Abstract
Purpose
Much research is now being conducted in order to understand the role of cytokines in the development of the inflammatory response following trauma. The purpose of this study was to evaluate whether serum levels of certain cytokines, measured immediately after initial injury, can be used as potential biomarkers for predicting the development and the degree of severity of the systemic inflammatory response (SIRS) in patients with moderate and severe trauma.
Methods
We conducted a prospective study with 71 individuals of whom 13 (18.3 %) were healthy controls and 58 (81.7 %) were traumatized orthopaedic patients who were categorized into two groups: 31 (43.6 %) with moderate injuries and 27 (38.1 %) patients with severe orthopaedic trauma. Thirty cc of heparinized blood were drawn from each individual within a few hours after the injury. Serum levels of pro-inflammatory, regulatory and anti-inflammatory cytokines were measured in each individual participant.
Results
High levels of pro-inflammatory cytokines IL-1β,-6,-8,-12, tumour necrosis factor alpha and interferon gamma were found in all injured patients compared to healthy controls. Only IL-6 and IL-8 were significantly higher in the injured patients. Levels of the regulatory cytokines, transformed growth factor beta (TGF-β) and IL-10 were higher in the injured patients, but significant only for TGF-β. Levels of IL-4 were significantly lower in the injured groups as compared to the controls.
Conclusions
Secretion of large amounts of pro-inflammatory cytokines and decreased level of anti-inflammatory cytokines during the acute phase of trauma may lead to the development of systemic inflammatory response syndrome (SIRS) in unstable polytraumatized patients. SIRS may result in life threatening conditions as acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF). High levels of IL-6, IL-8, TGFβ and low levels of IL-4 were found to be reliable markers for the existence of immune reactivity in trauma patients. More research is needed to study pattern of cytokine levels along the acute period of injury, after surgical interventions and during recovery.
Keywords: Cytokines, Polytrauma, Systemic inflammatory response (SIRS), Early total care (ETC), Damage control orthopaedics (DCO), Interleukines
Introduction
Recently, an increasing research interest has been focused on the issue of cytokines and their role in immune activity in trauma patients [1–5]. Skeletal and tissue injuries may cause a hyper-inflammatory reaction of the immune system manifested by elevation in levels of pro-inflammatory cytokines and may lead to the development of systemic inflammatory reaction syndrome (SIRS). The degree of this reaction depends on the severity of the trauma and is known as the “first hit” [2, 6]. The regular mode of treatment in the last few decades of patients with multiple injuries consists of immediate prolonged and extensive surgical procedures of isolated or combined head, chest and abdominal injuries followed by fixations of fractures of the pelvis and/or long bones of these patients and is called “early total care” (ETC). The secondary inflammatory reaction in such polytraumatized patients during and after surgical procedures, including immediate fracture fixation, is known as the “secondary hit” [2, 3, 5].
It was also found that ETC in polytraumatized patients with major blood loss may increase significantly the severity of this inflammatory response (SIRS) as described by various authors [2, 3, 5, 7, 8]. The development of this hyper-inflammatory reaction is manifested by activation of the immune system which triggers increased secretion of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and IL-8 [2, 4]. The increase in pro-inflammatory cytokines is evident soon after injuries (six hours) and lasts usually for 24–48 hours in most cases. The duration of this reaction depends on the severity of the trauma. In patients with severe injuries this increase may persist longer [2, 3]. In such patients the “secondary hit” inflammatory reaction, following ETC procedures, may aggravate the acute systemic inflammatory reaction syndrome (SIRS), and may lead to development of acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF) with a relatively high morbidity and mortality [2, 9–12].
The massive secretion of pro-inflammatory cytokines usually induces up-regulation of anti-inflammatory cytokines such as IL-4, and regulatory cytokines such as transforming growth factor-beta (TGF-beta) and IL-10, which results in a decrease of the severity of the inflammatory reaction [2, 4]. An imbalance between the early systemic inflammatory response and the later compensatory anti-inflammatory response may be responsible for organ dysfunction and increased susceptibility to infections [2, 4].
These findings led in the last decade to the development of a new concept of treatment of polytraumatized patients which is known as “damage control orthopaedics” (DCO). The basic principles of DCO are control of the life threatening injuries combined with minimal invasive stabilization of the fractures mainly by external fixators and then only after few days of metabolic and respiratory recovery to perform the definitive management of fracture fixation [2, 5, 7].
