Abstract
Objectives/Hypothesis
Tissue-engineered septal cartilage may provide a source of autologous cartilage for repair of nasal defects. Production of clinically useful neocartilage involves multiple steps that include manipulating the culture environment. Partial pressure of oxygen (ppO2) is a property that has been shown to influence cartilage development. Specifically, studies suggest low ppO2 augments in vitro growth of articular cartilage. Although in vivo measurements of articular cartilage ppO2 have demonstrated hypoxic conditions, measurements have not been performed in septal cartilage. The objective of this study was to determine the ppO2 of septal cartilage in vivo.
Study Design
Prospective, basic science.
Methods
The ppO2 was measured in 14 patients (mean ± standard deviation age, 35.9 ± 14.5 years; range, 18–63 years) during routine septoplasty or septorhinoplasty using the OxyLab pO2 monitor (Oxford Optronix Ltd., Oxford, UK). Measurements were taken from the septum and inferior turbinate. Each patient’s age and sex were recorded.
Results
The average ppO2 measured at the septum and inferior turbinate was 10.5 ± 10.1 mm Hg (1.4 ± 1.3%) and 27.6 ± 12.4 mm Hg (3.6 ± 1.6%), respectively. The ppO2 of these locations was significantly different (P < .005). Advancing age was positively correlated with septal ppO2 (R2 = 0.42; P < .05). Septal ppO2 showed no significant sex variation.
Conclusions
This is the first report of in vivo measurement of ppO2 in septal cartilage. The data demonstrate reduced oxygenation of septal cartilage relative to the inferior turbinate. This elucidates an important characteristic of the in vivo milieu that can be applied to septal cartilage tissue engineering.
Keywords: Cartilage, tissue engineering, nasal septum, oxygen content, in vivo
INTRODUCTION
Cartilaginous defects of the head and neck can result from congenital anomalies, trauma, or surgical resection. The repair of these defects poses a complicated reconstructive dilemma for head and neck surgeons, as ideal cartilage grafting material must be obtained to rebuild the deformity. Autologous, allogenic, and synthetic implants and grafts have been used for cartilage reconstruction. Due to the risk of immune rejection and disease transmission, allogenic grafts are not commonly used. The use of synthetic grafts may be complicated by infection, extrusion, and foreign body reactions.1-4 Autologous cartilage continues to be the preferred grafting material for reconstruction of nasal defects with common donor sites including the nasal septum, auricle, and rib. Of these, nasal septal cartilage is favored due to its ease of harvest, ideal structural properties, and minimal donor-site morbidity.5 Nasal septal cartilage is firm and nonmalleable, allowing it to resist deformity during the healing process. Conversely, auricular cartilage is more elastic and curvilinear in shape. Attainment of costal cartilage involves significant patient morbidity, and costal cartilage grafts may undergo unpredictable absorption and warping during healing. Although nasal septal cartilage possesses favorable properties for reconstruction, its use is limited by the finite amount of available tissue. Tissue engineering of autologous neocartilage may offer the potential to produce adequate quantities of autologous cartilage from a small donor specimen to create grafts in defined shapes and sizes.
