Abstract
Purpose
Little is known about the behavior of the endothelial cell (EC) layer following keratoplasty. In vitro experiments suggested that the peripheral endothelium might have a higher regenerative capacity than the central endothelium, and some authors hypothesized that endothelial progenitor cells are present in the limbal area. Thus, we analyzed the corneal endothelial regenerative capacity in vivo in a rat model of bullous keratopathy using either bullous central grafts or bullous peripheral recipient corneas to analyze differences in EC regeneration depending on central versus peripheral cell origin.
Methods
Bullous keratopathy was induced in Lewis rats with an intracameral injection of benzalkonium chloride (0.05%; BAK). Three days later, the central area of the bullous cornea was excised and used as a bullous graft, transplanted to a healthy, green fluorescent protein (GFP)-transgeneic Lewis receipient rat (group ‘bullous graft’). In return, the mentioned rat eye with the bullous keratopathy received a healthy GFP-transgeneic corneal transplant (group ‘bullous host’). A subgroup of these animals received a healthy, excentrically trephined including parts of the limbus (group ‘bullous host, limbo-keratoplasty’). The grafts were monitored clinically for 7 weeks. Subsequently, the mean EC density was calculated on corneal whole mounts with Alizarin Red S staining. GFP was analyzed with confocal microscopy to determine EC origin. Untreated fellow eyes served as controls.
Results
BAK injection led to a significant decrease in the mean EC density with subsequent bullous keratopathy. In the control eyes, the mean EC density was 3,744 cells/mm2 in the center and 2,811 cells/ mm2 in the periphery. In eyes with bullous keratopathy, only 233 cells/mm2 in the center and 622 cells/mm2 in the periphery were observed three days after BAK-injection. Bullous transplants in the group ‘bullous graft’ cleared, and GFP-positive cells were detected on the transplant. In contrast, no GFP-positive ECs were detected on the host cornea in the groups ‘bullous host’.
Conclusions
ECs from the peripheral cornea have the ability to cross the graft border and compensate for the pathologically altered/absent endothelium on the graft. In contrast, ECs derived from the central cornea remain central on the graft and do not replace or regenerate peripheral ECs in our model of bullous keratopathy.
Introduction
The corneal endothelial cell (EC) layer is crucial for corneal clarity: If the corneal EC density falls below a critical threshold, the barrier and pump function of the endothelium cannot be maintained, and the cornea becomes edematous and presents as bullous keratopathy. This is a major indication of orthotopically placed penetrating keratoplasty, or posterior lamellar keratoplasty [1,2]. However, even after corneal transplantation, which has a good prognosis for most low-risk indications [3,4], corneal decompensation due to chronic EC loss can occur and might lead to transplant failure [5]. Even though the EC layer is important for corneal clarity and thus visual acuity, little is known about the endothelial reactions following corneal transplantation.
In rodents and humans, some degree of reparative mechanisms are present in the EC layer. Even though human EC rarely divide physiologically, they have the ability to migrate and enlarge to compensate for smaller defects in the EC layer [6]. Ex vivo experiments suggest that the location of ECs might play a role in their regenerative capacity: Different groups have demonstrated that ECs obtained from the corneal periphery compared to the center have a higher regenerative capacity in vitro [7-9], and the presence of endothelial precursor cells in the corneal periphery or limbal area has been proposed. These data obtained in vitro support the hypothesis that the peripheral and central endothelium might differ in their regenerative capacity.
To further clarify this issue and its relevance in vivo, we analyzed central and peripheral endothelial regeneration following keratoplasty in a rat corneal transplant model. EC density was reduced before keratoplasty by treatment of either the corneal graft or the host with benzalkonium chloride (BAK), leading to bullous keratopathy. Subsequently, either a BAK-damaged corneal button was grafted to a healthy recipient rat or an untreated transplant was transplanted into a BAK-damaged recipient cornea. We also evaluated EC regeneration using untreated limbocorneal grafts in BAK-treated recipients to assess the possible role of limbal ECs in repopulating the damaged peripheral recipient endothelium. Transplantations were performed between green fluorescent protein (GFP) transgenic and wild-type rats to monitor EC origin aside from cell density and morphology. An immune response was excluded by syngeneic grafting.