The purpose of this study was to evaluate the degree of the systemic inflammatory response in patients following moderate and severe trauma. Therefore, differences in the levels of serum pro-inflammatory, anti-inflammatory and regulatory cytokines were assessed in injured patients with various types of trauma and compared to healthy controls.
Patients and methods
This study was approved by the Institutional Review Board (Helsinki Committee) in the Western Galilee Hospital. It consisted of 71 participants, all in the age range of 20–55, with mean age of 36.1 years (±10.4 SD). Thirteen (18.3 %) were healthy individuals that served as controls, and 58 (81.7 %) were patients, of whom 35 were males (60.3 %) and 23 females (39.7 %). Patients and controls were similarly distributed by age and gender. All patients were injured in traffic or work accidents and were categorized into two groups: 31 (43.6 %) patients with a moderate injury including isolated fractures of ankle or humerus and 27 (38.1 %) patients with severe trauma injuries including fractures of the femur or tibia or both femora, or of femur and tibia together. Inclusion criteria were age 20–60 years and ability to answer the questionnaires; exclusion criteria were chronic diseases that may affect immune functions. Patients with any head, chest, abdominal or pelvic injuries were also excluded.
Abbreviated injury scale (AIS)
Abbreviated injury scale (AIS) was used to assess the severity of injuries [13, 14]. This scale is an anatomically based consensus derived global severity scoring system that classifies each injury by body region according to its relative importance on a 6-point ordinal scale, 1 being minor, 2 being moderate, 3 serious, 4 severe, 5 critical, and 6 a non-survivable injury [13, 14]. In this study the injury severity scores (ISS) were not evaluated since this series of patients with fractured limbs had not any additional injuries of any other different body regions such as head, face, neck, chest, and abdomen. The ISS is an overall score for patients with multiple injuries; in each of them the AIS of six different body regions such as head and neck, face, chest, abdomen, extremities and external are evaluated [15]. The highest AIS scores of the three most severe injured regions are then squared and added together and the sum of them produces the ISS score. The AIS therefore is the most appropriate system in this paper dealing with various types of bone fractures without any other associated body injuries. The mean severity AIS injury score was 2.7 (SD ± 0.8). The AIS of the moderate group was 2, whereas that of the severe group was 3.44 (SD ± 0.5).
Serum cytokine levels
A few hours following injury (T1) 30 cm3 of heparinized venous blood samples were drawn for measurement of cytokine levels. A multiple analyte detection system based on fluorescent bead immunoassay was used as described previously by the present authors [16] for quantitative detection of the serum cytokines IL-1β, Il-2, IL-4, IL-6, IL-8, IL12, TGF-β, interferon-gamma (INFγ) and tumor necrosis factor-alpha (TNFα) as follows: (1) beads were coated with antibodies that specifically react with each of the analytes to be detected in the multiplex system, (2) a mixture of coated beads for each of the analytes was incubated with the samples or standard mixture, (3) a biotin-conjugated second antibody mixture was added, and (4) streptavidin–phycoerythrin was added, which bound to the biotin conjugate and emitted fluorescent signals. Data analysis was collected on a FACSCalibur and analysed using FlowCytomix Pro 2.2 software from Bender MedSystems [16].
Statistical analysis
The SPSS 19 software package was used for data analysis. Log transformed values of cytokine levels were used in t-test and ANOVA test. T-test for independent samples was used to assess differences between the injured and the control groups in levels of serum cytokines. Analysis of variance (ANOVA) was applied for comparison of the severe injured, moderate injured and the control groups. This analysis was followed by Scheffe post-hoc test in order to assess differences between pairs of groups. T-test for paired samples was applied for comparison between the measures at T1 (at injury) and T2 (six months later).
