Abstract
Purpose
Although kinematic changes in the sagittal plane of the osteoarthritic knee (OA) have been elucidated, very few studies have analysed changes in the frontal and horizontal planes. Therefore, the aim of this study was to investigate in vivo 3D knee kinematics during walking in patients wth knee OA.
Methods
Thirty patients with medial knee OA and a control group of similarly aged individuals were prospectively collected for this study. All participants were assessed with KneeKGTM system while walking on a treadmill at a self-selected speed. In each trial, we calculated the angular displacment of flexion/extension, abduction/adduction and external/internal tibial rotation. Statistical analysis was performed to determine differences between the knee OA group and the control group.
Results
Patients with knee OA had reduced extension during the stance phase (p < 0.05; 8.5° and 4.4°, OA and control group, respectively) and reduced flexion during pushoff and initial swing phase (p < 0.05; 41.9° and 49.4°, respectively). Adduction angle was consistently greater for OA patients (p < 0.05; 3.4° and −0.9°, respectively). Frontal laxity for OA patients was positively correlated with varus deformity (r = 0.42, p < 0.05). There was a significant difference (p) < 0.05 in tibial rotation during the midstance phase; OA patients retained a neutral position (−0.4°), while the control group presented internal tibial rotation (−2.2°).
Conclusion
Weight-bearing kinematics in medial OA knees differs from that of normal knees. The knee OA group showed an altered “screw-home” mechanism by decreased excursion in sagittal and axial tibial rotation and posterior tibial translation.
Keywords: Knee osteoarthritis, Kinematics, Gait, In vivo assessment
Introduction
Knee function can be quantified with either patient-based scales (questionnaires) or performance-based measures. Seeking to improve knee-function evaluation, numerous studies analysed movment of intact, pathological and treated knees. Quantitative kinematic analysis is an important tool for a thorough understanding of joint function [1]. Kinematics of osteoarthritic (OA) knees has been evaluated using surgical navigation systems, magnetic resonance imaging (MRI) and computed tomography (CT) [2–4], but these techniques cannot be used to study weight-bearing activities, and their results may be therefore be affected. With advances in sophisticated motion-capture technology, 3D knee motion during weight-bearing is now available. Gait, as the most common activity of daily living, has been analysed to clarify the biomechanical characteristics of OA knees.
Most previous studies of knee kinomatic changes in OA patients during gait focused on spatiotemporal parameters [5, 6]; demonstrating that knee OA patients walk slower, with reduced stride length and lower single-limb support compared with controls. Some studies examined kinematic alterations during phase-specific gait cycle and reported decreased knee excursion during flexion, decreased peak flexion during stance and increased knee flexion at heel strike [7–9]. Although kinematic changes in the sagittal plane were found, changes in frontal and axial planes and anterior–posterior (AP) translation remain unclear. Some studies reported an increase in knee adduction angle at initial contact and midstance and a smaller external tibial rotation angle at inital contact [10, 11]; however, both those studies concentrated only the stance phase. Because AP translation is small and can be affected by the choice of assessment system, few studies have analysed movments in this plane [2, 12]. The quasistatistcal fixation of our assessment system is the first to examine anterior–posterior translation during walking [13, 14]. The objective of this study was to use 3D motion analyses (KneeKGTM) to identify changes in kinematic variables of patients with OA of the knee during a complete gait cycle and to correlate them with clinical characteristics. We hypothesised that patients with an OA knee would exhibit altered knee kinematics in the sagittal, frontal and axial planes.
Materials and methods
Participants
This prospective study was performed between February and April 2011 in the biomechanical laboratory at our clinical centre. Thirty patients (18 women, 12 men; mean age 65.7 years) with varus malalignment and medial-compartment knee OA were recruited into the study. They had been scheduled for total knee arthroplasty (TKA). Diagnosis was based on clinical history, physical examination and radiographic evaluation that included comparative anteroposterior knee view with monopodal support, bilateral posteroanterior view at 45° flexion in weight-bearing, comparative lateral view at 30° flexion, axial view at 30° flexion and stress valgus and varus X-ray using a Telos system [15]. Malalignment was confirmed by measuring mechanical axis of the leg, hip–knee–ankle (HKA) angle, from bilateral weight-bearing anteroposterior long-leg films. A line is drawn from the centre of the femoral head to the midpoint of the tibial eminential spine and another from this midpoint to the centre of the tibial plafond. The medial angle between the lines is the HKA angle (varus < 180°, valgus > 180°). Study participants were classified in terms of OA disease severity using Ahlbäck’s radiographic grading system. The integrity of the anterior cruciate ligament (ACL) was assessed intra-operatively. The ACL was present in 21 knees, attenuated in five and ruptured in four (Table 1). Patients were studied if they could walk without a gait aid and were excluded if they had any neuromuscular disease, cardiovascular disorders or lower-limb surgeries that would affect their gait or put them at risk while participating.