Methods
Animals
All transplantations were performed in a syngeneic setting to avoid immune reactions using wild-type Lewis rats or GFP-transgenic Lewis rats (strain kindly provided by Professor Eiji Kobayashi, Japan [10]). Wild-type rats (>8 weeks old) were obtained from Charles River (Sulzfeld, Germany). All animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments were approved by the IACUC of the University of Freiburg, Germany.
Bullous keratopathy
To induce bullous keratopathy, BAK 0.05% was injected into the anterior chamber of rat eyes. Inbred Lewis rats were anaesthetized with inhalative isoflurane 4%. The anterior chamber of one eye was punctured using a fine glass needle and rinsed with BAK for 90 s, followed by rinsing with 0.9% sodium chloride for another 90 s. The corneal puncture was sealed with a small air bubble. Antibiotic ointment was applied, and a tarsorrhaphy was left in place for 3 days. The fellow eye was left untreated and served as the control. After 3 days, development of bullous keratopathy was confirmed using an operating microscope. Five animals were sacrificed at this time point to prepare flatmount preparations of treated and untreated corneas to evaluate mean EC density with Alizarin Red S stains. The other treated animals were used for the following keratoplasty experiments.
Groups
BAK-damaged bulbi received an untreated donor cornea from a GFP-transgenic Lewis rat (3.5 mm graft diameter, group ‘bullous host’), while the excised central part of the BAK-damaged cornea (3 mm) was in return used as a transplant with reduced EC density for a healthy GFP-transgenic host (excision 2.5 mm; group ‘bullous graft’).
Transplants were excised centrally and placed orthotopically to the central area of the recipient corneas in the usual fashion. However, since the effect of location of EC origin was to be analyzed, we also performed transplantations in the group ‘bullous host’ where we used excentrically trephined grafts including parts of the limbus as performed in penetrating central limbo-keratoplasty (group ‘bullous host, limbo-keratoplasty’) [11].
Corneal transplantations
Orthotopic corneal transplantations were performed as previously described [12]. Animals were anaesthetized using isoflurane inhalation followed by ketamine hydrochloride 100 mg/kg and atropine and xylazine 0.5 mg/kg intraperitoneally. Mydriatic eye drops were applied. Corneas were trephined using a 2.5, 3.0, or 3.5 mm trephine, respectively, to create an overlap of 0.5 mm between the donor and recipient cornea. All grafts were excised centrally, except the grafts from the group ‘bullous host, limbo-keratoplasty’. In these experiments, the grafts were excised excentrically after the conjunctiva was removed, including a portion of the limbus in one quadrant. The adhering iris root was removed using forceps. Twelve interrupted sutures (11.0 Ethilon; Ethicon, Norderstedt, Germany) were used to fix the allografts including the limbal grafts centrally. Antibiotic ointment was administered, and a tarsorrhaphy was left in place for 3 days. Grafts were repeatedly analyzed for corneal opacity according to an international score: 0: completely transparent corneal graft; 1: slight corneal graft opacity, but details of the iris vessel clearly visible; 2: moderate corneal graft opacity, iris vessels still visible; 3: strong corneal graft opacity, only pupil margin visible; 4: complete corneal graft opacity, pupil not visible. Rejection was defined as complete graft opacity (grade 4). Animals with surgical complications such as intraocular hemorrhage or cataract were excluded from further analysis.
Histological examination
Eyes were enucleated, and the corneas including the limbus were excised. Corneas were divided in half and prepared as corneal flatmounts with the endothelial side facing upward. One half of the corneas was used to stain the EC borders: Alizarin red S was applied for 1.5 min (pH = 4.3), and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Munich, Germany). Cell density was evaluated using a Carl Zeiss (Jena, Germany) microscope and analySIS software (Olympus, Hamburg, Germany): Cells were manually identified, and average cell density was calculated based on three counting frames for the graft and the host cornea, respectively. The other half of the corneas was fixated using methanol-free paraformaldehyde for 20 min at room temperature, washed in PBS (1x; 138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4-H2O, pH 7.0-7.3), and placed on glass slides with DAPI-Moviol to stain cell nuclei. The GFP positivity of the EC layer was analyzed using a confocal microscope and appropriate filters (Carl Zeiss).
