Abstract
Purpose
This study investigated the efficacy of platelet-rich plasma (PRP) on articular surfaces on which the mosaicplasty technique was performed. Our hypothesis was that PRP can accelerate the osseointegration process and enhance the quality of articular integrity after the mosaicplasty procedure.
Methods
Standard defects were created in the femoral groove of both patellofemoral joints of 12 New Zealand rabbits. PRP solution was placed inside the defect before fixation of the osteochondral autografts and injected inside the involved joint after capsular closure of the tested knees. The contralateral knees served as the control sides. The animals were euthanized three or six weeks after mosaicplasty, and both limbs were assessed according to Pineda’s histological grading scale. Significance level was set at p ≤ 0.05 a priori, and the Mann–Whitney U test was used for statistical analysis.
Results
Histologic findings at the interface between the transferred autograft and the original cartilage revealed better integration of the adjacent surfaces in the mosaicplasty with PRP group three weeks after the procedure; the difference was significant (p < 0.05). However, no significant difference in the transition zone was observed between the groups six weeks after the experiment (p = 0.59).
Conclusions
Our animal model showed that adjunctive use of PRP produced a better healing response and resulted in superior histological scores after three weeks compared with the mosaicplasty-only procedure. Interpretation of our results is important in terms of rapid return to previous activity levels. Thus, application of PRP can represent a valid therapeutic option for improving the efficacy of mosaicplasty by stimulating the local healing response.
Keywords: Platelet-rich plasma, Osteochondral defect, Mosaicplasty, Cartilage
Introduction
Management of full-thickness chondral defects of the knee remains a challenge for orthopaedic surgeons. The most important long-term result of this condition is osteoarthritis, which can impair quality of life [1]. The repair capacity of articular cartilage is limited due to its low mitotic activity, the absence of vessel and nerve supply, and the immobility of articular chondrocytes. Large defects generally fail to heal spontaneously, which makes surgical intervention necessary in order to avoid the progression of osteoarthritis [2]. Symptomatic articular cartilage defects have mostly been treated with microfracture, autologous chondrocyte implantation, and mosaicplasty. However, no treatment method has proven to be superior thus far [3, 4]. Drilling and microfracture have a wide range of uses for treating articular cartilage defects, but they do not restore normal hyaline cartilage, and treatment failures have been documented in the literature [5, 6].
Mosaicplasty, one of the most frequently used techniques for treating articular cartilage defects of many joints, can treat full-thickness defects with the host’s own cartilage [7]. This technique has the theoretical advantage of increased cellular viability. However, osseointegration and maintenance of the integrity of the implanted articular cartilage have been reported in the literature as the main reasons for failure [8, 9]. In addition, in vitro and in vivo studies have demonstrated that impaction on the grafts during press fit fixation can lead to chondrocyte death [10, 11]. Size of the lesion, time interval, and age of the patient are other important factors that affect outcomes, and little is known about the long-term viability of these grafts. In an animal osteochondral transplantation model, osseointegration between the recipient and donor bones was observed four weeks after transplantation [12]. This fairly long time period led us to investigate whether we could obtain a better outcome with the use of platelet-rich plasma (PRP).
Many growth factors, including transforming growth factor-B (TGF-B), fibroblast growth factor (FGF), and bone morphogenetic protein, have been found to be effective in cartilage regeneration and repair; however, clinical use of growth factors is not practical, due to their short lifespan and high cost [13, 14]. PRP is a rich source of growth factors, and its clinical use has become popular in recent years. PRP is defined as a volume of the plasma fraction of autologous blood that has a high concentration of platelet-derived growth factors and acts as a potential inducer of regeneration and healing of tissues. PRP contains growth factors such as platelet-derived growth factor, TGF-B, FGF, insulin growth factor, vascular endothelial growth factor, and epithelial cell growth factor [15]. In fact, platelet concentrate has been shown to promote bone healing, chondrocyte proliferation, and cartilage formation in orthopaedics [15–17]. Conversely, in a very recent review, the authors suggested that there is limited evidence to support the use of PRP in the management of chondral or osteochondral defects, whether alone or as an adjunct to surgical treatment [18].
The objective of this study was to investigate the efficacy of PRP on articular surfaces on which mosaicplasty was performed. In addition, the osseointegration process was assessed over the short term and relatively long term. Our hypothesis was that PRP can enhance the osseointegration process and articular integrity after mosaicplasty compared with mosaicplasty-only procedures.
Methods
For this study, we used 12 adult (22 weeks old) New Zealand rabbits weighing an average of 4.3 kg. The rabbits were genetically similar and were homogeneous for age, size, and feeding. The research was approved by the local ethics committee for animal experimentation. In order to provide standardization, the PRP was prepared using the Gravitational Platelet Separation System (GPS, Biomet, Warsaw, IN, USA). The rabbits were anaesthetized by intramuscular injection of 35 mg/kg ketamine and 5 mg/kg xylazine, and 27-mL blood samples were obtained in a 30-mL syringe containing 3 mL sodium citrate as an anticoagulant. Approximately 3 mL PRP was obtained for each rabbit. The PRP was prepared according to the manufacturer’s instructions, and no specialized instrument was required except the centrifuge machine and disposable cylindrical tubes, which included a section to collect the PRP. After the PRP was prepared, a platelet count analysis was performed on 1.0 mL of each PRP sample using a digital haematology analyser, and the mean platelet concentration was assessed and compared with the platelet concentration of the subject’s blood circulation.
