg, and AUCIV of 15.4 mg h/L (10,25,26). However, data were not from a crossover design.
The study used for voriconazole was an intravenous-to-oral switch design (20). In this study, patients were assigned intravenous dosing for the first 7 days of treatment, followed by oral dosing for the following 7 days. Both study arms involved the same patients. Finally, use of ciprofloxacin HCl literature data presented many of these same challenges as well. Of note, the oral and intravenous ciprofloxacin HCl studies were conducted in cystic fibrosis patients, who exhibit a higher oral bioavailability and clearance than normal children (37).
An additional important limitation is that no pharmacokinetic study included all pediatric subpopulations. For example, the dolasetron study included only children and adolescents between the ages of 3 and 18 years, and did not include neonates and infants (19). The oral and intravenous ketoprofen studies included only infants and children (10,25,26). The voriconazole study included only children aged between 2 and 12 years to calculate exposure. The azithromycin oral study included only infants and children between the ages of 6 months and 5 years, whereas the intravenous study included 32 subjects ranging from infants to adolescents. The ciprofloxacin study included only ten subjects that were either children or adolescents.
Permeability research contributed greatly to the adult BCS framework, including the mapping of adult oral fraction dose absorbed to laboratory permeability methods (1,2,43,44). A pediatric BCS would benefit from a mapping of pediatric oral fraction dose absorbed to laboratory permeability methods.
Utility of a Pediatric BCS
This work highlights some of the significant challenges that would be encountered in order to develop a pediatric BCS, such that the motivating factors for a pediatric BCS need to be clear and convincing. A main reason for a pediatric BCS is to promote pediatric product development. It has been advocated that biopharmaceutic risk assessment should be conducted early in pediatric development, including synchronization with the adult development program (5).
Scientists at the National Institute of Child Health and Human Development (NICHD) and the US Food and Drug Administration (FDA) have investigated oral formulations platforms, and summarized drug biopharmaceutic information of the 382 approved pediatric products. The investigation focused on liquid formulations for pediatric patients, although approved pediatric formulations also include sprinkles, capsules, injectables, chewable tablets, orally disintegrating tablets, metered dose inhalers (MDI), dry powdered inhalers (DPIs), and orally dissolving films. The investigation concluded that most liquid products are adult BCS class 1 and class 3. Few class 2 and class 4 formulations are formulated as oral solutions or syrups.
A second, related reason for a pediatric BCS is to assess risk of adult product failure in the pediatric population. Many drugs that have no indications for the pediatric population are used in the pediatric population. Even where a drug is indicated for pediatric use, labeling may still employ a dosing algorithm that does not perfectly coincide with the approved product dose. For example, dolasetron tablets are available as 50 mg and 100 mg tablets, yet the label indicates oral dosing in terms of 1.8 mg/kg within 1 h before chemotherapy in pediatric patients. Another example is ketoprofen, which is commonly used in pediatric patients (38,39). However, ketoprofen is only available in 25, 50 mg, and 75 mg capsules and is not indicated for use in children.
Figure 1 illustrates formulations in the normal course of a drug product’s life cycle, where even shortly after marketing of a new drug, the marketed formulation differ from the clinical trial formulation that demonstrated drug safety and efficacy, due to formulation changes in later development and scale-up and post-approval changes (SUPAC changes). Products routinely rely on equivalence methods (e.g., in vivo bioequivalence in adults, in vitro BCS based on adults), since individual products experience post-approval changes in formulation/manufacturing (42).
Fig. 1.
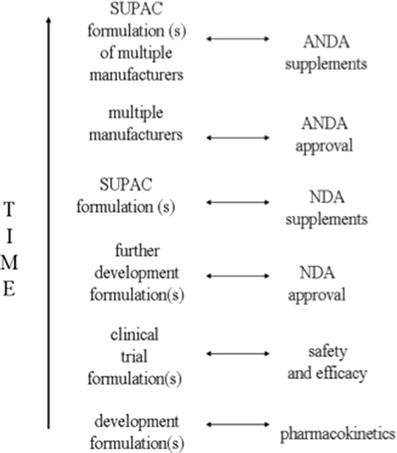
Formulations in the normal course of a drug product’s life cycle. Even shortly after marketing of a new drug, the marketed formulation differs from the clinical trial formulation that demonstrated drug safety and efficacy, due to formulation changes in later development and SUPAC changes. In later stages of drug market life, formulations from several generic manufacturers are available, including generic formulations with SUPAC changes. Products with indications for adults employ bioequivalence methods for adults, rather than the pediatric population. From Ref. (3)
An outstanding question is whether, for post-approval changes, bioequivalence in adults assures bioequivalence in pediatrics. A SUPAC change carries potential risk of bioinequivalence. Dolasetron, azithromycin, voriconazole, and ciprofloxacin are indicated in pediatrics, although azithromycin and ciprofloxacin may be frequently used off-label. Ketoprofen does not carry an indication for pediatric patients, although is used as for the treatment of pain and fever, peri- and postoperative pain, and inflammatory pain conditions (26).