Cartilage tissue engineering involves several keys steps. A cartilage sample is first digested to isolate the chondrocytes from the extracellular matrix (ECM). These cells are then proliferated in monolayer culture to increase cell number for the production of adequate quantities of tissue. During monolayer expansion, the chondrocytes undergo a shift toward a fibroblastic phenotype in a process called dedifferentiation. This is accompanied by a change from the production of type II collagen (characteristic of native chondrocytes) to type I collagen (characteristic of fibroblasts).6,7 If a dedifferentiated chondrocyte regains its native phenotype, it is referred to as redifferentiated. To produce clinically useful neocartilage, chondrocytes must redifferentiate and produce ECM after monolayer culture. Multiple factors influence chondrocyte redifferentiation, including media composition, cell seeding density, three-dimensional (3D) scaffold properties, and culture environment.8-10 In previous investigations in our laboratory, media composition, cell seeding density, and 3D scaffolds have been optimized for the culture of human nasal septal chondrocytes.11-13
Identifying the ideal culture environment to promote chondrogenesis may lead to the production of neocartilage that more closely resembles native tissue. Oxygen tension is a key characteristic of the culture environment and has been shown to influence development, including the formation of cartilage.14-16 The mechanism by which this occurs has not been completely elucidated. Nasal septal cartilage is avascular and receives its blood supply from the adjacent perichondrium. Similarly, articular cartilage obtains its oxygen supply from surrounding synovial fluid. Measurements of oxygen tension in healthy articular cartilage vary from 6% at the joint surface to 1% in the deep layers near the bony-cartilaginous junction.17 Chondrocytes are well adapted to this hypoxic environment and adjust their metabolism accordingly.18 Multiple studies using articular chondrocytes have shown that low oxygen tension promotes improved functional ECM production over standard 21% oxygen and anoxic conditions.19-21 Additionally, a hypoxic environment has been shown to promote redifferentiation of dedifferentiated bovine articular chondrocytes.22
There are limited data addressing in vitro growth of human nasal septal chondrocytes in a hypoxic milieu. One study by Malda and colleagues showed that septal chondrocytes cultured at 1% and 5% oxygen exhibited significantly increased expression of type II collagen and glycosaminoglycan content compared with those cultured at 21% oxygen. This study supports the tendency that low oxygen tension may stimulate the redifferentiation of dedifferentiated adult human nasal septal chondrocytes.23 The oxygen tension used for the culture environment in this study is based on measurements obtained in articular cartilage. The in vivo oxygen tension in human septal cartilage has not been previously reported. To most effectively mimic the native environment of septal cartilage, the in vivo oxygen tension must be determined.
Recently, a system used to measure the partial pressure of oxygen (ppO2) became commercially available. This device, the OxyLab pO2 monitor (Oxford Optronix Ltd., Oxford, UK), measures ppO2 using a fluorescence quenching technique. Short pulses of light-emitting diode light are transmitted along the fiber optic sensor to excite a platinum-based fluorophore situated at the sensor tip. The fluorophore is permanently immobilized and enclosed within a silicone matrix. The resulting emission of fluorescent light, quenched by the presence of oxygen molecules, travels back up the fiber and is detected by the instrument. The lifetime of fluorescence is inversely proportional to the concentration of dissolved oxygen and is interpreted to provide an absolute value for ppO2 in mmHg. The use of this device for determination of ppO2 in living tissue is well supported in the literature.24,25
The objective of this study was to measure the oxygen tension of septal cartilage in vivo using the OxyLab pO2 monitor. It is anticipated that this information can be used to optimize the culture environment of human septal neocartilage, thereby producing grafts that are comparable to native tissue.
MATERIALS AND METHODS
Patient Selection
Fourteen patients scheduled to undergo routine elective septoplasty or septorhinoplasty at the University of California, San Diego Medical Center Outpatient Surgery Center were recruited for the study. Institutional review board approval was obtained, and each patient signed a consent form agreeing to participate in the study. Patients known to be infected with human immunodeficiency virus, hepatitis B, hepatitis C, cyto-megalovirus, or syphilis were excluded. Any patient with a preexisting condition potentially affecting the integrity of the nasal septum, such as septal perforation, history of intranasal cocaine abuse, Wegener’s granulomatosis, midline granuloma, or lupus were also excluded. There were no limitations to inclusion based on age, gender, or ethnicity.