While the homologous PRP was being prepared, both knees of the animals were incised under sterile conditions through a medial parapatellar incision, and 4-mm defects were created in the femoral groove of both patellofemoral joints, as described by Sun et al. [19]. Standard defects were created at a depth of 4 mm and a diameter of 4 mm with a standard disposable osteochondral autograft transport system (DePuy, Warsaw, IN), and the rest of the process was performed as suggested by Nam et al. [20]. For all experiments, the right knee was the test and the left knee was the control. In the test knee, in order to mimic mosaicplasty, a harvested graft from the contralateral knee was transplanted into the socket of the test knee (Fig. 1) and applied to the defect with press-fit fixation by tamping it into the host bed (Fig. 2). Care was taken to ensure that the articular side of the graft was at the same level as the host cartilage. No exogenous activation technique was performed prior to implantation, and 1 mL of the PRP solution was placed inside the defect prior to fixation; a small amount of the solution overflowed out of the defect during compression.
Fig. 1.
Macroscopic appearance of the 4-mm graft harvested from the contralateral knee
Fig. 2.
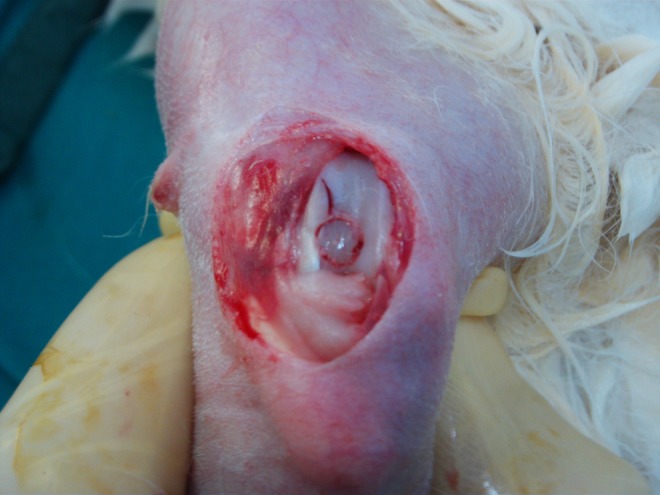
Macroscopic appearance of one sample after press-fit fixation of the graft
In addition, 1 mL of the PRP solution was injected into the joint following tight closure of the capsule, in order to prevent leakage of the PRP solution from the joint. A harvested graft from the test knee was transplanted into the control knee with press-fit fixation, without applying PRP solution to either the defect or the joint. Adequate hemostasis and surgical wound closure were then performed, respecting the anatomical layers.
The animals were allowed to move freely in their cages, and they were all able to bear weight on both extremities immediately after surgery. General health status was monitored during recovery by a veterinarian. Following the mosaicplasty procedure, the rabbits in group 1 were euthanised at three weeks, and those in group 2 were euthanised at six weeks. The distal femurs of the test and contralateral control limbs were harvested and prepared for histological assessment.
The distal femurs of the animals were fixed and decalcified with 10 % ethylenediaminetetraacetic acid (EDTA) solution. Samples were dehydrated and embedded in paraffin wax and cut along the sagittal plane of the defect area. Histologic samples were obtained from the central third of the defect. Three slices (4-μm thick) were stained with hematoxylin-eosin (H-E) and examined under a light microscope. Assessments were based on gross macroscopic and microscopic examinations. In the macroscopic evaluation, surface regularity, continuity, and arthritic changes were assessed.
The samples were evaluated by a well-experienced pathologist and scored according to Pineda’s histological grading scale [21]. The observer was blinded to the treatment technique. For each sample, a mean score was obtained from the pathologist based on observation of all of the histological sections, and the mean of the three scores was calculated. The histological scores of the two groups were evaluated and compared to determine whether the values were statistically significant. Subchondral bone healing and osseointegration were determined by noting the presence of bony trabecular interdigitation between the graft and the surrounding host subchondral bone [20]. The significance level was set a priori at p ≤ 0.05, and because all of the experimental animals underwent surgery on both knees, the contralateral knees served as the control group. The Mann–Whitney U test was used for statistical analysis.
Results
One rabbit died one week after the surgical procedures of unknown causes and was excluded from the study. Due to the loss of this animal, one more age- and weight-matched animal was included in group 1.