Qualitatively, it would appear that SUPAC changes for pediatric subpopulations may be of greater, similar, or less risk, compared to adult patients. In Table IX, azithromycin is BCS class 3 for both adults and all pediatric subpopulations. Hence, given their same BCS classification, SUPAC changes for pediatric patients would appear to be of similar risk compared to adult patients. Meanwhile, dolesetron is BCS class 1 for adults but class 3 for all pediatric subpopulations. Hence, SUPAC changes for pediatric patients would appear to be of greater risk compared to adult patients. Likewise, pediatric patients would appear to be of greater risk in the case of each ketoprofen and voriconazole. For adults and pediatrics, ciprofloxacin is class 4, such that assessing relative risk is difficult. Of course, these interpretations should also consider the general conservative nature designed into the BCS framework (41).
Additionally, multiple generic products are typically introduced when allowed. Hence, the marketplace often involves products that rely on bioequivalence methods based on adults. An additional unresolved question is whether, for abbreviated new drug applications, does bioequivalence in adults assure bioequivalence in pediatrics. A pediatric BCS would have promise to address risk of bioinequivalence for pediatric patients taking products with life cycle changes (e.g., SUPAC changes, generic products).
In conclusion, there was agreement in adult and pediatric BCS class for two drugs, azithromycin (class 3) and ciprofloxacin (class 4). Since all pediatric permeabilities were low, even when adult permeability class was high, there was discordance for the three drugs that have high adult permeability: dolasetron (class 3 in pediatric), ketoprofen (class 4 in pediatric), and voriconazole (class 4 in pediatric). The five studied drugs were selected since both oral and intravenous pharmacokinetic data was available for each drug, which is in contrast with the vast majority of drugs which do not have such literature data. Nevertheless, findings for these five drugs highlights challenges in devising pediatric BCS class boundaries, as well as challenges in using drug literature data. Experience here indicates that devising a pediatric BCS would be particularly challenging in identifying a solubility class boundary, where a dose volume based on subpopulation BSA was applied. Identifying a solubility class boundary was further complicated by differing algorithms for dose (7,40,41). Additionally, literature data of each drug required assumptions and decisions about data collection, which would appear even more problematic for most other drugs, which suffer from even less available literature data.
Footnotes
The views expressed are that of the authors and do not represent the policy of the Agency at this time.
REFERENCES
- 1.Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. 2000. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf. Accessed 21 May 2013.
- 2.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 3.Polli JE. In vitro studies are sometimes better than conventional human pharmacokinetic in vivo studies in assessing bioequivalence of immediate-release solid oral dosage forms. AAPS J. 2008;10:289–99. doi: 10.1208/s12248-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JA, Davit BM, Polli JE. Impact of biopharmaceutics classification system-based biowaivers. Mol Pharm. 2010;7:1539–44. doi: 10.1021/mp1001747. [DOI] [PubMed] [Google Scholar]
- 5.Purohit VS. Biopharmaceutic planning in pediatric drug development. AAPS J. 2012;4:519–22. doi: 10.1208/s12248-012-9364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Intra-Agency Agreement Between the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the U.S. Food and Drug Administration (FDA) Oral Formulations Platform—Report 1. 2011. http://bpca.nichd.nih.gov/collaborativeefforts/initiatives/upload/Formulations_Table_for_Web_11-02-11.pdf. Accessed 21 May 2013.