Results
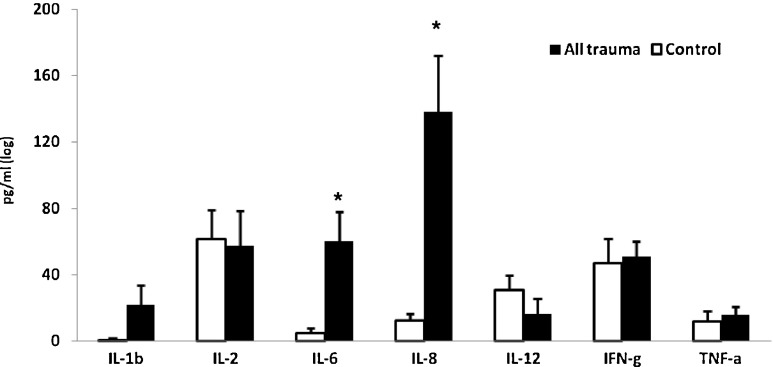
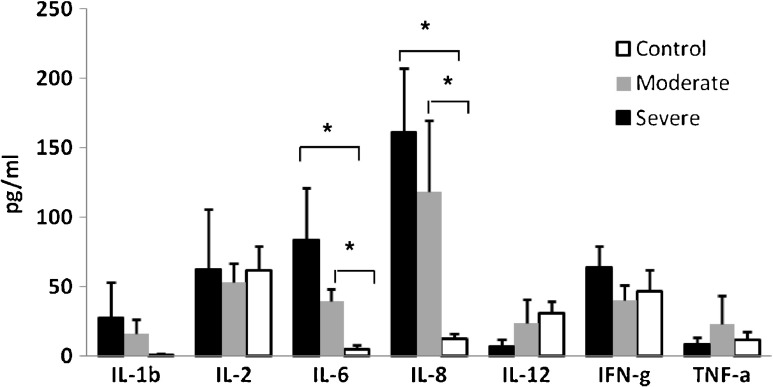
Levels of cytokines in serum were not significantly associated with age, except for levels of IL-4 that were lower with higher age (r = -0.28, p = 0.044). The findings of the present study show that patients with moderate and severe orthopaedic injuries present a distinct pattern of cytokine levels in serum as compared with healthy age- and sex-matched controls. The mean levels of the pro-inflammatory, anti-inflammatory and regulatory cytokines in all injured participants were compared to the control group. The levels of the pro-inflammatory cytokines IL-6 and IL-8 were significantly higher in the injured group compared to controls (p < 0.001 and p < 0.05, respectively), but the differences for the other pro-inflammatory cytokines were not statistically significant (Table 1, Fig. 1). The means and differences in the pro-inflammatory cytokines in the moderate injured, the severe injured and the control groups are presented in Table 2 and Fig. 2. The analysis of variance (ANOVA) showed that significantly higher levels of pro-inflammatory IL-6 and IL-8 were found in the severe and moderate injured groups as compared to controls (p < 0.01). Scheffe post-hoc comparisons between pairs among the three groups indicated that the differences were significant for each of the injured groups as compared to controls. The differences between the severely injured group and the moderately injured group themselves were not statistically significant (Fig. 2).
Table 1.
Levels of cytokines in injured and control groups, immediately after trauma (t1)
| Cytokines | Injured (n = 58) | Controls (n = 13) | t-test | ||
|---|---|---|---|---|---|
| M | SE | M | SE | t (69) | |
| IL-1β | 21.9 | 12.8 | 0.8 | 0.4 | 0.9 |
| IL-2 | 57.8 | 21.1 | 61.6 | 17.5 | -1.4 |
| IL-6 | 60.2 | 18.2 | 5.0 | 3.4 | 3.7*** |
| IL-8 | 138.6 | 34.7 | 12.2 | 4.3 | 3.2* |
| IL-12 | 16.4 | 9.2 | 19.1 | 8.6 | -1.2 |
| IFN-γ | 51.4 | 9.4 | 47.2 | 14.9 | 0.0 |
| TNF-α | 16.2 | 11.5 | 11.9 | 6.4 | 1.0 |
| IL-4 | 17.0 | 5.2 | 32.4 | 6.0 | -3.5* |
| IL-10 | 8.6 | 6.4 | 4.9 | 2.3 | -1.2 |
| TGF-β | 23.1 | 3.2 | 13.7 | 3.4 | 2.8** |
*p < 0.01, **p < 0.01, ***p < 0.001
Levels of cytokines are presented in pg/ml, except for TGFβ presented in ng/ml; t-test was conducted for log transformed measures
Fig. 1.
Means (±SE) of levels of pro-inflammatory cytokines in injured and control groups immediately after trauma (t1) *p < 0.01
Table 2.
