Abstract
This study examined the effect of an aqueous extract of Pulicaria undulata on the 1,4-dithiothreitol (DTT)-induced aggregation of proteins. The effects of the chaperone properties of P. undulata extract on protein aggregation were determined by measuring light scattering absorption, fluorescence, and circular dichroism (CD) spectroscopy. The aqueous extract of P. undulata possesses good chaperone properties but the protection effect was varied in different protein. The extract showed a higher level of protection in high molecular weight proteins than in those of low molecular weight. Using a fluorescence study, the present study provides information on the hydrophobic area of proteins interacting with the P. undulata extract. In fact, by increasing the concentration of the P. undulata extract, the hydrophic area of the protein decreased. CD spectroscopy also revealed that DTT caused changes in both the tertiary and the secondary structure of the proteins, while in the presence of P. undulata extract, there was little change. Our finding suggests the possibility of using P. undulata extract for the inhibition of aggregation and the deposition of protein in disease.
KEY WORDS: amorphous, P. undulata, protein aggregation
INTRODUCTION
Protein aggregation is a biological phenomenon in which protein is misfolded and aggregates either intra- or extracellularly. These protein aggregates are often toxic and have been implicated in a wide variety of diseases known as amyloidoses, including Alzheimer’s, Parkinson’s, and prion disease (1).
After synthesis, proteins typically fold into a particular three-dimensional conformation. This is their native state and it is the only one in which they are functional. Newly synthesized proteins, however, may not fold correctly, or properly folded proteins can spontaneously misfold and aggregate (2). Protein aggregation can result from various kinds of stress, such as agitation and exposure to extremes of pH, temperature, ionic strength, or various interfaces (e.g., air–liquid interface). High protein concentrations can also increase aggregation (3).
Pulicaria undulata (L.) C.A.Mey. [syn. Pulicaria crispa (forssk.) Olive. Francoeuria crispa (forssk.) Cass] is an annual herb, sometimes categorized as a perennial sub-shrub belonging to the Asteraceae family, and it produces small bright yellow flowers. This species can be found in Iran, Saudi Arabia, Kuwait, Iraq, Egypt, Afghanistan, Pakistan, India, and parts of North and West tropical Africa (4,5). Among the flora of Iran, five species of the genus Pulicaria (family Compositae, tribe Inulea) are reported (6). P. undulata has been used by the people of southern Egypt and Saudi Arabia as a medicinal plant to treat inflammation. It can also be used as an insect repellent and a herbal tea (7,8). Chemical study of different types of Pulicaria demonstrated that it contains several sesquiterpenoids, diterpenoids, and flavonoids (9–12). The analyses of the oil component of P. undulata showed that the oil is rich in phenolic compounds and monoterpene hydrocarbons, and comparatively low in sesquiterpene hydrocarbons (13). It showed a high content of the oxygenated monoterpenes carvotanacetone (91.4%) and 2,5-dimethoxy-p-cymene (2.6%) (14,15). In addition according to previous phytochemical studies, this herb is a considerable source of eudesmanolide, sesquiterpene lactones of the guaianolide and xanthanolides family (16–18). The antioxidant activity of the extract of this plant has been reported using DPPH (19). Antioxidants have been shown to have the ability to prevent the damage caused by oxidative stress (20–22). Considering antioxidant activity assays, we were interested in examining the possible effect of P. undulata extracts on the aggregation and deposition of protein. In this study, we have explored the potential for P. undulata extracts against the aggregation of proteins. We also used different proteins with different molecular weights to investigate and compare the effects of P. undulata extract on different size proteins. Of particular note, the result of this study shows that in all target proteins, P. undulata extracts inhibited the aggregation.
The present results indicate that P. undulata extract has the potential of being a useful lead component for the design of molecule inhibitors of protein aggregation.
MATERIAL AND METHODS
Bovine insulin (5.7 kDa), α-lactalbumin (14 kDa), ovotransferrin (87 kDa), 1,4-dithiothreitol (DTT), NaN3, and 1-anilino-8-naphthalene sulfonic acid (ANS) were obtained from Sigma-Aldrich (St. Louis, USA).
Botanical Material
P. undulata was collected in November 2011 from the Saravan area located in the Sistan and Baluchestan province of Iran. The taxonomic identification of plant materials was confirmed by Dr. Valizadeh in the Department of Biology at the University of Sistan and Baluchestan. The collected plants were dried in the shade and aerial parts were separated from the root. The voucher specimen has been deposited at the herbarium.