On gross examination, no grafts lost fixation in this study. Three weeks after osteochondral implantation, the transition zone of the autograft and the adjacent articular cartilage showed impressive integration in the mosaicplasty with PRP group. In addition, the osteochondral autografts appeared shiny white, and no arthritic changes were apparent in this group. Gross examination of the mosaicplasty only group (control group) revealed a linear margin in one knee, on one side of the autograft, indicating imperfect integration (Fig. 3). Some indistinct superficial fissures and an opaque white appearance, indicating microscopic low cellularity, were present in the samples from two animals (Fig. 4).
Fig. 3.
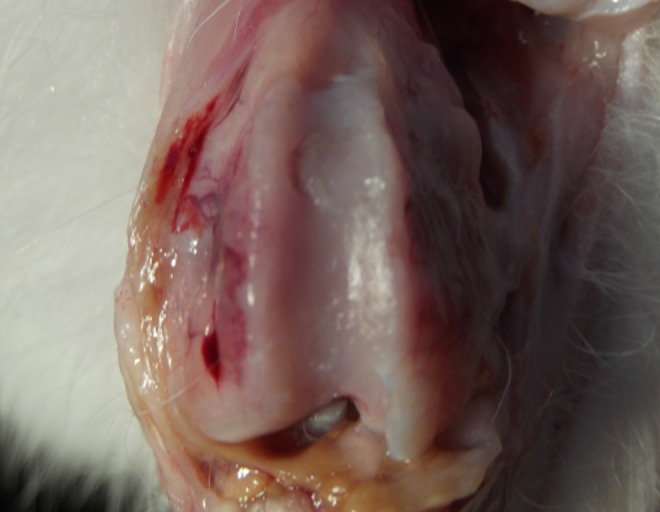
One sample of mosaicplasty alone group shows macroscopic gap formation (lack of cartilage integration) at just one end of the adjacent cartilage
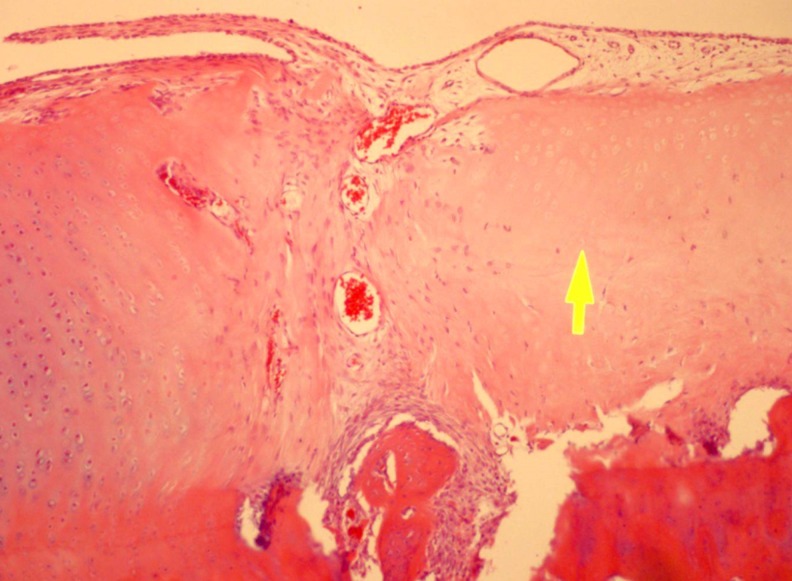
Fig. 4.
Microscopic images demonstrated low cellularity (yellow arrow) when compared with the host cartilage. (H-E staining. Original magnification 100×)
Six weeks after the surgical procedure, gross macroscopic evaluation showed a similar gross appearance between the mosaicplasty with PRP group and the mosaicplasty-alone group. On gross inspection of both knee joints at six weeks, the transition zone between the graft and the host cartilage was identified macroscopically. No apparent synovitis or evidence of infection were observed. The osteochondral autografts were shiny white in color (Fig. 5).
Fig. 5.
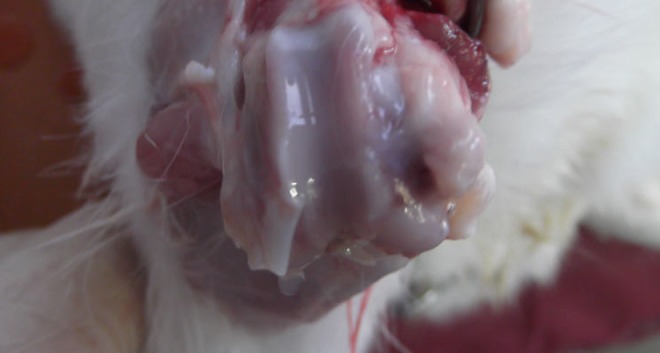
Similar appearance and colour to the normal cartilage and good integration with shiny appearance of the osteochondral graft can be seen macroscopically
Histological findings at the interface between the cartilage of the transferred autograft and the host cartilage revealed excellent integration of the adjacent surfaces in the mosaicplasty with PRP group three weeks after the experiment. All six animals in this group exhibited sufficient regeneration and integration (Fig. 6). However, in the mosaicplasty-only group, the cartilage of four of the six animals was significantly thinner than normal (p < 0.05) (Fig. 7), and cartilage integration had only occurred at one end of the adjacent cartilage (Fig. 8). The differences in histological scores between the groups were statistically significant (p < 0.05). A total index of healing score was derived by totaling the subcategories of Pineda’s histological grading scale, with a maximum possible worst score of 14. The histological scores of each group are summarized in Table 1.