Statistical analysis
Graft survival was analyzed using the Kaplan–Meier method. Cell counts were compared between groups using the t test. Opacity scores at different time points were compared using the Kruskal–Wallis test. p<0.05 was regarded as statistically significant.
Results
Induction of bullous keratopathy
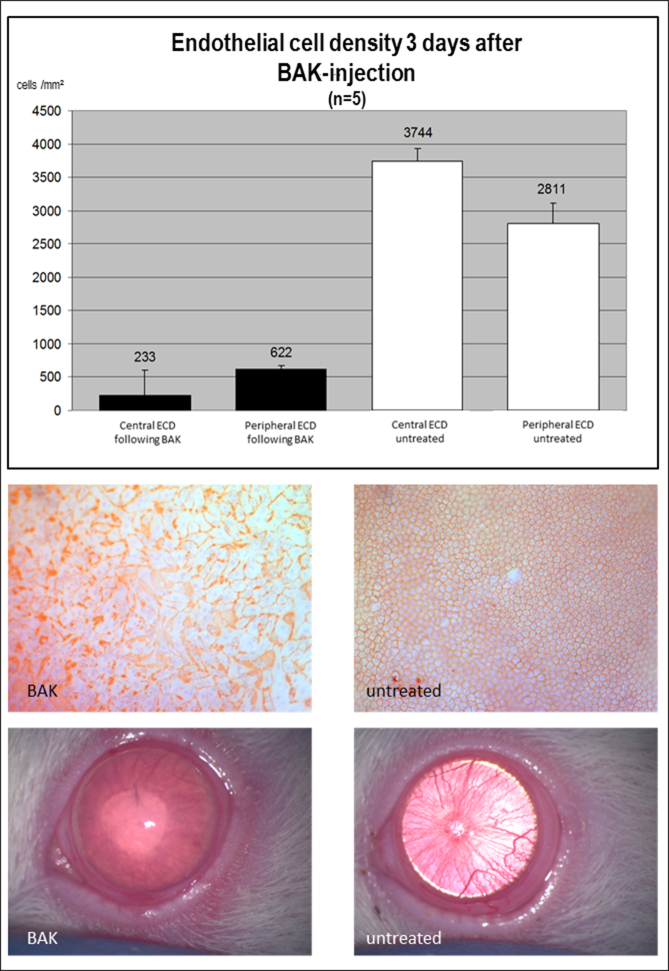
Injection of BAK into the anterior chamber resulted in bullous keratopathy, which was clinically evident after 3 days. Alizarin Red S stainings of corneal flatmount preparations at this time point (n = 5) demonstrated a pronounced decrease in EC density. While the untreated fellow eyes, which served as controls, had a mean EC density of 3,744/mm2 centrally and 2,811/mm2 peripherally, it was reduced following BAK treatment to 233/mm2 centrally and 622/mm2 peripherally, respectively (Figure 1).
Figure 1.
Induction of bullous keratopathy with intracameral benzalkonium chloride injection. A decrease was observed in the endothelial cell density (ECD) in the eyes treated with benzalkonium chloride (BAK) compared to the untreated fellow eyes. Cell counts (top), Alizarin Red S staining (middle), and clinical picture (bottom) are shown.