Preparation of the Aqueous Extract
Ten grams of the herb was ground into powder and extracted with distilled water (150 mL) by magnetic stirring (4,000 rpm) for 24 h at room temperature. The extracts were filtered through filter paper (Whatman no. 42). After filtration, the mixture was centrifuged at 10,000×g for 20 min to remove the debris. The solvent evaporated under reduced pressure and the residues were freeze-dried. The extracts were sealed in glass bottles and stored at +4°C until use. Extraction was done once and a single batch of aqueous extract was used for all experiments in this study.
Visible Absorption Spectroscopy (Light Scattering)
The ability of P. undulata extract to prevent the aggregation of different proteins was measured via visible light absorption spectroscopy, as described previously (23,24). Insulin, α-lactalbumin, and ovotransferrin at 0.42, 2, and 1.4 mg/mL, respectively (in a 50 mM sodium phosphate buffer, pH 7.4 , 0.05% NaN3, in the presence or absence of (1:1 w/w) ratio of P. undulata extract, with the addition of 100 mM NaCl and 1 mM EDTA for α-lactalbumin), were incubated at 37°C. The unfolding and aggregation of proteins was initiated by adding DTT to a final concentration of 20 mM. Light scattering was then observed at 340 nm at 37°C using an Elisa plate reader (Biotek E808, USA).
The relative protection activity of P. undulata extract was calculated as a percentage of protection against aggregation at the end of each assay, using the formula: % protection = ((A0 − A)/A0) × 100, where A0 and A represent the apparent saturation absorption (at 340 nm) in the absence and presence of P. undulata extract, respectively. All experiments were done at least three times and the results are shown as means ± SEM.
Fluorescence Spectroscopy
The intrinsic fluorescence intensity of insulin, α-lactalbumin, and ovotransferrin was monitored in the presence or absence of P. undulata extract to investigate the effect of P. undulata extract on the environment of the tryptophan (Trp) residues on the target proteins. Samples containing α-lactalbumin (10 μM), ovotransferrin (10 μM), and P. undulata extract, separately or in the presence of each other, with 20 mM DTT were incubated in a 50 mM sodium phosphate buffer, 0.05% (w/v) NaN3 and pH 7.4 for 3 h. Insulin (10 μM) and P. undulata extract (1:1 w/w), separately or in the presence of each other, were incubated in a 50 mM sodium phosphate buffer, 0.05% (w/v) NaN3 and pH 8.5 for 1 h after the addition of DTT. Fluorescence intensity was measured on a Varian fluorescence spectrofluorimeter. Tryptophan residues were excited at 295 nm using a 2.5-nm slit width, and emission spectra were recorded from 300 to 400 nm with a 5-nm slit width. Samples were placed in a 10-mm path length quartz cuvette. The spectrofluorimeter was set to 700 V with a scan speed of 240 nm/min.
The ANS binding assay was used to assess changes in the clustered exposed hydrophobicity upon interaction of the target proteins with the P. undulata extract. Samples containing insulin (10 μM), α-lactalbumin (10 μM), ovotransferrin (10 μM), and P. undulata extract (in a 1:1 weight ratio) separately or in the presence of each other, with 20 mM DTT, were incubated in a 50 mM sodium phosphate buffer, 0.05% (w/v) NaN3 and pH 7.4 for α-lactalbumin and ovotransferrin, and pH 8 for insulin. The ANS fluorescence was monitored on a Varian spectrofluorimeter. The excitation and emission wavelengths were set to 400–600 nm, with 2.5 and 5 nm slit widths. The fluorescence emission intensity was read in a 10-mm path length quartz cuvette with a volume of 350 μL titrated with 1 μL of a 10 mM ANS solution in a 50 mM sodium phosphate buffer, 0.05% (w/v) NaN3, pH 7.4, with 1 min of stirring after each addition. Titration was continued until the fluorescence intensity reached a plateau.
Circular dichroism spectroscopy
The overall secondary and tertiary structures of proteins in the presence and absence of P. undulata extract was examined using far- (190–250) and near-UV circular dichroism (CD) spectroscopy (250–350 nm) with 0.2 and 0.4 mg/mL protein. The measurements were taken in a 1-cm path length cuvette using a JASCO J-810 spectropolarimeter (JASCO, Victoria, Canada). The spectra were recorded using a resolution of 1 nm and a scanning speed of 100 nm/min, with a response time of 4 s and a bandwidth of 1.5 nm. The spectra presented were an average of four consecutive measurements with a baseline scan subtracted.