Table 1.
Clinical characteristics of the knee osteoarthritis (OA) group
| Clinical characteristics of knee OA patients | ||
|---|---|---|
| Perimeter of walking in daily life (number of patients) | < 0.5 km | 4 |
| 0.5–1 km | 8 | |
| ∼1 km | 14 | |
| >1 km | 4 | |
| Ahlbäck’s classification of knee OA (number of patients) | Stage I | – |
| Stage II | 8 | |
| Stage III | 16 | |
| Stage IV | 6 | |
| Status of ACL (number of patients) | Present | 21 |
| Attenuated | 5 | |
| Ruptured | 4 | |
| Range of motion (n = 30) (mean ± SD) | Extension | 5.6° (12.6) |
| Flexion | 110.8° (18.5) | |
ACL anterior cruciate ligament, SD standard deviation
A control group of 12 similar-aged individuals with varus malalignment was selected (Table 2). The asymptomatic individuals were evaluated by a trained orthopaedic surgeon and were excluded if they had orthopaedic (joint fracture, joint laxity, OA, lower-leg discrepancy and arthritis) or neurological problems that could affect their gait pattern.
Table 2.
Demographic characteristics of the study groups
| Knee OA group (mean ± SD) | Control group (mean ± SD) | Significance (p) | |
|---|---|---|---|
| Age | 65.73 years (10.0) | 61.67 years (3.1) | > 0.5 |
| Weight | 81.8 kg (14.2) | 73.5 kg (9) | < 0.05* |
| Height | 167.3 cm (7.2) | 166.2 cm (5.7) | > 0.5 |
| BMI | 29.08 kg/m2 (4.1) | 26.5 kg/m2 (1.8) | < 0.05* |
| Side | Right: 14 Left: 16 |
Right: 6 Left: 6 |
|
| Gender | Female: 18 Male: 12 |
Female: 8 Male: 4 |
BMI body mass index, SD standard deviation
In vivo kinematic evaluation
Patients’ knee motions were recorded using KneeKGTMsystem [14, 16], a noninvasive system for 3D analysis of the knee, in a weight-bearing and dynamic condition. It consists of an exoskeleton (Fig. 1) (femoral arch with lateral sensors; tibial sensor attachment and sacral belt); infrared camera (Polaris Spectra camera, Northern Digital Inc.) and a computer equipped with Knee3DTM software suite (Emovi, Inc.). It measures and analyses the 3D position and movment of markers embodied in a harness that is placed on the knee of patients whose movment function is to be assessed. The knee-marker attachment system is designed to reduce skin-motion artifacts [13]. Several studies have assessed its accuracy and reproducibility and validated it [13, 14, 17, 18]. Mean interobserver repeatability value ranged between 0.4° and 0.8° for rotation angles and between 0.8 and 2.2 mm for translation [14, 17]. This clinical tool enables an accurate and objective assessment of the 3D function of the knee joint.
Fig. 1.