Perforating keratoplasty in hosts with bullous keratopathy (group ‘bullous host’)
Clinical follow-up
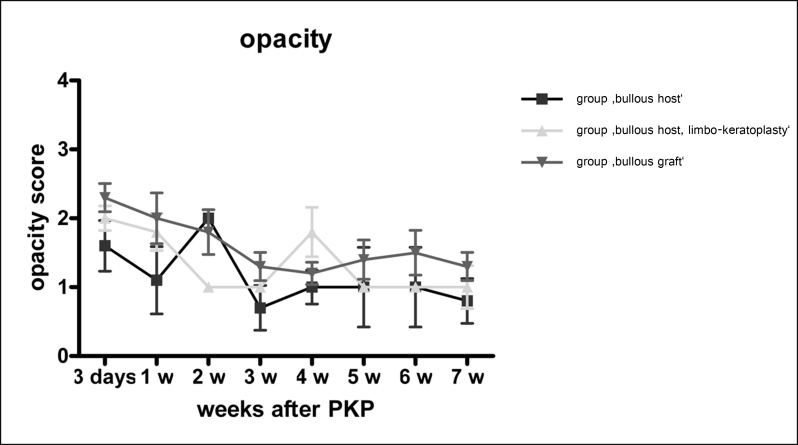
Eyes with bullous keratopathy received a corneal transplant with a healthy endothelium. Clinical follow-up for 7 weeks showed no signs of rejection. The mean initial opacity of 1.6 and 2.0 (group ‘bullous host’ (n=6) and group ‘bullous host, limbo-keratoplasty’ (n=5)), subsequently decreased to a mean of 1.0 and 1.3 for the group ‘bullous host’ and the group ‘bullous host, limbo-keratoplasty’, respectively, at 7 weeks follow-up (Figure 2). The difference between the groups was not statistically significant.
Figure 2.
Opacity: Clinical follow-up of all animals. Animals with benzalkonium chloride (BAK)-damaged corneas were used for (1) donating a corneal graft with a greatly reduced endothelial cell layer, which was transplanted to a healthy recipient (group ‘bullous graft’, n=6), and (2) serving as a recipient with bullous keratopathy that received a healthy graft (group ‘bullous host’, n=6). A subgroup of the bullous host group received a healthy graft including a limbal portion (group ‘bullous host, limbo-keratoplasty’, n=5). Animals were followed clinically for 7 weeks for opacity of the graft. Opacity score: 0: completely transparent corneal graft; 1: slight corneal graft opacity, but details of iris vessel clearly visible; 2: moderate corneal graft opacity, iris vessels still visible; 3: strong corneal graft opacity, only pupil margin visible; 4: complete corneal graft opacity, pupil not visible. Shown: mean±standard deviation.
Endothelial cell origin
Confocal microscopy of the endothelial cell layer revealed an intact EC layer on the grafts in this group. All transplants demonstrated GFP positivity for the ECs. The GFP-positive ECs expanded only into the graft border zone and did not expand onto the host cornea, in neither the group ‘bullous host’ (Figure 3) nor the group ‘bullous host, limbo-keratoplasty’. The host cornea remained covered with the previously BAK-damaged host endothelium.
Figure 3.
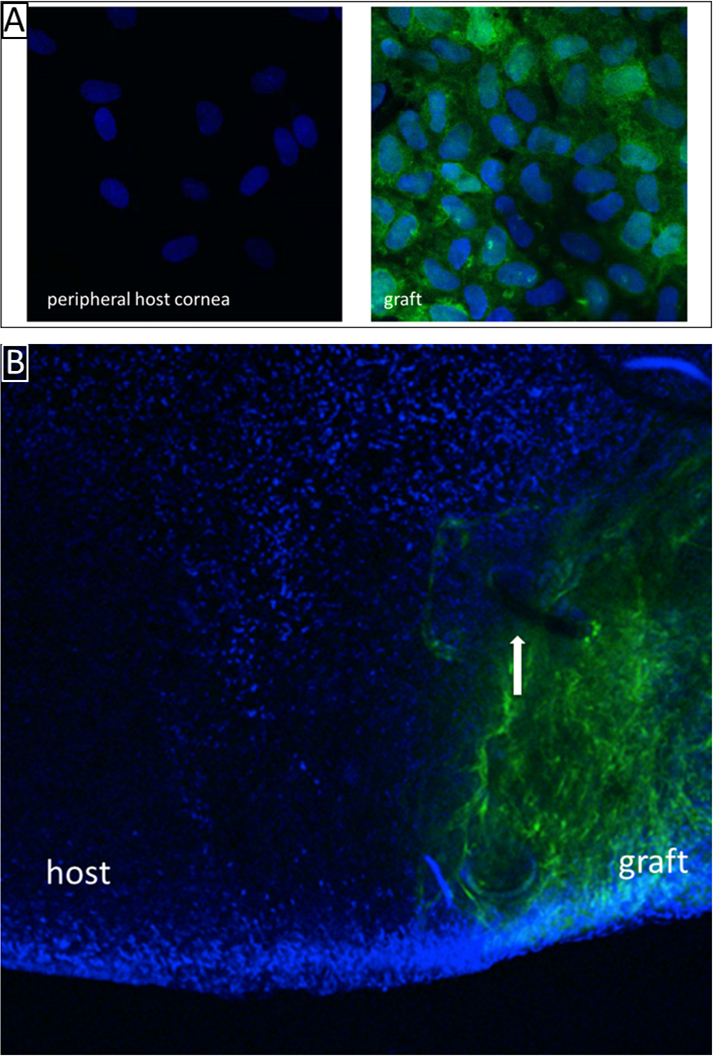
Healthy graft in a recipient cornea with bullous keratopathy (Group ‘bullous host’). The healthy graft endothelium remains on the graft. The peripheral benzalkonium chloride (BAK)-damaged endothelium is not replaced by the green fluorescent protein (GFP)-positive graft endothelium. A: High magnification of the endothelial cell layer of the host and the graft (original magnification: 1200×). B: Overview showing the graft border zone. The white arrow indicates one of the sutures in the host-graft junction (original magnification: 100×).