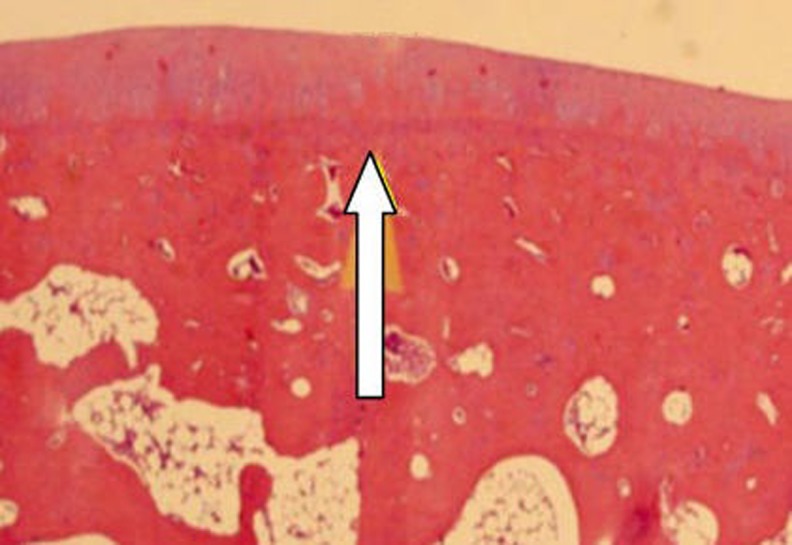
Fig. 6.
Regular appearance of the cartilage, bone, and the bone marrow can be seen at the transition zone, host, and the autograft. Sufficient integration at the edge of the transplanted cartilage was demonstrated (white arrow). (H-E staining. Original magnification 20×)
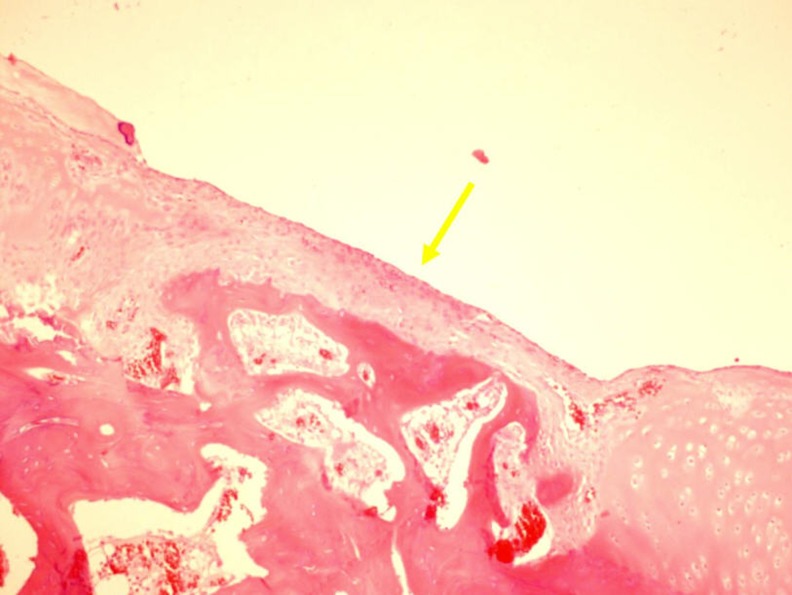
Fig. 7.
Normal cartilage tissue on the right side, and the transplanted cartilage on the left side. Thickness of the transition zone (in the middle) decreased. (H-E staining. Original magnification 400×)
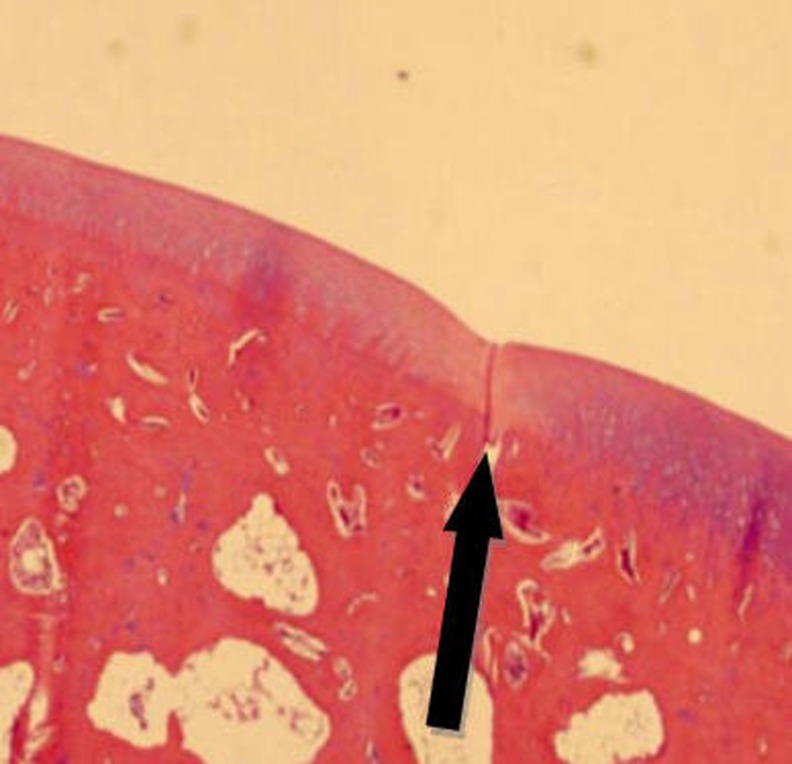
Fig. 8.