KneeKGTM trackers installed on the knee
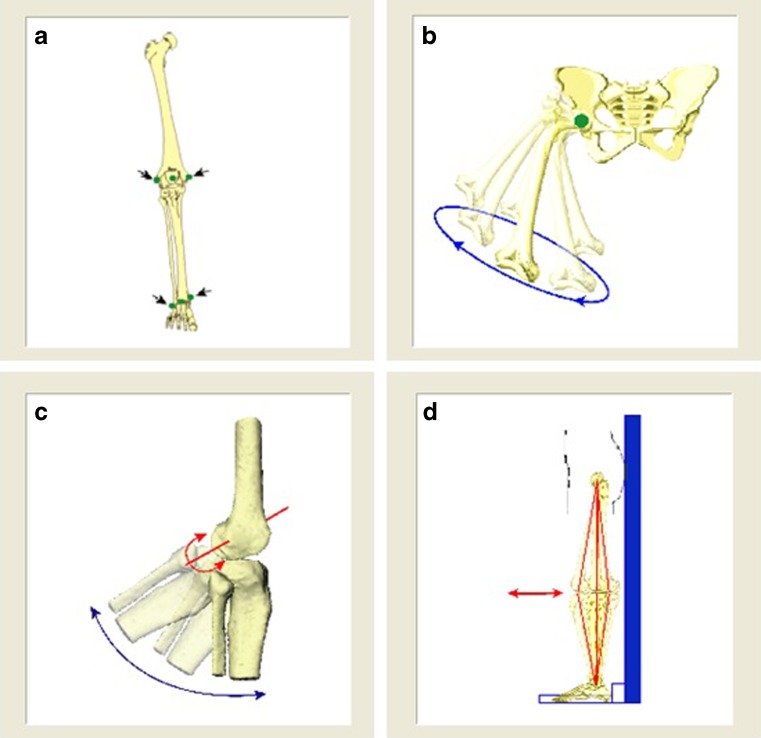
Kinematic analysis of the knee during gait was done with the study participant walking on the treadmill at a comfortable speed chosen by each participant. Firstly, because treadmill walking can be unfamiliar, a habituation period of ten minutes was initiated prior to data collection. After installation of the femoral, tibial and sacral trackers, the device was calibrated . The first step of the procedure was localising anatomical landmarks with the electromagnetic localiser (Fig. 2a). The second step was defining the centre of the hip, knee and ankle joints. The hip-joint centre (HJC) was defined using an optimisation computation method during leg circumduction (Fig. 2b). The knee-joint centre (KJC) was then determined to be the projection of the femoral epicondyle midpoint on the mean helical knee flexion–extension axis (again computed during flexion–extension) (Fig. 2c). The ankle-joint centre (AJC) was defined as the midpoint between the malleoli. Lastly, neutral transverse rotation was set when the knee was determined to be at 0° of flexion during a slight flexion–hyperextension movment (Fig. 2d). A sagittal plane forms from HJC to AJC vector joining; femoral longitudinal axis is defined from HJC to KJC; tibial longitudinal axis is defined from KJC to AJC; projections of longitudinal axes onto the sagittal plane are calculated, as are absolute angle between projections. With the patient in this posture, anterior–posterior axes are defined lying in the sagittal plane perpendicular to longitudinal axes, respectively, for femur and tibia. Mediolateral axes are defined for femur and for tibia perpendicular to the other two axes. Finally, the origin of the axes is fixed at KJC.
Fig. 2.
Calibration of measurments: a localising anatomical landmarks; b defining hip centre; c determining knee-joint centre; d setting neutral transverse rotation
Once system installation and calibration was finalised, the study participant was asked to walk at the preselected speed, and 3D displacments of the reflective markers were recorded by the operator. Once calibration and measurment is performed, the Knee3D suite computes various angle values on anatomical axes between tibia and femur and provides a chart that contains the study individual’s curves. The entire procedure lasts 20-25 minutes. after which the trackers were taken off and the individual was dismissed. A database in Microsoft Excel 2010 was created for each participant that contained the four biomechanical patterns consisting of the three knee angles: flexion–extension, abduction–adduction, internal–external tibial rotation (Fig. 3) and anterior–posterior translation.
Fig. 3.
Knee rotation axes
Using these data, we analysed movment in sagittal, frontal, axial and transverse planes during walking and their correlations with clinical data.
Statistical analysis
The assumptions of normality and equality of variance were assessed using Shapiro-Wilk and Levene’s tests, respectively. If data met parametric assumptions, the t test was used; if not, nonparametric Mann–Whitney test was used to compare differences in age, height, weight and body mass index (BMI). Analysis of variance (ANOVA) and post hoc (Tukey) tests were used to compare kinematic characteristics. Pearson’s correlations (r) were used to examine relationships between clinical and kinematic gait parameters. The statistical difference was set as p < 0.05. All data were analysed using SPSS v21 (SPSS Inc., Chicago, IL, USA).