Bullous grafts transplanted to healthy green fluorescent protein transgenic recipient rats (group ‘bullous graft’)
Clinical follow-up
Bullous grafts initially showed a mean opacity of 2.3 after 3 days, followed by a subsequent decrease to 1.3 at 7 weeks follow-up (n = 6). No graft reached grade 4 opacity, which is defined as corneal rejection (Figure 2).
Endothelial cell origin
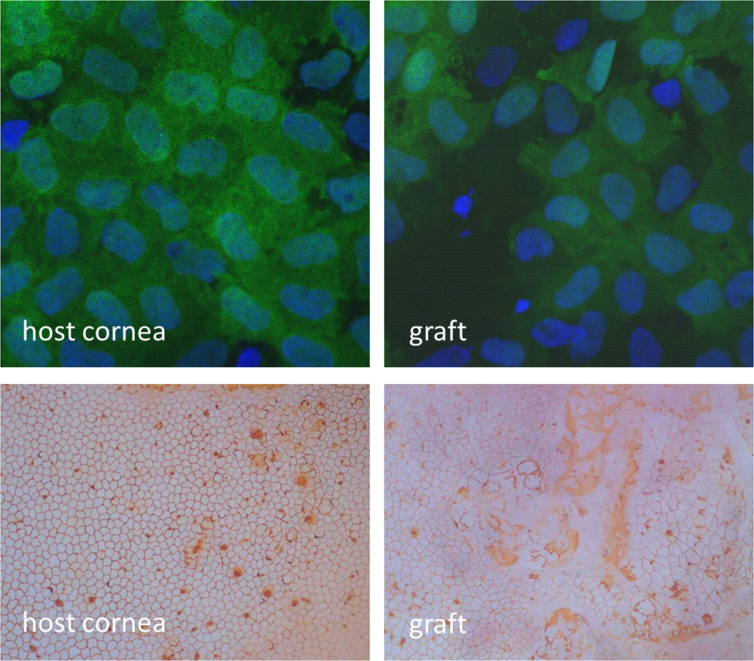
Confocal analysis of the EC layer 7 weeks following keratoplasty revealed a regular GFP-positive EC layer in the untreated host. On the graft, which was derived from a GFP-negative rat, GFP-positive ECs were detected in all animals. Thus, the EC layer on the graft was restored by the host. The regenerated ECs showed increased cell polymorphism and polymegathism (Figure 4). The EC density on the graft was 1,767±477/mm2 and 2,878±381/mm2 on the host cornea.
Figure 4.
Graft with bullous keratopathy transplanted to a healthy recipient (Group ‘bullous graft’). The healthy peripheral host endothelium replaces the central, benzalkonium chloride (BAK)-damaged graft endothelium. Top: Green fluorescent protein/4′,6-diamidino-2-phenylindole (GFP/DAPI) staining (original magnification 1200×). Bottom: Alizarin red S staining (original magnification 100×).