Insufficient cartilage integration was observed with a substantial margin. (H-E staining. Original magnification 20×)
Table 1.
Five individual parameters are listed according to the Pineda grading scale
| Score parameter | Three weeks (group I) | Six weeks (groups II) | ||
|---|---|---|---|---|
| Control group (n = 6) | PRP + mosaicplasty (n = 6) | Control group (n = 6) | PRP + mosaicplasty (n = 6) | |
| Cell morphology | 0.16 | 0.0 | 0.0 | 0.16 |
| Matrix-staining (metachromasia) | 0.0 | 0.0 | 0.0 | 0.0 |
| Surface regularity | 0.5 | 0.0 | 0.0 | 0.0 |
| Thickness of cartilage | 0.66 | 0.0 | 0.16 | 0.16 |
| Integration of donor with host | 0.16 | 0.33 | 0.83 | 0.66 |
| Total | 1.5 | 0.33 | 1.33 | 1 |
All values are given as the mean for each parameter
No significant differences in the histological grading of the osteochondral transplant area were observed between the mosaicplasty with PRP group and the mosaicplasty-only group six weeks after implantation (p = 0.59). A comparison of the different time periods within each group showed no significant difference in either group (p = 0.162 for the involved knees and p = 0.654 for the uninvolved knees). For both time periods and groups, all of the transplanted autografts demonstrated excellent trabecular interdigitation with the host subchondral bone.
Data analysis showed at least a four-fold greater platelet concentration in the PRP (mean, 1319 × 103/mL); the difference was significant (p < 0.05). In a very recent classification of PRP (PAW classification) [22], PRP solutions were staged as P4-Aα with a mean platelet count of more than 1250 × 103 and above baseline of total WBC-neutrophils (Table 2).
Table 2.
Total number of platelets, WBC, and neutrophil counts and the corresponding symbols according to PAW classification
| Animal number | Platelet concentration in whole blood | Platelet concentration in PRP | WBC count | Neutrophil count |
|---|---|---|---|---|
| 1 | 270 × 103 | 1253 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 2 | 292 × 103 | 1402 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 3 | 275 × 103 | 1318 × 103 (P4) | Above baseline (A) | Below baseline (α) |
| 4 | 260 × 103 | 1101 × 103 (P3) | Above baseline (A) | Above baseline (α) |
| 5 | 286 × 103 | 1273 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 6 | 290 × 103 | 1453 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 7 | 231 × 103 | 1197 × 103 (P3) | Below baseline (B) | Above baseline (α) |
| 8 | 237 × 103 | 1294 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 9 | 299 × 103 | 1399 × 103 (P4) | Above baseline (A) | Above baseline (α) |
| 10 | 268 × 103 | 1253 × 103 (P4) | Below baseline (B) | Above baseline (α) |
| 11 | 250 × 103 | 1411 × 103 (P4) | Above baseline (A) | Below baseline (α) |
| 12 | 264 × 103 | 1475 × 103 (P4) | Above baseline (A) | Above baseline (α) |
According to PAW classification, P3 indicates a platelet concentration of >750,000–1,250,000 and P4 indicates a platelet number of more than 1,250,000
Discussion
The aim of this study was to evaluate the effects of autologous PRP on the healing of cartilage and the osseointegration process in mosaicplasty procedures. To date, no previous studies that applied PRP to chondral defects [19, 23, 24] combined the mosaicplasty technique with PRP to assess the histological results in an animal model. Therefore, there was no information available regarding the effectiveness of PRP in this clinical condition.