RESULTS
Effect of P. undulata on the Aggregation of Insulin, α-Lactalbumin, and Ovotransferrin
The aggregation of proteins and the possible effect of P. undulata extract on protein aggregation were examined by light scattering absorption. The increase in absorbance at 340 nm over time reflects the aggregation of a stressed target protein.
An increase in the light scattering of reduced insulin over time was observed (Fig. 1a) and this indicates the aggregation and precipitation of reduced insulin. P. undulata extract had a protective effect, however, on the aggregation of insulin and this was observed by a reduction in light scattering (Fig. 1a, Table I). The addition of P. undulata extract to insulin at a 1:0.25 w/w ratio of insulin/P. undulata extract decreased the aggregation and precipitation of insulin about 20%. The ability of P. undulata extract to protect against this aggregation was increased by increasing the concentration of the P. undulata extract, such that at 1:2 w/w ratio of insulin/P. undulata extract, the aggregation decreased by 58% (Fig. 1a, Table I).
Fig. 1.
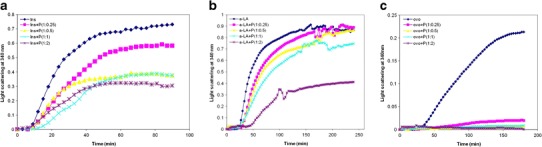
Visible absorption profiles at 340 nm of target protein aggregation in the presence or absence of different concentration of P. undulata extract for a insulin; b α-lactalbumin; and c ovotransferrin. All experiments were conducted in a 50 mM sodium phosphate buffer, 0.05% NaN3, pH 7.4 With the addition of 100 mM NaCl and 1 Mm EDTA for α-lactalbumin at 37°C. The w/w ratio of protein/P. undulata extract is indicated. Note that all the experiments were done in three times. The error bars were very low and not plotted in the graph
Table I.
Summary of Protection Percentage for Insulin, α-Lactalbumin, and Ovotransferrin in Spectroscopy Results in Different Concentration of P. undulata Extract
| Samples | % Protection |
|---|---|
| Ins + P (1:0.25) | 20.14 |
| Ins + P (1:0.5) | 48.49 |
| Ins + P (1:1) | 49.50 |
| Ins + P (1:2) | 58.22 |
| α-Lac + P (1:0.25) | 6.57 |
| α-Lac + P (1:0.5) | 17.05 |
| α-Lac + P (1:1) | 22.23 |
| α-Lac + P (1:2) | 59.56 |
| Ovo + P (1:0.25) | 90.61 |
| Ovo + P (1:1) | 92.24 |
| Ovo + P (1:2) | 100 |
Similar results were observed for reduced α-lactalbumin. α-Lactalbumin aggregates (Fig. 1a) after the addition of DTT to reduce its four disulfide bonds. P. undulata extract at 1:0.25 w/w of α-lactalbumin/P. undulata extract decreased the aggregation by 7% (Fig. 1b). As shown in Fig. 1, the protective ability of P. undulata extract against this aggregation was increased by increasing the concentration of P. undulata extract so that in the 1:2 weight ratio of α-lactalbumin/P. undulata extract, the aggregation decreased by 60% (Table I).
Ovotransferrin aggregates 27 min after the addition of DTT (Fig. 1c). P. undulata extract showed very good protection in preventing the aggregation of ovotransferrin, so that at 1:0.25 w/w ratio of ovotransferrin/P. undulata extract, the aggregation of the protein decreased by 90% (Fig. 1c, Table I).
Intrinsic Fluorescence Spectroscopy of Target Proteins in the Presence or Absence of P. undulata
In order to investigate the effect of P. undulata extract on the environment of the Trp residue of proteins, the intrinsic fluorescence of target proteins in the presence and absence of P. undulata extract was measured. The intrinsic fluorescence of insulin could not be measured due to the lack of Trp in its structure.
The intrinsic fluorescence of α-lactalbumin was compared in the absence and presence of different concentrations of P. undulata extract in order to investigate the effect of P. undulata extract on reduced α-lactalbumin. Since P. undulata extract alone showed very little fluorescence intensity (not shown) and the contribution of P. undulata extract to the fluorescence intensity is negligible relative to the proteins, changes in the fluorescence intensity must be due to the conformational changes in the target proteins. Figure 2a shows the fluorescence intensity of reduced α-lactalbumin. Adding P. undulata extract, at a concentration of 1:0.25 of α-lactalbumin/P. undulata extract, showed a decrease in the fluorescence intensity of α-lactalbumin of about 11%. As can be seen in Fig. 2a, the fluorescence intensity of α-lactalbumin decreased as the concentration of P. undulata extract increased (Table II).