Discussion
Ex vivo experiments have indicated that peripheral ECs might have a higher capacity to proliferate or compensate for endothelial wounds, and have even led to the hypothesis that EC precursor cells are located in the corneal periphery or limbal area: As early as 1998, Bednarz et al. cultured human corneal ECs and detected different characteristics of ECs depending on their position on the human cornea. Cells isolated from the central part of the cornea showed cell enlargement but no cell division to cover defects in the monolayer. In contrast, ECs isolated from the peripheral part of the cornea seemed not as tightly packed, and space was filled due to cell division rather than cell enlargement [13]. Later, Mimura et al. examined healing responses to scrape wounds of whole ex vivo cultured human corneas. The researchers noticed that human cultured ECs from the peripheral area retained higher replication competence compared to central cells, regardless of donor age [9]. The observation of cells with increased regenerative capacity finally led to the hypothesis of endothelial precursors in the posterior limbal area, from where these cells might migrate to the endothelial periphery and, perhaps, to wounded areas of the corneal endothelium when needed [8,14,15].
It was our goal to explore potential differences in the regenerative capacity of the central and peripheral corneal endothelium in vivo. Therefore, we used a syngeneic rat keratoplasty model. Our own recent experiments (data not shown) confirmed that syngeneic transplantation between Lewis rats using GFP-positive donors and GFP-negative littermates did not lead to rejection of the graft, and the host and graft endothelium remained intact. To study corneal endothelial regeneration, EC damage was induced by BAK, resulting in bullous keratopathy in either donors or recipients.
BAK is toxic for ECs [16] and has been used to experimentally induce bullous keratopathy by intracameral injection in rabbits [17-19]. Recently, the bullous keratopathy model has also been adapted for use in mice [20]. Yang et al. produced bullous keratopathy in one eye of a male rabbit, and the graft from a female rabbit was transplanted. Four months later, the detection frequency of the sex chromatin stained with acetic orcein was similar between the cells in the graft and in the bullous host cornea, indicating that the cells in the host had migrated from the graft [18]. In contrast, Nakahori et al. performed exchange keratoplasties between rabbits with damaged graft endothelium and damaged host endothelium. The authors found that an EC layer was apparent on previously damaged grafts after 4 weeks, while the former host endothelium did not recover [19]. These results are similar to our own observations, even though their experiments did not show whether the regenerated cells on the graft were host derived or equaled recovered graft endothelium.
In humans, little is known about the fate of donor or host-derived ECs following transplantation. A distinct marker is needed to distinguish donor and recipient cells. Some studies have used fluorescence in situ hybridization of sex chromosomes on corneal graft explants in a sex-mismatched situation between the original donor and the host to analyze whether the ECs on the graft are graft or host derived. The first study found no donor ECs 1–30 years after the original keratoplasty (in ten out of these 14 cases endothelial failure/rejection was the reason for graft explantation) [21]. In contrast, Lagali et al. demonstrated that 26 out of 35 grafts retained graft ECs up to 3 years after transplantation in varying percentages. Donor cells were reported to be distributed throughout the corneal sections in a seemingly random fashion [22]. Thus, although these experiments showed that graft ECs in humans can either remain intact or be replaced by the host endothelium (reasons for either reaction are not clear thus far), these experiments cannot answer the question whether graft endothelium can repopulate the host cornea just as well [23] since the peripheral host cornea is not accessible for fluorescence in situ hybridization in living humans.
In our set of experiments, GFP expression allowed us to monitor the cell origin of the graft and the host cornea. Transplantation of a BAK-damaged graft into a healthy host resulted in replacement of the damaged graft endothelium by GFP-positive host ECs. Previously, we have shown that when transplanting an EC-free graft (mechanical EC removal), the healthy peripheral rat corneal endothelium is capable of repopulating this transplant. When performing allogeneic rat keratoplasties, the immune reaction leads to EC loss of the graft, and then is likewise followed by a host-derived EC repopulation [12,24]. All these results consistently demonstrate that the peripheral rat endothelium has the potency to recover the EC layer of a graft devoid of or with a strongly damaged endothelium.
Although proliferation was not specifically assessed again in the current study, immunohistochemical staining in our previous study clearly showed that EC regeneration in the rat keratoplasty model is based on not only cell migration but also EC proliferation [12].