Levels of pro- and anti-inflammatory cytokines in severe and moderate trauma and in control group immediately after trauma (t1)
| Cytokines | Moderately injured (n = 31) | Severely injured (n = 27) | Controls (n = 13) | ANOVA | |||
|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | F (2,68) | |
| IL-1β | 16.4 | 10.3 | 28.3 | 25.0 | 0.8 | 0.4 | 0.3 |
| IL-2 | 53.2 | 13.8 | 63.0 | 42.9 | 61.6 | 17.5 | 2.4 |
| IL-6 | 39.6 | 9.1 | 83.9 | 37.5 | 5.0 | 3.4 | 7.2* |
| IL-8 | 118.5 | 51.4 | 161.6 | 46.0 | 12.2 | 4.3 | 6.5* |
| IL-12 | 24.1 | 16.9 | 7.6 | 4.3 | 19.1 | 8.6 | -1.2 |
| IFN-γ | 40.4 | 11.3 | 64.1 | 15.2 | 47.2 | 14.9 | 0.1 |
| TNF-α | 23.0 | 21.3 | 8.5 | 5.0 | 11.9 | 6.4 | 0.6 |
| IL-4 | 14.3 | 4.6 | 20.1 | 9.9 | 32.4 | 6.0 | 6.1* |
| IL-10 | 1.8 | 0.7 | 16.4 | 13.6 | 4.9 | 2.3 | 1.2 |
| TGF-β | 23.6 | 4.8 | 22.4 | 4.4 | 13.7 | 3.3 | 1.7 |
* p < 0.01
ANOVA was conducted for log transformed measures
Levels of cytokines are presented in pg/ml, except for TGFβ presented in ng/ml
Fig. 2.
Means (±SE) of levels of IL-4 and IL-10 in injured and control groups immediately after trauma (t1). Note: Levels of TGFβ are presented in ng/ml; * p < 0.01
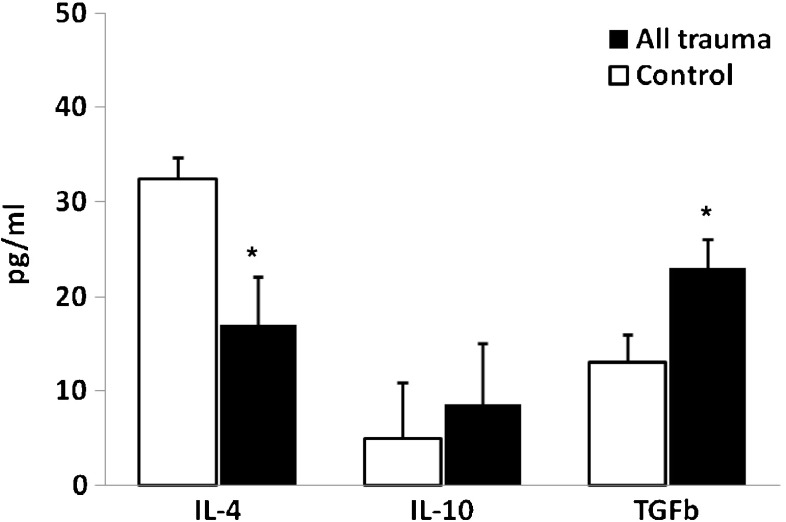
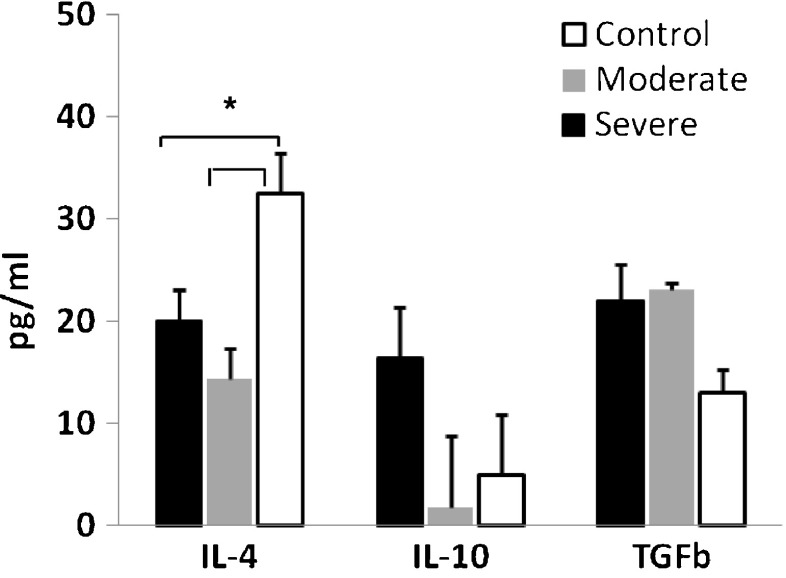
Mean values for the anti-inflammatory cytokine IL-4, and regulatory cytokine TGF-β and IL-10 for all injured participants compared to controls are presented in Table 1 and Fig. 3. The levels of IL-4 were significantly lower (p < 0.05) and the levels of TGF-β were significantly higher (p < 0.01) in the injured patients as a whole group, compared to controls, whereas IL-10 levels were not statistically different between the injured group and the controls. Table 2 and Fig. 4 show the means of levels of IL-4, IL-10 and TGF-β for the severe, moderate and control groups. ANOVA and Scheffe post-hoc test showed that the levels of IL-4 were significantly lower in each of the two injured groups as compared to controls (p < 0.01).