The primary outcome of this study was histological evaluation of the transition zone. Although press-fit fixation of the plugs can be achieved during mosaicplasty, there seems to be a lack of cartilage at the transition zone due to the implementation of the technique. During mosaicplasty, the tip of the blade creates a microscopic cleft between the host and the autograft, and this issue needs to be resolved for a proper healing process and osseointegration. The results of our study showed that intra-articular use of PRP during mosaicplasty led to better cartilage integration at the transition zone and improved quality of the repaired tissue three weeks after the experiments than did mosaicplasty alone. However, there was no significant difference between the groups after six weeks, and the histological results were similar to those of a previously reported study in which 2.7-mm autografts were used [20]. In the previous study, cartilage bonding seemed to be the major problem, which was defined as the histological appearance of perfect apposition between the graft and the host cartilage surface [25]. That study demonstrated how the histological properties of transplanted osteochondral autografts behaved six weeks and 12 weeks after osteochondral transfer; it was found that none of the transplanted grafts were completely bonded on all sides. However, at six weeks, only two knees in our study presented histologically insufficient binding on both sides of the autografts. The larger autografts (4 mm) used in our study might be a factor that contributed to better results.
The time effect analysis did not indicate any significant difference in the two groups. This finding might be due to several reasons. First, the amount of PRP solution is controversial, and might not be enough for an adequate response to impact the long term. Although there is not a consensus regarding the amount of PRP for use in animal studies, a 1-mL intra-articular injection of PRP is a comparable amount with the previous studies [23, 24]. Secondly, PRP might affect the healing process in the acute and subacute time period, and a steady state obtained at the proliferative phase of the healing process. Thereafter, the deterioration process might continue in both groups in the chronic setting, leading to an insignificant difference at six weeks. Our explanation was already supported by Gulotta et al., who suggested that the majority of patients who undergo this surgery experience immediate chondrocyte necrosis after surgery, followed by a reincorporation of their grafts [11]. In this process, PRP may have a reinforcement on chondral regeneration and proliferation of chondrocytes as a biologic inducer in the short term [16, 26].
Interpretation of our results is important in terms of improved healing response over a three-week period when compared with the controls. Naturally, cartilage disturbances might be expected, according to previous studies. Those in vitro studies showed significant chondrocyte necrosis and apoptosis shortly after the autologous osteochondral transplantation because the impaction on articular cartilage has a fatal effect on chondrocytes [10, 27]. Gulotta et al., in their rabbit model, confirmed that chondrocyte necrosis and cartilage degradation occur during the early periods of autologous osteochondral transplantation after impaction. They reported that only 63.3 % of chondrocytes remain viable immediately after the procedure, and that only 51.6 % are viable after four days. In ovine and pig models, researchers have reported that the grafts exhibit greater degeneration and deterioration than the host articular cartilages do [28, 29]. Interestingly, those studies demonstrated that severe degeneration can occur as early as two weeks after osteochondral transplantation. In fact, those results might explain the inferior results achieved in the control group in our study after three weeks and the superior results obtained in the mosaicplasty with PRP group.
A lack of repeated PRP injections might be another reason, and further study may be needed to address this deficiency. Milano et al. suggested administering repeated platelet concentrate injections after application of microfractures to full-thickness cartilage injuries and reported superior and more durable reparative responses than isolated microfractures in an animal model [24]. They performed repeated injections to obtain long-term effects during the study and had better histological scores in the followup period from three to six months, but not at 12 months’ followup. Thus, in order to obtain a permanent effect, multiple injections make more sense; however, the duration and intervals of the PRP applications is not clear yet.
The main goal of osteochondral transfer is to restore the original articular cartilage and maintain the original joint biomechanics. However, during the osseointegration process, many factors affect the final outcome, and once an osteochondral lesion or a chondral defect occur, the regenerating potential differs among individuals. Whatever the affecting factor, incomplete healing of the spaces between the graft and surrounding tissue and fibrillation of the repair tissue have been reported [20, 30]. Researchers have attributed their inferior results to insufficient adaptive remodeling of the cartilaginous portion of the graft and insufficient time to allow remodeling. Limitation of movement and weight bearing after mosaicplasty are the most important causes of this condition. However, a restricted time period is essential for protecting the autografts, and prolonged restriction of weight bearing has been a necessity for adequate healing response of osteochondral autografts. This protective time period might be shortened by providing a better and more rapid healing response with the use of biological inducers.
It has been shown that osseointegration between the recipient and donor bones can occur four weeks after transplantation [12]. This is a fairly long time period for patients with high expectations who want to return to previous activity levels or sports as soon as possible. Under these circumstances, our aim in this animal model was to influence the osseointegration process with the use of PRP. Previous studies have reported controversial results regarding the effectiveness of PRP in bone healing [15, 31–33], but none was supported by level I clinical evidence. Consistently, in the two time periods in our study, there were no histological differences in osseointegration that were shown to be responsible for failures [8]. Bone-to-bone healing is a special condition and differs from cartilage healing in many ways. Adequate osseointegration had already occurred in all of our specimens at three weeks independently of the PRP application. Although osseointegration was perfect in all of the animals, even at three weeks, integrity and adequate healing of the implanted cartilage emerged as a major problem in the control group in our study. Although our study did not demonstrate any significant histological differences six weeks after PRP injection, as a clinical relevance, improved histological results at three weeks might allow early rehabilitation, including weight bearing and rapid activity modification, and lead to better clinical outcomes in the long term.