Interestingly, when the exchange keratoplasty was performed (transplanting a healthy graft into a host with a damaged endothelium, which equals the common clinical implication of keratoplasty for bullous keratopathy), the graft endothelium did not replace the peripheral host ECs in our rat model. This might be due to the higher regenerative capacity of peripheral Ecs, and might support the hypothesis of peripheral endothelial precursor cells. However, peripherally trephined transplants including a limbal portion did not change this result. This observation does not necessarily refute the hypothesis of the increased peripheral regenerative capacity of ECs. There are several possibilities why these transplants did not show increased EC regeneration. First, the transplants were placed centrally due to technical reasons in rat keratoplasty, and whether limbal areas transplanted to a different area of the cornea function unchanged is unknown. We observed that the epithelium (data not shown) of the graft was rapidly replaced by the host epithelium, directed from the periphery to the center, just as in grafts without a limbal portion. This failure to preserve the epithelial regenerative capacity of the transplanted limbal area in this rat keratoplasty model might also be true for the endothelial regenerative capacity of the peripheral endothelium. Second, EC regeneration might be directional, and the regeneration of transplanted limbal area might still be directed toward the center and thus not extend onto the host cornea. This hypothesis is emphasized by the work of He et al., which suggested a continuous slow centripetal migration of ECs throughout life [25].
Taken together, our in vivo experiments in the rat indicate that EC repopulation of damaged corneal grafts occurs by the host, while the repopulation of damaged peripheral ECs by a graft is not as easily accomplished. This further supports the hypothesis hat there are differences between central and peripheral corneal ECs in vivo.
Acknowledgments
Funding support for this study was provided by Ernst-und-Berta-Grimmke-Foundation, Düsseldorf, Germany. Part of this study was presented at the annual meeting of the German Ophthalmology Society, Berlin 2012 and acknowledged with a poster award. We thank Prof. Eiji Kobayashi, Center for Development of Advanced Medical Technology, Jichi Medical University, Tochigi, Japan for providing the strain of GFP+ Lewis rats for our experiments.
References
- 1.Wang J, Hasenfus A, Schirra F, Bohle RM, Seitz B, Szentmáry N. Changing indications for penetrating keratoplasty in Homburg/Saar from 2001 to 2010–histopathology of 1,200 corneal buttons. Graefes Arch Clin Exp Ophthalmol. 2013;251:797–802. doi: 10.1007/s00417-012-2117-2. [DOI] [PubMed] [Google Scholar]
- 2.Keenan TDL, Jones MNA, Rushton S, Carley FM. Trends in the indications for corneal graft surgery in the United Kingdom: 1999 through 2009. Arch Ophthalmol. 2012;130:621–8. doi: 10.1001/archophthalmol.2011.2585. [DOI] [PubMed] [Google Scholar]
- 3.Reinhard T, Böhringer D, Enczmann J, Kögler G. S Mayweg S, Wernet P, Sundmacher R. Improvement of graft prognosis in penetrating normal-risk keratoplasty by HLA class I and II matching. Eye (Lond) 2004;18:269–77. doi: 10.1038/sj.eye.6700636. [DOI] [PubMed] [Google Scholar]
- 4.Reimer A, Langenbucher A, Cursiefen C. Long-term outcome after penetrating keratoplasty for bullous keratopathy - influence of preoperative visual acuity on final outcome. Klin Monatsbl Augenheilkd. 2012;229:149–57. doi: 10.1055/s-0031-1281760. [DOI] [PubMed] [Google Scholar]
- 5.Claerhout I, Beele H, Kestelyn P. Graft failure: I. Endothelial cell loss. Int Ophthalmol. 2008;28:165–73. doi: 10.1007/s10792-007-9087-0. [DOI] [PubMed] [Google Scholar]
- 6.Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81:629–38. doi: 10.1016/j.exer.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Engelmann K, Bednarz J, Böhnke M. Endothelial cell transplantation and growth behavior of the human corneal endothelium. Ophthalmologe. 1999;96:555–62. doi: 10.1007/s003470050452. [DOI] [PubMed] [Google Scholar]