Fig. 3.
Means (±SE) of levels of pro-inflammatory cytokines in severe and moderate trauma and in control groups immediately after trauma (t1); * P < 0.01
Fig. 4.
Means (±SE) of levels of anti-inflammatory cytokines, by severe, moderate and control groups immediately after trauma (t1). Note: Levels of TGFβ1 are presented in ng/ml; * p < 0.01
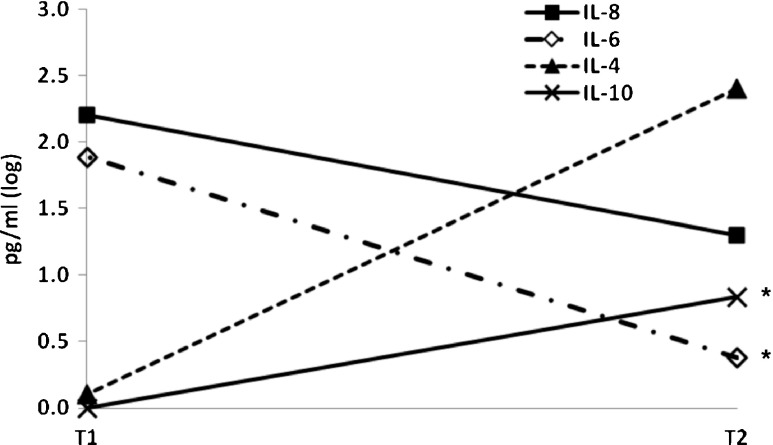
In addition, a preliminary assessment of the first four injured individuals for serum cytokines six months after initial trauma was done. The differences between the two time points (T1 after initial trauma and T2 six months later) for IL-6, IL-8, IL-4 and IL-10 are presented in Table 3 and Fig. 5. The levels of pro-inflammatory cytokines IL-6 and IL-8 decreased from T1 to T2, and the differences were statistically significant for measurements of IL-6 (p < 0.05). The levels of regulatory (IL-10) and anti-inflammatory (IL-4) cytokines substantially increased from T1 to T2 but the differences were statistically significant only for IL-10.
Table 3.
Differences in levels of selected pro- and anti-inflammatory cytokines across two time points—at injury and after additional six months post-injury
| Cytokines | On injury | After six months | t-test | ||
|---|---|---|---|---|---|
| M | SE | M | SE | t (3) | |
| IL-6 | 80.0 | 16.0 | 1.7 | 0.8 | 4.7* |
| IL-8 | 415.0 | 145.5 | 85.9 | 42.9 | 2.5 |
| IL-4 | 0.0 | 0.0 | 330.7 | 189.7 | -1. 7 |
| IL-10 | 0.0 | 0.0 | 6.0 | 0.5 | -13.0** |
* p < 0.05, ** p < 0.01
Paired t test for t1-t2 was conducted on log transformed measures (injured only, N = 4)
Fig. 5.
Differences in levels of cytokines between hospitalization (t1), and six months (t2). Note: the scale was log transformed * p < 0.01
Discussion
The present results show that patients with various orthopaedic injuries present a distinct pattern of cytokine levels as compared with healthy matched controls. Significantly higher serum levels of pro-inflammatory cytokines IL-6 and IL-8 and of the regulatory cytokine TGF-β and low levels of anti-inflammatory IL-4 were found in each type of injured group compared to controls, while the difference in the levels of IL-10 was not significant. The higher levels of pro-inflammatory cytokines in the injured patients accord with the reported pro-inflammatory state in reaction to injury. Secretion of anti-inflammatory cytokines are induced by IL-6, in order to attenuate the inflammatory activity [9–12].