Fig. 2.
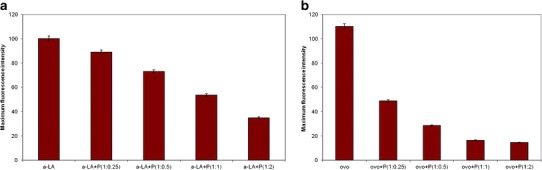
The maximum intrinsic fluorescence of target proteins (1 mg/mL) and P. undulata extract at different concentration. All experiments were incubated in a 50 mM sodium phosphate buffer, 0.05% NaN3 and pH 7.4 at 37°C for insulin in 1 h (a), for α-lactalbumin and ovotransferrin in 3 h (b, c)
Table II.
Secondary Structural Prediction from Far-CD Spectra for Insulin, α-Lactalbumin, and Ovotransferrin in Presence of P. undulata Extract Using the JASCO Program
| Samples | % α-helix | % β-sheet | % β-turn | % random coil |
|---|---|---|---|---|
| Ins | 18.7 | 34.9 | 11.3 | 35.1 |
| Ins + DTT | 2 | 52.8 | 5 | 41.2 |
| Ins + DTT + P | 10.2 | 43.1 | 10.2 | 36.5 |
| α-LA | 29.6 | 37.5 | 9.1 | 28.8 |
| α-LA + DTT | 23.6 | 47.4 | 15.4 | 29.6 |
| α-LA + DTT + P | 25 | 31.4 | 11.2 | 32.4 |
| ovo | 26.3 | 5.4 | 14.7 | 53.6 |
| ovo + DTT | 5.2 | 42.5 | 12.4 | 39.9 |
| ovo + DTT + P | 12 | 32.3 | 26.4 | 39.3 |
The relative fluorescence intensities of the ovotransferrin with different concentrations of P. undulata exhibited the same trends (Fig. 2b). As the concentration of P. undulata extract increased, the maximum of the fluorescence intensity decreased (Table II).
ANS Binding of Target Proteins in the Presence or Absence of P. undulata Extract
ANS have proven to be sensitive probes for partially folded intermediates in protein-folding pathways (25). In the present study, ANS binding assays have been used to investigate the characteristics of folding and unfolding pathways of proteins through the changes in surface hydrophobicity of the proteins interacting with P. undulata extract (Fig. 3). All reduced target proteins on their own showed ANS binding, which is indicative of the exposure of hydrophobic regions to the solution. Adding P. undulata extract to the reduced proteins decreased the fluorescence intensity but the effect varied. This implies the formation of a complex between the proteins and P. undulata extract that indicates that the exposed hydrophobicity of the protein interacted with the P. undulata extract, leading to the formation of a complex.
Fig. 3.
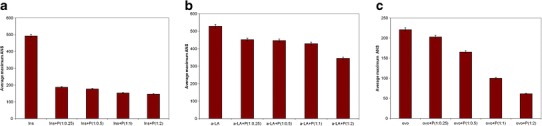
Average maximum ANS fluorescence bound of target protein (1 mg/mL) and P. undulata extract at different concentration. All experiments were conducted in a 50 mM sodium phosphate buffer, 0.05% NaN3 and pH 7.4 at 37°C for insulin (a), for α-lactalbumin (b), and ovotransferrin in (c)
Circular Dichroism Spectroscopy of Proteins in the Presence and Absence of P. undulata
CD spectroscopy in near-UV of the native and reduced target protein in the presence and absence of P. undulata extract was performed to get more information on the tertiary structure of the protein, especially in the environment of aromatic (e.g., tryptophan) residues. Far-UV spectroscopy was also used to probe the effect of P. undulata extract on the secondary structure of the stressed protein.
A CD spectrum of bovine insulin showed negative ellipticity around 275 nm due to four tyrosine residues (Fig. 4a). A spectrum recorded after the reduction of the insulin showed an increase in the Tyr signal at around 275 nm, indicating some change in the environment of the tyrosine residue. CD spectra of reduced insulin in the presence of P. undulata extract revealed that this effect decreased so that negative ellipticity around 275 nm changes was observed indicating a small conformational transition.