Measurement of ppO2
The surgical procedure was performed in the typical fashion with induction of anesthesia and intubation. The patient’s septal mucosa and skin soft-tissue envelope (for open nasal approach) were injected with 7 to 10 mL of 1% lidocaine with epinephrine 1:100,000. The patient was then prepped and draped in the usual sterile fashion. Standard intranasal or extranasal incisions were performed based on the surgical approach for the patient’s condition. A mucoperichondrial flap was raised, exposing the septal cartilage. At this time, tissue oxygenation was determined using the OxyLab pO2 monitor. A sterile measurement needle probe was inserted into the wider inferior portion of the septal cartilage adjacent to the maxillary crest that was intended to be excised (Fig. 1). The needle probe was attached to the OxyLab monitor for data acquisition. The measurement was allowed to stabilize and then recorded. Following that, a measurement was also taken from the inferior turbinate as a control. Once the data was acquired, the probe was removed and the surgical procedure continued as planned. Each patient’s age and sex were recorded along with the ppO2 measurements.
Fig. 1.
OxyLab needle probe inserted into the inferior portion of the septal cartilage adjacent to the maxillary crest.
Statistical Analysis
Analysis was performed using Systat 10.2 (Systat Software, Chicago, IL). Means are presented ± the standard deviation. A paired-samples t test was used to compare the measured ppO2 of the septum and inferior turbinate. Linear regression analysis was used to analyze the relationship between ppO2 and age. An analysis of variance test was used to determine the effect of age and sex on ppO2. A difference was considered significant when P ≤ .05.
RESULTS
The ppO2 was measured in 14 patients with a mean age of 35.9 ± 14.5 years (range, 18–63 years). There were seven males and seven females in this group of patients. The average ppO2 measured at the septum was 10.5 ± 10.1 mm Hg (1.4 ± 1.3%) in all 14 patients. The measured ppO2 at the inferior turbinate was 27.6 ± 12.4 mm Hg (3.6 ± 1.6%). The oxygen concentration at these locations was significantly different (P .002).
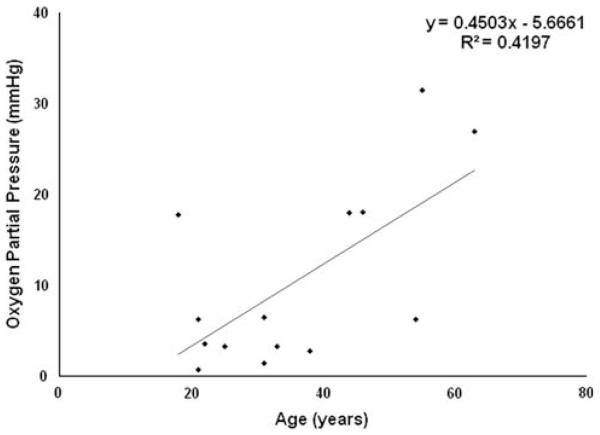
The measured oxygen concentration significantly varied with age, with an average septal ppO2 of 5.1 ± 5.1 mm Hg (0.7 ± 0.7%) in patients younger than 40 years and 20.2 ± 9.7 mm Hg (2.6 ± 1.2%) in patients older than 40 years (P = .006). The positive correlation between advancing age and septal ppO2 was statistically significant (Fig. 2; R2 = 0.42; P < .05). The oxygen tension did not vary significantly with the sex of the patient (P = .28).
Fig. 2.
Relationship of septal partial pressure of oxygen (ppO2) and patient age. The regression line and corresponding R2 value is shown. There was a significant correlation between advancing age and septal ppO (R2 = 0.42; P < .05).
DISCUSSION
In this study, we measured the in vivo oxygen level in the nasal septum of 14 patients. Measurements were only taken from the inferior strip of septum (just superior to the maxillary crest) to ensure complete entry of the probe tip into this area of thicker tissue. This circumvented the problem of the probe tip piercing through to the other external side of the septal cartilage. Additionally, cartilage from this anatomic location is used for expansion during cartilage tissue engineering, and therefore measuring the oxygen level in this region is most relevant for the application of this information to the in vitro culture environment. We found that the oxygen concentration in human septal cartilage is relatively hypoxic when compared with the oxygen concentration in the inferior turbinate and ambient oxygen levels. As patient age increases, the measured oxygen level also increases. However, there is no correlation between oxygen level and sex. This is the first report of in vivo ppO2 measurements in human septal cartilage.