The increase of pro-inflammatory cytokine levels following injuries indicates their role in the body reaction to trauma [2, 3]. The pro-inflammatory cytokines are produced during early immune response and upregulate the expression of endothelial cells adhesion molecules and the activation of neutrophils. TNFα and IL-1β induce epithelial adhesion molecules and chemokines, recruiting leukocytes to infected tissues. IL-6 is produced by T lymphocytes and macrophages and induces B lymphocytes proliferation and antibody production and the proliferation and maturation of T cells. IL-8 is released by activated monocytes and serves as a chemokine for neutrophils and macrophages and plays a key role in the accumulation of leukocytes at sites of inflammation [9, 10]. IL-12 is produced by dendritic cells and macrophages and is involved in the differentiation of Th1 cells and in stimulating natural killer activity and production of IFN-γ from natural killer cells. IFN-γ is produced predominantly by natural killer and Th1 cells and upregulates their activity and is critical for innate and adaptive immunity. IL-2 is secreted by Th1 cells and is necessary for the growth, proliferation, and differentiation of Th1 cells and induces B cell proliferation [6, 17].
The lower levels of the anti-inflammatory cytokines IL-4 found in the injured group compared to controls accords with previous studies that show suppression of these cytokines during the immediate immune reaction to acute injury. IL-4 is secreted by Th2 helper cells and is depressed when levels of pro-inflammatory cytokines increase. Increase in IL-4 in later stages of the immune response induces suppression of pro-inflammatory cytokines [2, 4].
TGF-β and IL-10 are regulatory cytokine secreted by T cells and macrophages, and have a central role in regulation of proliferation and differentiation of cells, wound healing, and angiogenesis [18]. Our findings of high levels of TGF-β and IL-10 in the injured patients are supported by previous reports demonstrating increase in serum concentrations during the early response to injury [19–22]. Evidence exists that the high serum concentrations of TGF-β and IL-10 are involved in the further down regulation of the inflammatory cytokines. In addition, TGF-β is involved in the process of fracture healing [20–22]. It enhances the proliferation and differentiation of mesenchymal stem cells and increases the production of extracellular matrix. It has a key role in the promotion of cartilage formation and increases the formation of callus and bone strength [20].
Our other findings are that in a subsample of four injured patients checked six months after initial injury (T2) the levels of the pro-inflammatory cytokines decreased (the difference was statistically significant for IL-6 only) and that levels of anti-inflammatory cytokines substantially increased (the difference was statistically significant for IL-10 only). These preliminary findings are supported by previous findings reporting differences during the first week after the initial trauma [2–9, 23, 24]. However, we consider our findings as an only pilot observation of a small group of patients and it should be submitted to replication and further investigations in a larger groups of patients.
The significant increase in the levels of pro-inflammatory cytokines IL-6 and IL-8 in all injured patients, with the highest levels in the severe injured group, is very important as fracture management in patients with multiple injuries continues to be of crucial importance. Early treatment of unstable traumatized patients with head, chest, abdomen or pelvic injuries and major blood loss that consists of immediate life saving surgical procedures and fracture fixation of long bones mainly with reamed nails (early total care) may be associated with the development of a secondary life threatening posttraumatic systemic inflammatory response syndrome (SIRS). This may be followed by acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF) with a relatively high morbidity and mortality [1–4]. Primary external fixation of long bone fractures in such patients is safe and a short procedure. Therefore, the current recommendation in unstable poly-traumatized patients is to use modular and minimally invasive external frames for the initial fracture fixation. This approach is named “damage control orthopaedic surgery” (DCO). The basic principles of DCO in such patients consist of immediate treatment of life threatening conditions and primary minimal invasive fixation of long bone fractures, followed by metabolic and respiratory stabilization of the patients. After an additional few days of metabolic and respiratory recovery the final definitive management of fracture fixation is performed. A significant reduction in incidence of general systemic complications such as ARDS and MOF has been described in the DCO groups of patients despite higher ISS in comparison with the ETC group.
The study has some limitations that should be acknowledged. It is a one time-point assessment; therefore the markers correlation to clinical course was not feasible. The sample consists of patients with moderate or severe fractures of the limbs, without involvement of head, chest, abdominal or pelvic injuries. Therefore the abbreviated injury scale (AIS) was used to assess the severity of injuries [13, 14]. The injury severity score (ISS), which is the sum of the AIS squared values of the three most severe injured regions, was not evaluated since this series of patients with fractured limbs did not have any additional injuries of any other different body regions. We initially and purposefully excluded patients with complicated injuries in order to achieve homogeneity in severity levels. The severity of AIS is therefore much lower than the ISS. Further assessments of cytokines as biomarkers of systemic inflammatory response in multiple trauma injuries including orthopaedic injuries are needed.