Fig. 4.
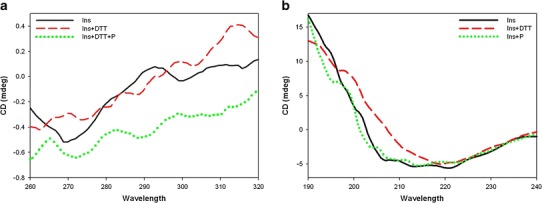
Near-UV CD spectra (a) and far-UV CD spectra (b) of insulin: unstressed (solid line), stressed (dashed line), stressed in the presence of P. undulata extract (dotted line)
The far-UV spectrum of insulin is shown in Fig. 4b. It contains two negative ellipticities at 208 and 222 nm and one positive ellipticity at 192 nm, characteristic of an α-helical secondary structure, similar to the result observed previously (26,27). Incubation of insulin in the presence of DTT caused the α-helical content of the insulin to decrease, as indicated by the lack of the minima in the CD spectra. It also shows a negative ellipticity at 218 nm, which is characteristic of the β-sheet secondary structure. The CD signals show that insulin exhibited a transformation from an α-helix to a β-sheet structure by reduction. In the presence of P. undulata extract, however, no significant changes were observed by far-UV CD (Fig. 4b). The content of the secondary structure of insulin in the presence and absence of P. undulata extract is shown in Table II.
CD spectra of native α-lactalbumin (Fig. 5a) showed the characteristic 275 nm tryptophan maximum and the 292 nm tyrosine minimum (28). The reduced α-lactalbumin after 3-h incubation showed the characteristic loss of signal in the tyrosine and tryptophan region consistent with the protein becoming partially unfolded.
Fig. 5.
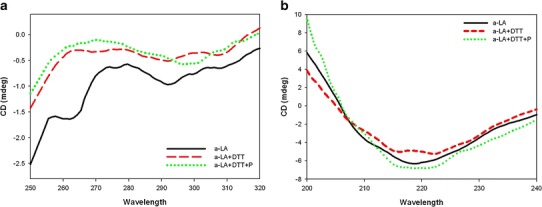
Near-UV CD spectra (a) and far-UV CD spectra (b) of α-lactalbumin: unstressed (solid line), stressed (dashed line), stressed in the presence of P. undulata extract (dotted line)
By adding P. undulata extract to the reduced α-lactalbumin, however, the change in ellipticity at 275 and 292 nm was not significant (Fig. 4b), implying little alteration in the tertiary structure.
The far-UV spectrum of α-lactalbumin (Fig. 5b) shows strong negative ellipticity around 222 nm, characteristic of an α-helix structure with general features similar to those already observed in our previous study (29). Table II shows the content of the secondary structure of the reduced α-lactalbumin in the presence and absence of the P. undulata extract. Figure 5b and Table II show that in the presence of DTT, a decrease in the α-helix content of α-lactalbumin with an increase in the content of β-sheet occurred. Then, a transition from an α-helix to a β-structure in the α-lactalbumin was seen in the reduced form of the protein. Adding P. undulata extract to the protein, however, led to an increase in the size of the negative CD signal over the range of 220–230 nm, reflecting the increased stability of the helical regions of the protein.
The near-UV CD spectrum of the native state of the ovotransferrin (Fig. 6a) reveals two negative ellipticity peaks at 275 and 290 nm due to tyrosin and tryptophan residues, respectively (30). In the presence of DTT, the intensity of the peak at these wavelengths responds quite differently to change in DTT. The shift of peak maxima and the reduction of band intensity illustrate the loss of native structure that indicates the formation of a molten globule state. In the presence of the P. undulata extract, however, the peak is similar to that observed in native protein with a decrease in overall intensity.
Fig. 6.
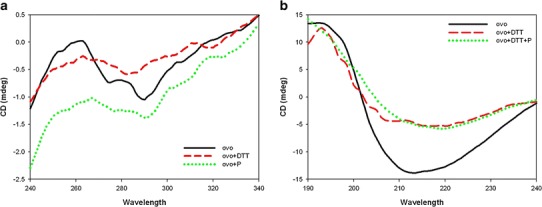
Near-UV CD spectra (a) and far-UV CD spectra (b) of ovotransferrin: unstressed (solid line), stressed (dashed line), stressed in the presence of P. undulata extract (dotted line)
The far-CD spectrum of native ovotransferrin exhibited a minimum around 210 nm, a characteristic typical of an α-helical structure (Fig. 6b) (31). By adding DTT, there occurred crucial alternations in the CD signals, as can be observed from actual changes in the content value of the secondary structure (Table II). In the presence of the P. undulata extract, however, this deviated pattern is still similar to that obtained for native protein.