Results
Table 3 summarises spatiotemporal and kinematic data for OA and control groups.
Table 3.
Kinematic data of study participants
| Kinematic characteristics (mean ± SD) | Knee OA group | Control group | Significance (p) |
|---|---|---|---|
| Speed | 1.2 km/h (0.3) | 2.1 km/h (0.2) | < 0.05* |
| Flexion angle at initial contact | 19° (7.7) | 17.4° (12.5) | > 0.05 |
| Maximum flexion during stance | 22.4° (8.1) | 28.1° (7.9) | < 0.05* |
| Maximum extension during stance | 7.6° (4.1) | 2.2° (4) | < 0.05* |
| Maximum flexion during swing | 48.2° (6.3) | 54.4° (5.3) | < 0.05* |
| Maximum extension during swing | 14.9° (6.5) | 12.5° (6.7) | > 0.05 |
| Range of flexion–extension | 40.6° (6.1) | 52.2° (5.3) | < 0.05* |
| Adduction (+)/abduction (−) angle at initial contact | 5.7° (7.3) | −0.4° (2.8) | < 0.05* |
| Range of adduction–abduction | 7.7° (5) | 5.5° (1.6) | > 0.05 |
| Internal(−)/external(+) Rotation at initial contact | 0.3° (3.6) | −0.1° (2.4) | > 0.05 |
| Range of internal–external rotation | 7.6° (3.1) | 9.3° (2.4) | < 0.05* |
| Anterior (+)/posterior (−) translation of tibia at initial contact | −2.9 mm (5.4) | 0.4 mm (2.2) | < 0.05* |
| Range of anterior–posterior translation | 7.9 mm (4.1) | 9.3 mm (4.4) | > 0.05 |
SD standard deviation, OA osteoarthritis
Flexion–extension
The range of motion in the sagittal plane was significantly lower for the OA (40.6° ± 6.1) than the control (52.2° ± 5.3; p < 0.05) group. Maximum flexion during stance was significantly lower for the OA than the control (22.4° ± 8.09 and 28.1° ± 7.97, respectively, p < 0.05). group During the gait cycle, we observed a significant difference during terminal stance, which corresponds to maximum knee extension (p < 0.05; 8.5° and 4.4°, OA and control group, respectively). During the initial swing phase, OA patients had reduced flexion (p < 0.05; 41.9° and 49.4°, OA and control groups, respectively) (Fig. 4).
Fig. 4.
Knee flexion (+)/extension (−) angle during gait cycle
Adduction–abduction
At initial contact, OA patients showed an adduction angle of 5.7° ± 7.3, whereas the control group stayed in a relatively neutral position (−0.4° ± 2.8, p < 0.05). This difference was observed during the entire gait cycle (Fig. 5). There was a negative correlation between HKA and range of adduction–abduction (r = −0.42, p < 0.05), which means that a higher varus deformity is associated with a higher frontal laxity.
Fig. 5.
Knee adduction (+)/abduction (−) angle during gait cycle
Internal–external rotation
Range of motion of internal–external rotation of OA patients was significantly lower than that of the control group (7.6° ± 3.1; 9.3° ± 2.4, respectively; p < 0.05). In terms of internal–external rotation during the gait cycle, the OA group tended to behave in contrast with the control group. During the stance phase, the OA group remained in a relatively neutral position (mean −0.5° ± 0.4), whereas the control group showed internal rotation (mean −2° ± 0.7), p < 0.05. On the other hand, during the swing phase, the control group started to rotate externally, with the peak at 86 % of the gait cycle at midswing (2.1°); the OA group firstly rotated internally then restored the neutral position, with the peak at 93 % of the gait cycle at terminal swing phase (0.5°) (Fig. 6).
Fig. 6.