The causes and effects of changes in balance of pro- and anti-inflammatory cytokines due to injury should be further studied. One example is the need to study the role of genetic polymorphism in inducing hyper-inflammation. Our preliminary results provide an indication for the involvement of genetic factors, such as polymorphism in certain genes (TLR9), that regulate immune responses in accelerated hyperinflammation in response to injury [25]. Another direction is to study the long-term effect of hyperinflammation due to injury on psychological reactions to trauma. Our previous studies as well as others showed that high serum levels of pro-inflammatory cytokines IL-6 and IL-8, and low levels of the regulatory cytokine TGF-β and anti-inflammatory IL-4 and IL-10 predicted higher levels of acute stress symptoms soon after injury [16, 26]. Moreover, when controlling for age and severity of injury, higher serum levels of IL-8 and lower serum TGF-β predicted higher posttraumatic stress symptoms one month after injury [16]. According to previous findings that certain pro-inflammatory cytokines, such as IL-6, may cause or intensify psychological symptoms [27], these results suggest that high levels of inflammation due to injury may be a risk factor for post trauma psychological symptomatology. Other studies described other conditions with significant levels of various cytokines in the synovial fluid of patients with rheumatoid arthritis and also in patients with osteoarthritis, supporting a pro-inflammatory process in the pathogenesis of osteoarthritis [28–30]. Elevated plasma levels of pro-inflammatory cytokines, such as IL-1beta, IL-8 and TNF-alpha, were also observed as useful markers of bone resorption in patients with aseptic loosening of large joint prosthesis [31].
Based on the findings of this study of such significant increases in the levels of pro-inflammatory cytokines IL-6, TGFβ and IL-8, with the highest levels in the severely injured group, combined with significant low levels of IL-4 in all injured patients, it is suggested that serum high levels of these cytokines can be used as potential reliable biomarkers for predicting the development of systemic inflammatory response syndrome (SIRS) in patients with multiple trauma. Secretion of large amounts of pro-inflammatory cytokines and decreased level of anti-inflammatory cytokines during the acute phase of trauma, mainly in unstable polytraumatized patients, may lead to the development of systemic inflammatory response syndrome (SIRS). This may result in life threatening conditions as acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF). In such conditions this may suggest the use of DCO as the initial treatment of unstable poly-traumatized patients. DCO surgery appears to be a viable alternative for poly-traumatized patients with long bone fractures. More research is needed to study the pattern of specific changes in the levels of subgroups of cytokine along the acute period of injury, after surgical interventions and across several time points during recovery.
References
- 1.Giannoudis PV, Hildebrand F. Pape HC (2004): Inflammatory serum markers in patients with multiple trauma: Can they predict outcome? J Bone Joint Surg Br. 2004;86:313–323. doi: 10.1302/0301-620X.86B3.15035. [DOI] [PubMed] [Google Scholar]
- 2.Craig S, Roberts CS, Pape HC, Jones AL, Malkani AL, Rodriguez JL, Giannoudis PV. Damage control orthopaedics. Evolving concepts in the treatment of patients who have sustained orthopaedic trauma. J Bone Joint Surg Am. 2005;87:434–449. [PubMed] [Google Scholar]
- 3.Pape HC, Rixen D, Morley J, et al. Impact of the method of initial stabilization for femoral shaft fractures in patients with multiple injuries at risk for complications (borderline patients) Ann Surg. 2007;246:491–499. doi: 10.1097/SLA.0b013e3181485750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sears BW, Strover MD, Callaci J. Pathoanatomy and clinical correlation of the immunoinflamatory response following orthopaedic trauma. J AAOS. 2009;17:255–265. doi: 10.5435/00124635-200904000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichte P, Kobbe P, Dombroski D, Pape HC. Damage control orthopaedics; current evidence. Curr Opin Crit Care. 2012;18:647–650. doi: 10.1097/MCC.0b013e328359fd57. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis PV, Smith RM, Bellamy MC, Morrison JF, Dickson RA, Guillou PJ. Stimulation of the inflammatory system by reamed and unreamed nailing of femoral fractures: An analysis of the second hit. J Bone Joint Surg Br. 1999;81:356–361. doi: 10.1302/0301-620X.81B2.8988. [DOI] [PubMed] [Google Scholar]