DISCUSSION
In the present paper, we report on potential pharmacological properties of P. undulata extract against DTT-induced aggregation of different proteins (insulin, α-lactalbumin, and ovotransferrin). Recently, considerable attention has been focused on herbal medicine as a protective agent, mostly because of fewer side effects (32,33). To our knowledge, this study is the first to be published on the effect of P. undulata extract as an inhibitor for protein aggregation. The present results show that P. undulata aqueous extract inhibits aggregation and causes disaggregation of previously aggregated proteins. These effects are accompanied by marked protection against protein aggregation in light scattering results. Visible absorption spectroscopy revealed an increase in light scattering over time of the stressed ovotransferrin, insulin, and α-lactalbumin, due to their aggregation and precipitation. Light scattering results also indicated that P. undulata extract effectively prevented the aggregation of all target proteins in a concentration-dependent manner (Fig. 1, Table I).
It has been suggested that the anti-aggregation property of P. undulata extract may be due to its interaction with the hydrophobic surface of proteins, which are considered crucial to protein aggregation (34,35). Thus, based on the light scattering results described above, our investigation focused on the effect of P. undulata extract on the hydrophobic surface of the proteins. It was assumed that the compound should be sufficiently hydrophobic to interfere with key hydrophobic interaction in protein aggregation. Following this hypothesis, the intrinsic fluorescence of α-lactalbumin showed a high fluorescence intensity which indicates that a large structural change occurred within α-lactalbumin in the presence of DTT. Adding P. undulata extract to reduced α-lactalbumin, however, decreased the fluorescence intensity in a concentration-dependent manner. The same result was obtained for ovotransferrin. This means increasing interaction between denatured proteins and P. undulata extract.
Following the intrinsic fluorescence result, an ANS binding assay also showed that the presence of P. undulata extract decreased the exposure of the hydrophobic regions in all the target proteins. Thus, it can be concluded that P. undulata extract decreased the destabilization of the target proteins. This most likely arises from the reaction of P. undulata extract with the reduced target proteins due to its two phenolic compounds (terpenoids and flavonoids) (36,37). It is speculated that these two components interact with the aggregation-prone hydrophobic area and prevent hydrophobic interaction of nearby molecules that causes self-association and aggregation formation. This is probably due to interaction between the phenol groups of P. undulata extract with the hydrophobic residue, possibly by binding to and shielding the hydrophobic region from solvent and thus blocking associations with each other. The proposed mechanisms of interaction of P. undulata extract with reduced protein are shown in Scheme 1.
Scheme 1.
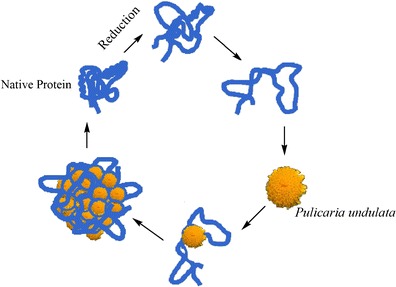
Proposed mechanism of chaperone action of P. undulata extract in preventing protein aggregation
This scheme shows reduction modification of protein caused by DTT. Protein under stress adopts a partially folded state which exposes the hydrophobic area to the solution. This exposed area has the propensity to aggregate. In this proposed mechanism, P. undulata extract interacts with the partially folded protein and refolds it to its native state.
Also, consistent with the intrinsic and extrinsic fluorescence data, near and far-UV CD spectroscopy of the proteins in the presence and absence of P. undulata extract showed little or no perturbation of the protein structure when the P. undulata extract was present. In contrast, significant loss of protein structure was observed in the stressed formulation.
Near-UV CD spectra of α-lactalbumin showed, for example, differences in tryptophan emission intensity bands in the presence and absence of DTT. In this study, however, when adding P. undulata extract to the reduced α-lactalbumin there was little change in the ellipticity of the Trp residue in the near-UV region of the CD spectrum. Similar changes were observed in the tertiary structure of the insulin and the ovotransferrin.