- 7.Hoff DS, Jensen PD. Pediatric pharmacotherapy. In: Hoff DS, Jensen PD, editors. Pharmacotherapy self-assessment program. 4. Kansas City: American College of Clinical Pharmacy; 2003. p. 13. [Google Scholar]
- 8.Abdel-Rahman SM, Amidon GL, Kaul A, Lukacova V, Vinks AA, Knipp GT, et al. Summary of the National Institute of Child Health and Human Development—best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop—Pediatric Biopharmaceutics Classification System Working Group. Clin Ther. 2012;34(11):S11–24. doi: 10.1016/j.clinthera.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ANZEMET® (dolasetron mesylate) Tablets. Sanofi-Aventis U.S. LLC. October 2003. http://products.sanofi.us/anzemet_tablets/anzemettab.pdf. Accessed 24 August 2013.
- 10.Kokki H, Tuomilehto H, Karvinen M. Pharmacokinetics of ketoprofen following oral and intramuscular administration in young children. Eur J Clin Pharmacol. 2001;57(9):643–7. doi: 10.1007/s002280100339. [DOI] [PubMed] [Google Scholar]
- 11.Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin Infect Dis. 2010;50(1):27–36. doi: 10.1086/648679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ZITHROMAX® (azithromycin tablets and azithromycin for oral suspension). Pfizer Inc. February 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=511. Accessed 24 August 2013.
- 13.CIPRO® (Ciprofloxacin Hydrochloride) TABLETS, CIPRO® (Ciprofloxacin) ORAL SUSPENSION. Bayer HealthCare Pharmaceuticals Inc. 2008. http://www.univgraph.com/bayer/inserts/ciprotab.pdf Accessed 21 May 2013.
- 14.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–47. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curatolo W. Interdisciplinary science and the design of a single-dose antibiotic therapy. Pharm Res. 2011;28(9):2059–71. doi: 10.1007/s11095-011-0382-0. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3(6):631–43. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2 to 20 years: Boys Stature-for-Age and Weight-for-Age Percentiles. 2000. http://www.cdc.gov/growthcharts/data/set1clinical/cj41c021.pdf Accessed 21 May 2013.
- 18.Centers for Disease Control and Prevention. Birth to 36 months: Boys Length-for-Age and Weight-for-Age Percentiles. 2000. http://www.cdc.gov/growthcharts/data/set1clinical/cj41l017.pdf Accessed 21 May 2013.
- 19.Coppes MJ, Yanofsky R, Pritchard S, Leclerc JM, Howard DR, Perrotta M, et al. Safety, tolerability, antiemetic efficacy, and pharmacokinetics of oral dolasetron mesylate in pediatric cancer patients receiving moderately to highly emetogenic chemotherapy. J Pediatr Hematol Oncol. 1999;21(4):274–83. doi: 10.1097/00043426-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll TA, Yu LC, Frangoul H, Krance RA, Nemecek E, Blumer J, et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob Agents Chemother. 2011;55(12):5770–9. doi: 10.1128/AAC.00531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahata MC, Koranyi KI, Luke DR, Foulds G. Pharmacokinetics of azithromycin in pediatric patients with acute otitis media. Antimicrob Agents Chemother. 1995;39(8):1875–7. doi: 10.1128/AAC.39.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer HG, Stass H, Wedgwood J, Hampel B, Fischer C, Kuhlmann J, et al. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40(1):29–34. doi: 10.1128/aac.40.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppes MJ, Lau R, Ingram LC, Wiernikowski JT, Grant R, Howard DR, et al. Open-label comparison of the antiemetic efficacy of single intravenous doses of dolasetron mesylate in pediatric cancer patients receiving moderately to highly emetogenic chemotherapy. Med Pediatr Oncol. 1999;33(2):99–105. doi: 10.1002/(SICI)1096-911X(199908)33:2<99::AID-MPO7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.KETOPROFEN® capsule. Mylan Pharmaceuticals Inc. 2011. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=198a4140-f4c0-4478-9157-ee1d68d0bb96. Accessed 24 November 2013.
- 25.Kokki H, Karvinen M, Suhonen P. Pharmacokinetics of intravenous and rectal ketoprofen in young children. Clin Pharmacokinet. 2003;42(4):373–9. doi: 10.2165/00003088-200342040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kokki H. Ketoprofen pharmacokinetics, efficacy, and tolerability in pediatric patients. Pediatr Drugs. 2010;12(5):313–29. doi: 10.2165/11534910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.VFEND® (Voriconazole) Tablets, Oral Suspension. Pfizer Ireland Pharmaceutical 2011. http://labeling.pfizer.com/ShowLabeling.aspx?id=618. Accessed 24 November 2013.