Knee external (+)/ internal (−) rotation angle during gait
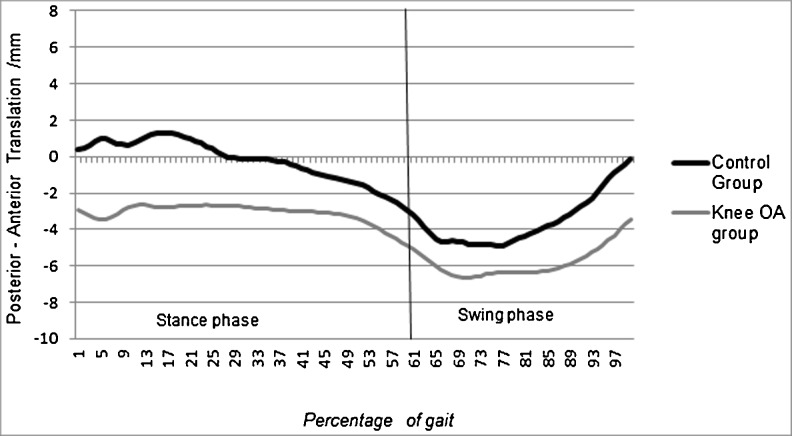
Anterior–posterior translation
At initial contact, the OA group were translated significantly more posteriorly (−2.9 mm ± 5.4) compared with the control group (0.4 mm ± 2.21); p < 0.05. In the OA group, the tibia stayed in a more posterior position during the entire whole gait cycle, but significance was reached at loading and midstance phases (Fig. 7).
Fig. 7.
Tibia anterior (+)/posterior (−) translation (mm) with respect to femur
There was no significant difference in kinematics of OA knees between intact/attenuated and ruptured ACL groups (Table 4).
Table 4.
Kinematic data of the knee OA group based on ACL status. There was no significant difference in either plan
| Kinematic data (mean ± SD) | ACL intact | ACL attenuated | ACL ruptured | Significance (p) |
|---|---|---|---|---|
| Range of flexion/extension | 40.9° (6.9) | 39.8° (4.4) | 40° (2.4) | >0.05 |
| Range of adduction/abduction | 7.7° (4.2) | 9.4° (8.7) | 4.5° (1.2) | >0.05 |
| Range of internal/external rotation | 7.1° (2.6) | 8.6° (5.2) | 8.4°(2.3) | >0.05 |
| Range of anterior/posterior translation | 8.2 mm (4.4) | 5.69 mm (2.8) | 9.03 mm (3.4) | >0.05 |
OA osteoarthritis, ACL anterior cruciate ligament, SD standard deviation
Discussion
The major finding of this study was that the OA knee group showed an overall stiffening gait strategy by exhibiting reduced motion not only in the sagittal but also in the axial plane. Patients with OA showed a reduction in flexion–extension range. Reductions in flexion excursion have been found in most studies that examined patients with OA knees [10, 19–21]. Heiden et al. found no difference in flexion–extension excursion, but they analysed only one phase of the gait cycle, from heel strike to midstance, and included all-stage OA knee patients [22]. Our OA knee group showed a significantly decreased maximum flexion angle during both stance and swing phases, which is in agrement with previous studies [7, 10]. The knee OA group in this study, as in the Schmitt and Rudolph study, showed less extension during the single-limb support-stance phase (34–51 %) than the control group. This lack of extension might be because of greater levels of flexor activation found in the study by Heiden et al. [22] and quadriceps weakness [7, 23] observed at severe OA knees.
Even though the range of adduction–abduction was greater in the OA group, it did not reach statistical significance. On the other hand, there was a positive correlation between varus deformity and adduction–abduction laxity. Results of this study revealed that knee OA patients showed a significantly increased knee adduction angle throughout the gait cycle. These data are consistent with the findings of previous studies [4, 10, 12]. This frontal instability associated with increased adduction angle may increase further degradation of the cartilage. Furthermore, Lewek, Rudolph and Snyder-Mackle showed that excessive frontal laxity was observed only on the medial side of the joint and was accompanied by greater medial muscle cocontraction as an effort to control this laxity [19].