PRP has recently come into use in a wide range of orthopaedic applications, such as bone healing, cartilage repair, and tendon repair [12, 13, 34]. Many studies have shown that PRP enhances proliferation and chondrogenic stem cell differentiation [35]. However, a limited number of in vivo studies have described the effect of PRP on the histologic appearance of the articular cartilage repair. Some of those studies concluded that PRP improved the histology of the cartilage repair [19, 23, 24, 36, 37], and a few reports stated that it worsened the histological scores [38, 39]. Sun et al. applied PRP in a rabbit model and reported that PRP has an effect on resurfacing cartilage defects and restoring the subchondral bone [19]. Saito et al. used PRP to demonstrate its preventive effect on osteoarthritis [23]. Contrary to these studies, however, Kon et al. reported that PRP decreased the type II collagen content of cartilage repair tissue and worsened the gross appearance [38]. In addition, although many positive results have been reported, there have also been unpredictable results, due to variable platelet concentrations in solutions and different preparation techniques [9, 40, 41, 42]. Nevertheless, in a very recent review by Sheth et al., they attributed uncertain results for orthopaedic indications to a lack of standardization of outcome measures, PRP production, and investigative protocols [42, 43]. In order to overcome these limitations and standardize the preparation of PRP, we used the same manufacturer’s kits; thus, we avoided creating a misleading factor, and we achieved an increase of at least four to five times over baseline platelet numbers, which was suggested as a sufficient cellular response by Marx [44].
We acknowledge several limitations to this study. First, the short time period is a weakness of our study, but we feel that the comparison of results three weeks after osteochondral transfer is important in terms of histological assessment during the chondrocyte degradation process. Six weeks is also an important time point that has been previously studied [20], and it gave us an opportunity to compare the results of this study. Second, while understanding the biomechanical properties and the detailed contents of the transplanted cartilage by using immunohistochemical evaluation could be beneficial, our study was based on histological findings and gross macroscopic examination because the main problem of a previous study indicated that cartilage bonding was the major issue. Third, the size of the lesion is an important issue, considering the variable results of mosaicplasty with different graft sizes [4], and the efficacy of the treatment on larger defects cannot be predicted from the results of the present study. Fourth, rabbit knee cartilage is thinner than human cartilage, and this difference might result in differences in the effectiveness of PRP. One last limitation is that we did not analyse the growth factor content of the PRP obtained from the animals, but rather, measured just the platelet contents.
Cartilage defects have been treated with many different techniques, as shown in previous animal models; however, treating chondral defects by transferring osteochondral autografts has only been described [20] and compared with other techniques in a rabbit model [45]. With our limited time points and limitation of animal models, we tried to simulate an osseochondral autograft transfer in an animal model to assess whether the PRP solution affects the integrity of articular cartilage and osseointegration.
Conclusions
The results of the present study showed that adjunctive use of PRP produced a better healing response and resulted in superior histological scores three weeks after PRP application when compared with a mosaicplasty-only procedure. In clinical use, improved histological results obtained in the earlier stages could lead to a faster return to previous activity levels. Application of PRP can represent a valid therapeutic option to improve the efficacy of the mosaicplasty technique by stimulating the local healing response. We believe that well-designed clinical trials are needed to translate the findings to clinical use.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, Aroen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Mankin HA. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 4.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223–230. doi: 10.1302/0301-620X.85B2.13543. [DOI] [PubMed] [Google Scholar]
- 5.Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade. 1999;28:26e32. doi: 10.1007/s001320050318. [DOI] [PubMed] [Google Scholar]
- 6.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2e12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 7.Altan E, Senaran H, Acar MA, Aydın K, Ozbaydar MU (2013) Mozaicplasty technique for treatment of reverse Hill-Sachs lesion. Tech Should Elbow Surg 14:1–4. doi:10.1097/BTE.0b013e318274962c
- 8.Marcacci M, Kon E, Zaffagnini S, Iacono F, Neri MP, Vascellari A, et al. Multiple osteochondral arthroscopic grafting (mosaicplasty) for cartilage defects of the knee: prospective study results at 2-year follow-up. Arthroscopy. 2005;21(4):462–470. doi: 10.1016/j.arthro.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Borzini P, Mazzucco L. Tissue regeneration and in loco administration of platelet derivatives: clinical outcome, heterogeneous products, and heterogeneity of the effector mechanisms. Transfusion. 2005;45:1759e67. doi: 10.1111/j.1537-2995.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Borazjani BH, Chen AC, Bae WC, et al. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am. 2006;88(9):1934–1943. doi: 10.2106/JBJS.E.00992. [DOI] [PubMed] [Google Scholar]