- 28.Jacobs RF, Maples HD, Aranda JV, Espinoza GM, Knirsch C, Chandra R, et al. Pharmacokinetics of intravenously administered azithromycin in pediatric patients. Pediatr Infect Dis J. 2005;24(1):34–9. doi: 10.1097/01.inf.0000148927.48680.fc. [DOI] [PubMed] [Google Scholar]
- 29.Pelotas H. Single-dose and steady-state pharmacokinetics of a new oral suspension of ciprofloxacin in, children. Pediatrics. 1998;101(4):658. doi: 10.1542/peds.101.4.658. [DOI] [PubMed] [Google Scholar]
- 30.Baines D. Postoperative nausea and vomiting in children. Paediatr Anaesth. 1996;6:7–14. doi: 10.1111/j.1460-9592.1996.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Sung Y. Risks and benefits of drugs used in the management of PONV. Drug Saf. 1996;14:181–97. doi: 10.2165/00002018-199614030-00005. [DOI] [PubMed] [Google Scholar]
- 32.Olutoye O, Jantzen EC, Alexis R, Rajchert D, Schreiner MS, Watcha MF. A comparison of the costs and efficacy of ondansetron and dolasetron in the prophylaxis of postoperative vomiting in pediatric patients undergoing ambulatory surgery. Anesth Analg. 2003;97(2):390–6. doi: 10.1213/01.ANE.0000072706.09194.5E. [DOI] [PubMed] [Google Scholar]
- 33.Kokki H, Karvinen M, Jekunen A. Diffusion of ketoprofen into the cerebrospinal fluid of young children. Paediatr Anaesth. 2002;12(4):313–6. doi: 10.1046/j.1460-9592.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- 34.Lexi-Comp OnlineTM . Pediatric & neonatal lexi-drugs OnlineTM. Hudson: Lexi-Comp; 2013. [Google Scholar]
- 35.Knipping S, Holzhausen H, Riederer A, Bloching M. Cystic fibrosis: ultrastructural changes of nasal mucosa. Eur Arch Otorhinolaryngol. 2007;264(12):1413–8. doi: 10.1007/s00405-007-0393-y. [DOI] [PubMed] [Google Scholar]
- 36.Polli JE, Yu LX, Cook JA, Amidon GL, Borchardt RT, Burnside BA, et al. Summary workshop report: biopharmaceutics classification system—implementation challenges and extension opportunities. J Pharm Sci. 2004;93:1375–81. doi: 10.1002/jps.20064. [DOI] [PubMed] [Google Scholar]
- 37.Christensson B, Nilsson-Ehle I, Ljungberg B, Linblad A, Malmborg AS, Hjelte L, et al. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother. 1992;36:2512–7. doi: 10.1128/AAC.36.11.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Medicines Agency. Revised provisional priority list for studies into off-patent paediatric medicinal products. http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500143970&mid=WC0b01ac058009a3dc. Accessed 29 April 2013.
- 39.Lindell-Osuagwu L, Korhonen MJ, Saano S, Helin-Tanninen M, Naaranlahti T, Kokki H. Off-label and unlicensed drug prescribing in three paediatric wards in Finland and review of the international literature. J Clin Pharm Ther. 2009;34(3):277–87. doi: 10.1111/j.1365-2710.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 40.Abernethy DR, Burckart GJ. Pediatric dose selection. Clin Pharmacol Ther. 2010;87:270–1. doi: 10.1038/clpt.2009.292. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood I. Interspecies pharmacokinetic scaling. Rockville: Pine House; 2005. [Google Scholar]
- 42.Yazdanian M, Briggs K, Jankovsky C, Hawi A. The "high solubility" definition of the current FDA guidance on biopharmaceutical classification system may be too strict for acidic drugs. Pharm Res. 2004;21(2):293–9. doi: 10.1023/B:PHAM.0000016242.48642.71. [DOI] [PubMed] [Google Scholar]
- 43.Polli JE, Ginski MJ. Human drug absorption kinetics and comparison to Caco-2 monolayer permeabilities. Pharm Res. 1998;15:47–52. doi: 10.1023/A:1011992518592. [DOI] [PubMed] [Google Scholar]
- 44.Lentz KA, Hayashi J, Lucisano LJ, Polli JE. Development of a more rapid, reduced serum culture system for Caco-2 monolayers and application to biopharmaceutics classification system. Int J Pharm. 2000;200(1):41–51. doi: 10.1016/S0378-5173(00)00334-3. [DOI] [PubMed] [Google Scholar]