Similar to the change in the tertiary structure seen by the near-UV CD spectra, the far-UV CD spectra revealed that the helical secondary structure of all target proteins was greatly decreased and the β-sheet increased in the presence of DTT. In the presence of P. undulata extract, however, only a slight distortion in the secondary structure was observed. This is could be because the polar group of P. undulata extracts acts to prevent structural change from helical to a β-sheet.
In conclusion, this study provides preliminary evidence of a therapeutic effect of P. undulata extract on protein aggregation. Our results show that P. undulata extract has anti-aggregation properties. Although in vivo the effectiveness of P. undulata extract remains to be investigated, it should be considered as a possible drug candidate or lead component of drugs to prevent or delay protein aggregation and amyloid disease.
REFERENCES
- 1.Dobson CD. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3:9–23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Brange J, Andersen L, Laursen ED, Meyn G, Rasmussen E. Toward understanding insulin fibrillation. J Pharm Sci. 1997;86:517–525. doi: 10.1021/js960297s. [DOI] [PubMed] [Google Scholar]
- 4.Boulos L. Flora of Egypt. Cairo, Egypt: Al-Hadara; 2002. [Google Scholar]
- 5.Al-Rawi A. Flora of Kuwait. Oxford: Alden; 1987. [Google Scholar]
- 6.Nematollahi F, Rustaiyan A, Larijani K, Nadimi M, Masoudi S. Essential oil composition of Artemisia biennis wild and Pulicaria undulata (L.) C.A. Mey., two compositae herbs growing wild in Iran. J Essent Oil Res. 2006;183:339–41.
- 7.Ross SA, El-Sayed KA, El-Sohly MA, Hamann MT, Abdel-Halim OB, Ahmed AF, et al. Phytochemical analysis of Geigeria alata and Francoeuria crispa essential oils. Planta Med. 1997;63:479–482. doi: 10.1055/s-2006-957743. [DOI] [PubMed] [Google Scholar]
- 8.Stavri M, Mathew KT, Gordon A, Steven D, Shnyder R, Falconer A, et al. Guaianolide sesquiterpenes from Pulicaria crispa (Forssk.) Oliv Phytochem. 2008;69:1915–1918. doi: 10.1016/j.phytochem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Bohlman F, Knoll KH, El-Emary NA. Newartige sesquiterpene lactone aus Pulicaria crispa. Phytochemistry. 1979;18:1231–1233. doi: 10.1016/0031-9422(79)80146-6. [DOI] [Google Scholar]
- 10.Rustaiyan A, Simozar E, Ahmadi A, Grenz M, Bohlmann MF. A hardwickiic acid derivative from Pulicaria ghaphalodes. Phytochemistry. 1981;20:2772–2773. doi: 10.1016/0031-9422(81)85286-7. [DOI] [Google Scholar]
- 11.Nurmukhamedova MR, Abdullaev ND, Sidyakin GP. Diterpenoids of Pulicaria salviifolia, II, structure of salvicin. Chem Nat Comp. 1986;22:277–279. doi: 10.1007/BF00598296. [DOI] [Google Scholar]
- 12.Mansour RMA, Ahmed AA, Melek FR, Saleh NAM. The flavonoids of Pulicaria incisa. Fitoterapia. 1990;61:186–187. [Google Scholar]
- 13.Mossa JS, Hifnawy MS, Al-Yahya MA, Al-Meshal IA, Mekkawi AG. GC/MS analysis of essential oils of Pulicaria arabica and P. undulata. Pharm Biol. 1987;25:113–119. doi: 10.3109/13880208709088136. [DOI] [Google Scholar]
- 14.EL-Kamali HH, Yousif MO, Ahmed OI, Sabir SS. Phytochemical analysis of the essential oil from aerial parts of Pulicaria undulata (L.) Kostel from Sudan. Ethnobotanical Leaflets. 2009;13:467–471. [Google Scholar]
- 15.Ali NA, Sharopov FS, Alhaj M, Hill GM, Porzel A, Arnold N, et al. Chemical composition and biological activity of essential oil from Pulicaria undulata from Yemen. Nat Prod Commun. 2012;7:257–260. [PubMed] [Google Scholar]
- 16.San Feliciano A, Medarde M, Gordaliza M, Del Olmo E, Miguel del Corral JM. Sesquiterpenoids and phenolics of Pulicaria paludosa. Phytochem. 1989;28:2717–2721. doi: 10.1016/S0031-9422(00)98074-9. [DOI] [Google Scholar]