- 11.Gulotta LV, Rudzki JR, Kovacevic D, Chen CC, Milentijevic D, Williams RJ., 3rd Chondrocyte death and cartilage degradation after autologous osteochondral transplantation surgery in a rabbit model. Am J Sports Med. 2009;37(7):1324–1333. doi: 10.1177/0363546509333476. [DOI] [PubMed] [Google Scholar]
- 12.Hangody L, Kish G, Karapati Z, Eberhart R. Osteochondral plugs: Autogenous osteochondral mosaicplasty for the treatment of focal chondral and osteochondral articular defects. Oper Tech Orthop. 1997;7:312–322. doi: 10.1016/S1048-6666(97)80035-3. [DOI] [Google Scholar]
- 13.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26:131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borovecki F, Pecina Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin XL, Smith CM, Costa ML. The clinical use of platelet rich plasma in the promotion of bone healing: A systematic review. Injury. 2009;40:158–162. doi: 10.1016/j.injury.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr Cartil. 2006;14:1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: Experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65:1951–1957. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Dold A, Zywiel M, Taylor DW, Dwyer T, Theodoropoulos J. Platelet-rich plasma in the management of articular cartilage pathology: a systematic review. Clin J Sport Med. 2014 doi: 10.1097/01.jsm.0000432855.85143.e5. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34(4):589–597. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam EK, Makhsous M, Koh J, Bowen M, Nuber G, Zhang LQ. Biomechanical and histological evaluation of osteochondral transplantation in a rabbit model. Am J Sports Med. 2004;32(2):308–316. doi: 10.1177/0363546503259616. [DOI] [PubMed] [Google Scholar]
- 21.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 22.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 23.Saito M, Takahashi KA, Arai Y, Inoue A, Sakao K, Tonomura H, et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol. 2009;27:201–207. [PubMed] [Google Scholar]
- 24.Milano G, Deriu L, Passino ES, Masala G, Manunta A, Postacchini R, et al. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy. 2012;28(5):688–701. doi: 10.1016/j.arthro.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. J Bone Joint Surg Am. 1986;68(7):1017–1035. [PubMed] [Google Scholar]
- 26.Drengk A, Zapf A, Sturmer EK, Sturmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009;189(5):317–326. doi: 10.1159/000151290. [DOI] [PubMed] [Google Scholar]
- 27.Whiteside RA, Jakob RP, Wyss UP, Mainil-Varlet P. Impact loading of articular cartilage during transplantation of osteochondral autograft. J Bone Joint Surg Br. 2005;87(9):1285–1291. doi: 10.1302/0301-620X.87B9.15710. [DOI] [PubMed] [Google Scholar]
- 28.Baumbach K, Petersen JP, Ueblacker P, et al. The fate of osteochondral grafts after autologous osteochondral transplantation: a one-year follow-up study in a minipig model. Arch Orthop Trauma Surg. 2008;128:1255–1263. doi: 10.1007/s00402-007-0532-3. [DOI] [PubMed] [Google Scholar]
- 29.Tibesku CO, Szuwart T, Kleffner TO, et al. Hyaline cartilage degenerates after autologous osteochondral transplantation. J Orthop Res. 2004;22(6):1210–1214. doi: 10.1016/j.orthres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Hurtig M, Pearce S, Warren S, Kalra M, Miniaci A. Arthroscopic mosaic arthroplasty in the equine third carpal bone. Vet Surg. 2001;30:228–239. doi: 10.1053/jvet.2001.23348. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Yang X, Huang G, et al. Platelet-rich plasma promotes healing of osteoporotic fractures. Orthopedics. 2013;36(6):e687–e694. doi: 10.3928/01477447-20130523-10. [DOI] [PubMed] [Google Scholar]
- 32.Arpornmaeklong P, Kochel M, Depprich R, Kübler NR, Würzler KK. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33(1):60–70. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 33.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–2420. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 34.Castricini R, Longo U, de Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: A randomized controlled trial. Am J Sports Med. 2011;39:258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 35.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15:431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milano G, Passino ES, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: An experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18:971–980. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Qi YY, Chen X, Jiang YZ, et al. Local delivery of autologous platelet in collagen matrix simulated in situ articular cartilage repair. Cell Transplant. 2009;18:1161–1169. doi: 10.3727/096368909X12483162197169. [DOI] [PubMed] [Google Scholar]
- 38.Kon E, Filardo G, Delcogliano M, et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;11:220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brehm W, Aklin B, Yamashita T, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14:1214–1226. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Weibrich G, Kleis WK, Hitzler WE, Hafner G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005;20:118e23. [PubMed] [Google Scholar]
- 41.Leitner GC, Gruber R, Neumüller J, Wagner A, Kloimstein P, Höcker P, et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135–139. doi: 10.1111/j.1423-0410.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 42.Pettersson S, Wetterö J, Tengvall P, Kratz GJ. Human articular chondrocytes on macroporous gelatin microcarriers form structurally stable constructs with blood-derived biological glues in vitro. Tissue Eng Regen Med. 2009;3(6):450–460. doi: 10.1002/term.179. [DOI] [PubMed] [Google Scholar]
- 43.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: A meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 44.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatr Orthop. 2004;24(4):427–433. doi: 10.1097/01241398-200407000-00014. [DOI] [PubMed] [Google Scholar]