In our study, the range of internal–external rotation in the knee OA group was significantly smaller than that of the control group. The same result was observed by Nagano et al. [10], who reported decreased excursion of axial tibial rotation in the OA group. There was no significant difference at the initial contact rotational angle, but OA knees differed from normal knees during the entire gait cycle. Firstly, during the stance phase, the knee OA group displayed a relatively neutral position, whereas the control group rotated internally, reaching a significant difference at midstance. This difference at this gait phase, which corresponds to maximal knee extension, may be due to the lack of full extension of OA knees. Hamai et al, evaluated rotational angles using CT and reported a femoral internal rotation bias compared with the control group [3]. Saari et al., using dynamic radiostereometric analysis (RSA), found a decreased internal rotation in the knee OA group. Secondly, during the swing phase, the knee OA group maintained a neutral position at the terminal swing phase, whereas the control group rotated externally. To our knowledge, this is the first study to provide information for external–internal rotation during the swing phase. These data show that the “screw-home” mechanism may be altered in the OA knee. Nagao et al. used ultrasound to measure the rotational angle in OA knees and reported that the rotation of screw-home decreases with progression of knee OA then moved more like a simple hinge joint [24].
There is no consensus in the literature concerning anterior–posterior translation in OA knees. In this study, the knee OA group displayed a significantly increased posterior translation from initial contact to midstance. During the swing phase, the tibia in patients with OA knees remained more posteriorly but to a lesser degree. Siston et al. found no differences in anterior–posterior translation between OA and control groups [2], but in their study, kinematics were evaluated with passive motion, and the control group comprised cadaveric knees, whereas we assessed dynamic motions in vivo. Hamai et al. observed a less posterior femoral translation in patients with medial knee OA [3]. Saari et al. concluded that the increased posterior displacment of knees with arthrosis tended to deviate from normal knees in the same way as previously observed in TKA [12]. There was no significant difference in anterior–posterior translation between intact/attenuated/ruptured ACL in OA knees. However, sample sizes of ruptured and attenuated ACL groups (four and five patients, respectively) were small for drawing frank conclusions. Further investigations with sufficient sample sizes are needed to compare the kinematics in OA knees with different ACL status.
This study has some limitations. First, OA patients were heavier and walked with a slower speed than those in the control group. BMI and speed difference is consistent with the literature for similar age groups with and without OA [25, 26]. Some studies compared gait trials between groups by selecting a speed usually near 1 m/s. The effect of walking speed on gait measures, particularly in the sagittal plane, has previously been studied [9, 27]. Whereas the “normal” group showed significant difference between 1 m/s and self-selected speeds for all variables, the severe OA group showed no differences between the two speeds. We did not select the speed for patients to walk because we wanted the individual to walk as they do in their normal daily activities. Another point is that gait speed is linked with the presence of knee OA, and it is difficult to separate the cofounding effect [7]. Secondly, artifacts from soft tissue could have affected results. Nevertheless, Sati and Larouche showed that skin-motion artifacts are reduced with this harness because it is fixed quasistatically in the thigh and calf [18]. Lustig et al. concluded that this evaluation system provides an objective assessment of the precise biomechanical behavior of the knee [14].
The material presented here provides insight into the profound alterations in knee kinematics during walking as a result of OA progression. This information could be taken into consideration in the development of new methods of OA treatment, either conservative or in the innovative designs of knee prostheses.
Conclusion
This study investigated in vivo kinematics of the OA knee during gait on a treadmill. The knee OA group showed an altered screw-home mechanism by decreased excursion in sagittal and axial tibial rotation, and tibial translation posteriorly. On the other hand, we observed an adduction angle during the entire gait cycle and an increased frontal laxity with increased varus malalignment. Analysing postarthroplasty knee function would be of interest to understand whether the changes described in this study could predict postarthroplasty knee kinematics and whether knee kinematics after arthroplasty return to normal.