The ppO2 of the septum and inferior turbinate were significantly different, which is a predictable finding as nasal cartilage receives its oxygen supply through diffusion from the adjacent perichondrium. Conversely, the inferior turbinate is supplied by arterial arcades derived from a descending branch of the sphenopalatine artery.26 To determine whether certain patient characteristics affect oxygen concentration in the nasal septum, this study investigated possible age- or sex-related variance in oxygen level. The septal oxygen level in males and females was comparable. In contrast, both regression analysis and analysis of variance showed a significant increase in septal ppO2 with advancing age. This age-related increase in oxygen level is supported by the decreased cellularity found in human septal cartilage with increasing age. Homicz and colleagues analyzed the biochemical constitution of inferior septal cartilage obtained from 33 patients and found that as patient age increases, cellularity decreases by 7.4% per decade.27 This relationship between age and cellularity has also been demonstrated in human articular cartilage.28 More-over, studies have shown that articular chondrocytes display an age-related decline in proliferation and synthetic capacity.29 Therefore, increased measured ppO2 in older patients may be due to reductions in chondrocyte cell number, proliferation, and metabolism with the advancement of age.
CONCLUSION
Multiple studies have demonstrated the benefits of culturing articular chondrocytes in a hypoxic environment. Hypoxia supports redifferentiation of dedifferentiated cells and the production of functional ECM. Given that the in vivo ppO2 in septal cartilage is also relatively hypoxic, the application of this property to the culture of human septal chondrocytes will better mimic the native septal environment. In turn, this may result in the production of clinically useful neocartilage constructs.
Acknowledgments
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (RR&D Merit Review Award; d.w.) and NIHR01 AR044058 (r.l.s.).
Footnotes
Presented at the Combined Otolaryngology Spring Meetings, San Diego, California, U.S.A., April 18–22, 2012.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Tardy ME, Jr, Denneny J, III, Fritsch MH. The versatile cartilage autograft in reconstruction of the nose and face. Laryngoscope. 1985;95:523–533. doi: 10.1288/00005537-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Komender J, Marczynski W, Tylman D, Malczewska H, Komender A, Sla-dowski D. Preserved tissue allografts in reconstructive surgery. Cell Tissue Bank. 2001;2:103–112. doi: 10.1023/A:1014333022132. [DOI] [PubMed] [Google Scholar]
- 3.Collawn SS, Fix RJ, Moore JR, Vasconez LO. Nasal cartilage grafts: more than a decade of experience. Plast Reconstr Surg. 1997;100:1547–1552. doi: 10.1097/00006534-199711000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Romo T, III, McLaughlin LA, Levine JM, Sclafani AP. Nasal implants: autogenous, semisynthetic, and synthetic. Facial Plast Surg Clin North Am. 2002;10:155–166. doi: 10.1016/s1064-7406(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 5.Sajjadian A, Rubinstein R, Naghshineh N. Current status of grafts and implants in rhinoplasty: part I. Autologous grafts. Plast Reconstr Surg. 2010;125:40e–49e. doi: 10.1097/PRS.0b013e3181c82f12. [DOI] [PubMed] [Google Scholar]
- 6.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesized as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 7.Homicz MR, Schumacher BL, Sah RL, Watson D. Effects of serial expansion of septal chondrocytes on tissue-engineered neocartilage composition. Otolaryngol Head Neck Surg. 2002;127:398–408. doi: 10.1067/mhn.2002.129730. [DOI] [PubMed] [Google Scholar]