- 8.Yamagami S, Yokoo S, Mimura T, Takato T, Araie M, Amano S. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology. 2007;114:433–9. doi: 10.1016/j.ophtha.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47:1387–96. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H, Ohsawa I, Murakami T, Kimura A, Hakamata Y, Sato Y, Kaneko T, Takahashi M, Okada T, Ozawa K, Francis J, Leone P, Kobayashi E. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun. 2005;329:288–95. doi: 10.1016/j.bbrc.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard T, Sundmacher R, Spelsberg H, Althaus C. Homologous penetrating central limbo-keratoplasty (HPCLK) in bilateral limbal stem cell insufficiency. Acta Ophthalmol Scand. 1999;77:663–7. doi: 10.1034/j.1600-0420.1999.770611.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwartzkopff J, Bredow L, Mahlenbrey S, Boehringer D, Reinhard T. Regeneration of corneal endothelium following complete endothelial cell loss in rat keratoplasty. 2010. Available at: http://www.molvis.org/molvis/v16/a254/ Accessed February 26, 2013. [PMC free article] [PubMed]
- 13.Bednarz J, Rodokanaki-von Schrenck A, Engelmann K. Different characteristics of endothelial cells from central and peripheral human cornea in primary culture and after subculture. In Vitro Cell Dev Biol Anim. 1998;34:149–53. doi: 10.1007/s11626-998-0097-7. [DOI] [PubMed] [Google Scholar]
- 14.Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–24. [PubMed] [Google Scholar]
- 15.McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13:1984–2000. [PubMed] [Google Scholar]
- 16.Hull DS. Effects of epinephrine, benzalkonium chloride, and intraocular miotics on corneal endothelium. South Med J. 1979;72:1380–1. doi: 10.1097/00007611-197911000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Maurice D, Perlman M. Permanent destruction of the corneal endothelium in rabbits. Invest Ophthalmol Vis Sci. 1977;16:646–9. [PubMed] [Google Scholar]
- 18.Yang HJ, Sato T, Matsubara M, Tanishima T. Endothelial wound-healing in penetrating corneal graft for experimental bullous keratopathy in rabbit. Jpn J Ophthalmol. 1985;29:378–93. [PubMed] [Google Scholar]
- 19.Nakahori Y, Katakami C, Yamamoto M. Corneal endothelial cell proliferation and migration after penetrating keratoplasty in rabbits. Jpn J Ophthalmol. 1996;40:271–8. [PubMed] [Google Scholar]
- 20.Hayashi T, Yamagami S, Tanaka K, Yokoo S, Usui T, Amano S, Mizuki N. A mouse model of allogeneic corneal endothelial cell transplantation. Cornea. 2008;27:699–705. doi: 10.1097/QAI.0b013e31815e722b. [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–6. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 22.Lagali N, Stenevi U, Claesson M, Fagerholm P, Hanson C, Weijdegård B, Strömbeck A. Donor and Recipient Endothelial Cell Population of the Transplanted Human Cornea: A Two-Dimensional Imaging Study. IOVS. 2010;51:1898–904. doi: 10.1167/iovs.09-4066. [DOI] [PubMed] [Google Scholar]
- 23.Böhringer D, Böhringer S, Poxleitner K, Birnbaum F, Schwartzkopff J, Maier P, Sundmacher R, Reinhard T. Long-term graft survival in penetrating keratoplasty: the biexponential model of chronic endothelial cell loss revisited. Cornea. 2010;29:1113–7. doi: 10.1097/ICO.0b013e3181d21d07. [DOI] [PubMed] [Google Scholar]
- 24.Bredow S. Reinhard. Host-derived endothelial regeneration of corneal transplants in the rat keratoplasty model. Ophthalmic Res. doi: 10.1159/000360739. In press. [DOI] [PubMed] [Google Scholar]
- 25.He Z, Campolmi N, Gain P, Ha Thi B, Dumollard S, Peoc'h M, Piselli S, Garraud O, Thuret G. Revisited microanatomy of the corneal endothelial periphery: new evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells. 2012;30:2523–34. doi: 10.1002/stem.1212. [DOI] [PubMed] [Google Scholar]