References
- 1.Ramsey DK, Wretenberg PF. Biomechanics of the knee: methodological considerations in the in vivo kinematic analysis of the tibiofemoral and patellofemoral joint. Clin Biomech. 1999;14:595–611. doi: 10.1016/S0268-0033(99)00015-7. [DOI] [PubMed] [Google Scholar]
- 2.Siston RA, Giori NJ, Goodman SB, et al. (2006) Intraoperative Passive Kinematics of Osteoarthritic Knees before and after Total Knee Arthroplasty. 1607–1614. doi: 10.1002/jor [DOI] [PubMed]
- 3.Hamai S, Moro-oka T-A, Miura H, et al. Knee kinematics in medial osteoarthritis during in vivo weight-bearing activities. J Orthop Res. 2009;27:1555–1561. doi: 10.1002/jor.20928. [DOI] [PubMed] [Google Scholar]
- 4.Chang AH, Chmiel JS, Moisio KC, et al. Varus thrust and knee frontal plane dynamic motion in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1668–1673. doi: 10.1016/j.joca.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ornetti P, Maillefert J-F, Laroche D, et al. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: a systematic review. Joint Bone Spine. 2010;77:421–425. doi: 10.1016/j.jbspin.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Segal G, Igolnikov I, et al. Differences in gait pattern parameters between medial and anterior knee pain in patients with osteoarthritis of the knee. Clin Biomech. 2012;27:584–587. doi: 10.1016/j.clinbiomech.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–341. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 8.Mündermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 9.Zeni JA, Higginson JS. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clin Biomech (Bristol, Avon) 2009;24:372–378. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagano Y, Naito K, Saho Y, et al. Association between in vivo knee kinematics during gait and the severity of knee osteoarthritis. Knee. 2012;19:628–632. doi: 10.1016/j.knee.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Briem K, Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27:78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- 12.Saari T, Carlsson L, Karlsson J, Kärrholm J. Knee kinematics in medial arthrosis. Dynamic radiostereometry during active extension and weight-bearing. J Biomech. 2005;38:285–292. doi: 10.1016/j.jbiomech.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Labbe DR, Hagemeister N, Tremblay M, de Guise J. Reliability of a method for analyzing three-dimensional knee kinematics during gait. Gait Posture. 2008;28:170–174. doi: 10.1016/j.gaitpost.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Magnussen RA, Neyret P, Cheze L, Lustig S. The KneeKG system: a review of the literature. Knee Surg Sports Traumatol Arthrosc. 2012;20:633–638. doi: 10.1007/s00167-011-1814-4. [DOI] [PubMed] [Google Scholar]
- 15.Alban P (2012) TKA outcomes after prior bone and soft tissue knee surgery. doi: 10.1007/s00167-012-2139-7 [DOI] [PubMed]
- 16.Mezghani N, Ouakrim Y, Fuentes A, et al. Knee osteoarthritis severity assessment using knee kinematic data classification. Osteoarthr Cartil. 2012;20:S97. doi: 10.1016/j.joca.2012.02.102. [DOI] [Google Scholar]
- 17.Hagemeister N, Parent G, Van de Putte M, et al. A reproducible method for studying three-dimensional knee kinematics. J Biomech. 2005;38:1926–1931. doi: 10.1016/j.jbiomech.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Sati M, De Guise J, Larouche S, Drouin G. Quantitative assessment of skin-bone movment at the knee. Knee. 1996;3:121–138. doi: 10.1016/0968-0160(96)00210-4. [DOI] [Google Scholar]
- 19.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Childs JD, Sparto PJ, Fitzgerald GK, et al. Alterations in lower extremity movment and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt LC, Rudolph KS, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2008;87:1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiden TL, Lloyd DG, Ackland TR. Knee joint kinematics, kinetics and muscle co-contraction in knee osteoarthritis patient gait. Clin Biomech (Bristol, Avon) 2009;24:833–841. doi: 10.1016/j.clinbiomech.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Baert IAC, Jonkers I, Staes F, et al. Gait characteristics and lower limb muscle strength in women with early and established knee osteoarthritis. Clin Biomech (Bristol, Avon) 2013;28:40–47. doi: 10.1016/j.clinbiomech.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Nagao N, Tachibana T, Mizuno K. The rotational angle in osteoarthritic knees. Int Orthop. 1998;22:282–287. doi: 10.1007/s002640050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman KR, Hughes C, Morrey BF, et al. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–915. doi: 10.1016/S0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 26.Deluzio KJ, Astephen JL. Biomechanical features of gait waveform data associated with knee osteoarthritis: an application of principal component analysis. Gait Posture. 2007;25:86–93. doi: 10.1016/j.gaitpost.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Lelas JL, Merriman GJ, Riley PO, Kerrigan DC. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture. 2003;17:106–112. doi: 10.1016/S0966-6362(02)00060-7. [DOI] [PubMed] [Google Scholar]