- 8.Pape HC, Hildebrand F, Pertschy S, Zelle B, Garapati R, Grimme K, Krettek C, Reed RL., 2nd Changes in the management of femoral shaft fractures in polytrauma patients: from early total care to damage control orthopedic surgery. J Trauma. 2002;53:452–462. doi: 10.1097/00005373-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Biffl W, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 11.Kumbhare D, Parkinson W, Dunlop B, Richards C, Kerr C, Buckley N, Adachi J. Injury measurement properties of serum interleukin-6 following lumbar decompression surgery. J Surg Res. 2009;157:161–167. doi: 10.1016/j.jss.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Reikeras O. Immune depression in musculoskeletal trauma. Inflamm Res. 2010;59:409–414. doi: 10.1007/s00011-010-0167-7. [DOI] [PubMed] [Google Scholar]
- 13.Copes WS, Lawnick M, Champion HR, Sacco WJ. A comparison of abbreviated injury scale 1980 and 1985 versions. J Trauma. 1988;28:78–86. doi: 10.1097/00005373-198801000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Civil ID, Schwab CW. The abbreviated injury scale, 1985 revision; a condensed chart for clinical use. J Trauma. 1988;28:87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1988;14:187–196. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42:117–131. doi: 10.2190/PM.42.2.b. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- 18.Sultani S, Stringer AM, Bowen JM, Gibson RJ (2012) Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemotherapy Res Practice 2012; Article ID 490804, 11 pages doi:10.1155/2012/490804 [DOI] [PMC free article] [PubMed]
- 19.Ayala A, Meldrum DR, Perrin MM, Chaudry IH. The release of transforming growth factor-beta following haemorrhage: its role as a mediator of host immunosuppression. Immunology. 1993;79:479–84. [PMC free article] [PubMed] [Google Scholar]
- 20.Sarahrudi K, Thomas A, Mousavi M, Kaiser G, Köttstorfer J, Kecht M, Hajdu S, Aharinejad S. Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury. 2011;42:833–837. doi: 10.1016/j.injury.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun T, Wang X, Liu Z, Chen X, Zhang J. Plasma concentrations of pro- and anti-inflammatory cytokines and outcome prediction in elderly hip fracture patients. Inj. 2011;42(7):707–713. doi: 10.1016/j.injury.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 23.Smith RM, Bellamy MC, Morrison JF, Dickson RA, Guillou PJ. Stimulation of the inflammatory system by reamed and unreamed nailing of femoral fractures: An analysis of the second hit. J Bone Joint Surg Br. 1999;81:356–361. doi: 10.1302/0301-620X.81B2.9154. [DOI] [PubMed] [Google Scholar]
- 24.Lee CC, Marill KA, Carter WA, Crupi RS. A current concept of trauma-induced multiorgan failure. Ann Emerg Med. 2001;38:170–176. doi: 10.1067/mem.2001.114313. [DOI] [PubMed] [Google Scholar]
- 25.Cohen M, Volpin G, Meir T, Klein E, Katz R, Assaf M, Pollack S. Possible association of Toll-like receptor 9 polymorphisms with cytokine levels and posttraumatic symptoms in individuals with various types of orthopaedic trauma: early findings. Inj. 2013;44(11):1625–1629. doi: 10.1016/j.injury.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Newport DJ, Nemeroff BC. Neurobiology of posttraumatic stress disorder. Curr Opin Neurobiol. 2000;10:211–218. doi: 10.1016/S0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 28.Hoff P, Buttgereit F, Burmester GR, Jakstadt M, Gaber T, Andreas K, Matziolis G, Perka C, Rohner E. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthopaedics (SICOT) 2013;37:145–151. doi: 10.1007/s00264-012-1724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto S, Muller-Ladner U, Gay RE, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996;23(8):1345–1352. [PubMed] [Google Scholar]
- 30.Rohner E, Matziolis G, Perka C, Fuchtmeier B, Gaber T, Burmester GR, Buttgereit F, Hoff P. Inflammatory synovial fluid microenvironment drives primary human chondrocytes to actively take part in inflammatory joint diseases. Immunol Res. 2012;52(3):169–175. doi: 10.1007/s12026-011-8247-5. [DOI] [PubMed] [Google Scholar]
- 31.Hundric-Hasple Z, Pecina M, Haspl M, Tomicic M, Jukic I. Plasma cytokines as markers of aseptic prosthesis loosening. Clin Orthop Relat Res. 2006;453:299–304. doi: 10.1097/01.blo.0000229365.57985.96. [DOI] [PubMed] [Google Scholar]