- 17.Dendougui H, Benayache S, Benayache F, Connoly JD. Sesquiterpene lactones from Pulicaria crispa. Fitoterapia. 2000;71:373–378. doi: 10.1016/S0367-326X(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Mogib M, Jakupovic J, Dawidar AM, Metwally MA, Abou-Elzahab M. Sesquiterpene lactones and kaurane glycosides from Francoeuria crispa. Phytochemistry. 1990;29:2581–2584. doi: 10.1016/0031-9422(90)85193-J. [DOI] [Google Scholar]
- 19.Ravandeh M, Valizadeh J, Noroozifar M, Khorasani-Motlagh M. Screening of chemical composition of essential oil, mineral elements and antioxidant activity in Pulicaria undulata (L.) C. A. Mey from Iran. J Med Plants Res. 2011;5:2035–2040. [Google Scholar]
- 20.Venkat Ratnam D, Ankola DD, Bhardwaj V, Sahana DK, Ravi Kumar MNV. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Wootton-Beard PC, Ryan L. Improving public health? The role of antioxidant-rich fruit and vegetable beverages. Food Res Int. 2011;44:3135–3148. doi: 10.1016/j.foodres.2011.09.015. [DOI] [Google Scholar]
- 22.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Bio Med. 2010;49:503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Ecroyd H, Carver JA. The effect of small molecules in modulating the chaperone activity of αB-crystallin against ordered and disordered protein aggregation. FEBS J. 2008;275:935–947. doi: 10.1111/j.1742-4658.2008.06257.x. [DOI] [PubMed] [Google Scholar]
- 24.Ghahghaei A, Bathaie SZ, Shahraki A, Rahmany Asgarabad F. Comparison of the chaperoning action of glycerol and β-casein on aggregation of proteins in the presence of crowding agent. Int J Pept Res Ther. 2011;17:101–111. doi: 10.1007/s10989-011-9247-y. [DOI] [Google Scholar]
- 25.Gasymov OK, Glasgow BJ. ANS fluorescence: potential to augment the identification of the external binding sites of proteins. BBA. 2007;1774:403–411. doi: 10.1016/j.bbapap.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray JE, Serge NT, Sterycharz ED. Optical activity of insulin. I. On the nature of circular dichroism bands. Biochemistry. 1971;10:824–831. doi: 10.1021/bi00781a015. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Su R, Qi W, He Z. Photo-induced inhibition of insulin amyloid fibrillation on online laser measurement. Biochem Bioph Res Co. 2011;409:229–234. doi: 10.1016/j.bbrc.2011.04.132. [DOI] [PubMed] [Google Scholar]
- 28.Ewbank JJ, Creighton TE. Structural characterization of the disulphide folding intermediates of bovine-lactalbumin. Biochemistry. 1993;32:3694–3707. doi: 10.1021/bi00065a023. [DOI] [PubMed] [Google Scholar]
- 29.Ghahghaei A, Divsalar A, Faridi N. The effects of molecular crowding on the amyloid fibril formation of α-lactalbumin and the chaperone action of α-casein. Protein J. 2010;29:257–264. doi: 10.1007/s10930-010-9247-3. [DOI] [PubMed] [Google Scholar]
- 30.Rabbani G, Ahmad E, Zaidi N, Hasan Khan R. pH-Dependent conformational transitions in conalbumin (ovotransferrin), a metalloproteinase from hen egg white. Cell Biochem Biophys. 2011;61:551–560. doi: 10.1007/s12013-011-9237-x. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani K, Yamashita H, Oe H, Hirose M. Structural characteristics of the disulfide-reduced ovotransferrin N-lobe analysed by protein fragmentation. Biosci Biotech Bioch. 1997;61:641–646. doi: 10.1271/bbb.61.641. [DOI] [Google Scholar]
- 32.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 33.Akhondzadeh S, Abbasi SH. Herbal medicine in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2006;21:113–118. doi: 10.1177/153331750602100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/S0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 35.Dobson CM. The structure basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:133–145. doi: 10.1098/rstb.2000.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabry TJ, Markham KR, Thomas WB. The systematic identification of flavonoids. 1. Heidelberg: Springer; 1970. [Google Scholar]
- 37.Kamali HH, Ahmed AH. Antibacterial properties of essential oil from Nigella sativa seeds, Cymbopogon citratus leave and Pulicaria undulata aerial parts. Fitoterapia. 1998;69:77–78. [Google Scholar]