- 8.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 9.Homicz MR, Chia SH, Schumacher BL, et al. Human septal chondrocyte redifferentiation in alginate, polyglycolic acid scaffold, and monolayer culture. Laryngoscope. 2003;113:25–32. doi: 10.1097/00005537-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chia SH, Homicz MR, Schumacher BL, et al. Characterization of human nasal septal chondrocytes cultured in alginate. J Am Coll Surg. 2005;200:691–704. doi: 10.1016/j.jamcollsurg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Chia SH, Schumacher BL, Klein TJ, et al. Tissue-engineered human nasal septal cartilage using the alginate-recovered-chondrocyte method. Laryngoscope. 2004;114:38–45. doi: 10.1097/00005537-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Alexander TH, Sage AB, Schumacher BL, Sah RL, Watson D. Human serum for tissue engineering of human nasal septal cartilage. Otolaryngol Head Neck Surg. 2006;135:397–403. doi: 10.1016/j.otohns.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Alexander TH, Sage AB, Chen AC, et al. IGF-1 and GDF-5-promote the formation of tissue-engineered human nasal septal cartilage. Tissue Eng Part C Methods. 2010;16:1213–1221. doi: 10.1089/ten.tec.2009.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malda J, Martens DE, Riesle J, Tramper J, van Blitterswijk CA. Cartilage tissue engineering: controversy in the effect of oxygen. Crit Rev Biotech. 2003;23:175–194. [PubMed] [Google Scholar]
- 15.Bassett CAL, Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. 1961;190:460–461. doi: 10.1038/190460a0. [DOI] [PubMed] [Google Scholar]
- 16.Pawelek JM. Effects of thyroxine and low oxygen tension on chondrogenic expression in cell culture. Dev Biol. 1969;19:52–72. doi: 10.1016/0012-1606(69)90070-0. [DOI] [PubMed] [Google Scholar]
- 17.Pfander D, Gelse K. Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr Opin Rheumatol. 2007;19:457–462. doi: 10.1097/BOR.0b013e3282ba5693. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CL, Sambanis A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001;7:791–806. doi: 10.1089/107632701753337735. [DOI] [PubMed] [Google Scholar]
- 19.Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10:825–832. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 20.Kurz B, Domm C, Jin M, Sellckau R, Schünke M. Tissue engineering of articular cartilage under the influence of collagen I/III membranes and low oxygen tension. Tissue Eng. 2004;10:1277–1286. doi: 10.1089/ten.2004.10.1796. [DOI] [PubMed] [Google Scholar]
- 21.Foldager CB, Nielsen AB, Munir S, et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011;82:234–240. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domm C, Schunke M, Steinhagen J, Freitag S, Kurz B. Influence of various alginate brands on the redifferentiation of dedifferentiated bovine articular chondrocytes in alginate bead culture under high and low oxygen tension. Tissue Eng. 2004;10:1796–1805. doi: 10.1089/ten.2004.10.1796. [DOI] [PubMed] [Google Scholar]
- 23.Malda J, van Blitterswijk CA, van Geffen A, Martens DE, Tramper J, Riesle J. Low oxygen tension stimulates the redifferentiation of dedifferentiated adult human nasal chondrocytes. Osteoarthritis Cartilage. 2004;12:306–313. doi: 10.1016/j.joca.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001;280:H2533–H2544. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 25.Badger WJ, Whitbeck C, Kogan B, Chichester P, Levin RM. The immediate effect of castration on female rabbit bladder blood flow and tissue oxygenation. Urol Int. 2006;76:264–268. doi: 10.1159/000091631. [DOI] [PubMed] [Google Scholar]
- 26.Padgham N, Vaughan-Jones R. Cadaver studies of the anatomy of arterial supply to the inferior turbinates. J R Soc Med. 1991;84:728–730. doi: 10.1177/014107689108401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homicz MR, McGowan KB, Lottman LM, Beh G, Sah RL, Watson D. A compositional analysis of human nasal septal cartilage. Arch Facial Plast Surg. 2003;5:53–58. doi: 10.1001/archfaci.5.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]