Abstract
The actin cytoskeleton is very dynamic and highly regulated by multiple associated proteins in vivo. Understanding how this system of proteins functions in the processes of actin network assembly and disassembly requires methods to dissect the mechanisms of activity of individual factors and of multiple factors acting in concert. The advent of single-filament and single-molecule fluorescence imaging methods has provided a powerful new approach to discovering actin-regulatory activities and obtaining direct, quantitative insights into the pathways of molecular interactions that regulate actin network architecture and dynamics. Here we describe techniques for acquisition and analysis of single-molecule data, applied to the novel challenges of studying the filament assembly and disassembly activities of actin-associated proteins in vitro. We discuss the advantages of single-molecule analysis in directly visualizing the order of molecular events, measuring the kinetic rates of filament binding and dissociation, and studying the coordination among multiple factors. The methods described here complement traditional biochemical approaches in elucidating actin-regulatory mechanisms in reconstituted filamentous networks.
1. INTRODUCTION
The actin cytoskeleton plays fundamental roles in a diverse set of cellular processes, rapidly responding to cellular or environmental cues, including directed cell migration, cell and tissue morphogenesis, endocytosis, cell division, and pathogen infection (Haglund & Welch, 2011; Pollard & Cooper, 2009; Ridley, 2011). Spatial and temporal control of these functions is achieved by an arsenal of actin-associated proteins, which regulate the nucleation, elongation, cross-linking, and disassembly of filaments (Siripala & Welch, 2007). For example, at the sites of endocytosis in yeast and mammals, more than 50 different proteins function to regulate the rapid assembly and turnover of a distinctive, architecturally complex actin network and drive endocytic vesicle internalization within 15–20 s (Boettner, Friesen, Andrews, & Lemmon, 2011; Weinberg & Drubin, 2012). New actin-regulatory factors are being discovered at an accelerated pace (Firat-Karalar & Welch, 2011), and it is now clear that a single animal cell can express hundreds of different proteins that directly bind actin. While conventional biochemical studies provide excellent tools for identifying interactions among actin-associated proteins, quantifying their equilibrium thermodynamic properties, and determining the efficacy of their control over actin dynamics in ensemble reactions, the question facing many researchers is: How do I better understand the activity and mechanism of my protein?
Direct observation of single-filament dynamics in real time (Amann & Pollard, 2001; Yanagida, Nakase, Nishiyama, & Oosawa, 1984) (see also the preceding chapters of this volume), using methods such as total internal reflection fluorescence (TIRF) microscopy, has led to major advances in our understanding of the regulation of actin filament nucleation, network architecture, and disassembly. Bulk assays (e.g., pyrene–actin) measure polymer mass and are not well suited to detecting changes in the interconnection of filaments that occurs during network remodeling. However, direct observation of the individual filaments, for example, within dendritic networks formed by Arp2/3 complex, has led to important discoveries about how branches are initially assembled and subsequently pruned or debranched (Blanchoin, Pollard, & Mullins, 2000; Cai, Makhov, Schafer, & Bear, 2008; Chan, Beltzner, & Pollard, 2009; Gandhi et al., 2010; Martin, Welch, & Drubin, 2006). Yet even with this advance, we are left to infer the molecular mechanisms, indirectly, from observations of filament dynamics. Similarly, mathematical modeling can provide valuable insights into whether a hypothesized mechanism can explain macroscopic behavior (Beltzner & Pollard, 2008; Mogilner, Wollman, & Marshall, 2006; Schaus, Taylor, & Borisy, 2007). However, the available data are often insufficient to accurately constrain all of the unknown and interdependent parameters in such models. What is often lacking is a means to directly validate the under-lying mechanistic assumptions and kinetic pathways of molecular interactions.
Single-molecule imaging has the exciting potential to circumvent the above limitations and advance our understanding of actin-regulatory mechanisms (Breitsprecher et al., 2012; Fujiwara, Remmert, & Hammer, 2010; Hansen & Mullins, 2010; Mizuno et al., 2011; Smith, Daugherty-Clarke, Goode, & Gelles, 2013; Smith, Padrick, et al., 2013). By monitoring the molecular sequence of events in filament formation or disassembly in real time, and quantifying the rate constants for each step, these studies can elucidate the precise functional mechanisms of actin-regulatory proteins. Dual observation of individual molecules and filaments provides a means to (1) measure filament binding and dissociation kinetics, (2) define the order of steps and the intermediates in a mechanism, (3) identify alternate pathways, and (4) detect rare events that may be critically important for function among a majority that are not. Furthermore, the roles of multiple factors in jointly regulating actin dynamics can be examined by either testing the effects of a secondary (unlabeled) factor on the behavior of the primary (labeled) factor or labeling both proteins and performing multicolor analysis. Recently reported examples that we highlight in this chapter include the coordination of Arp2/3 complex and its activator (the VCA domain of WASp family proteins) in actin filament branch formation (Smith, Daugherty-Clarke, Goode, & Gelles, 2013; Smith, Padrick, et al., 2013), the dual activity of the formin mDia1 and adenomatous polyposis coli (APC) in actin filament nucleation and elongation (Breitsprecher et al., 2012), and the cooperative severing and disassembly of filaments by cofilin and Srv2/cyclase-associated protein (Srv2/CAP) (Chaudhry et al., 2013). These studies have helped define critical pieces of the puzzle of cytoskeletal dynamics, but more importantly, they show that this approach can help researchers define the mechanisms of numerous other actin regulators.
The methods described here are intended for researchers who wish to perform single-molecule imaging and analysis of actin-associated proteins, but could be applied more generally to study any macromolecule-polymer interactions in the case where the polymer is elongated on optical length scales (filamentous polymers).
2. PROTEIN TAGS, LABELING, AND TETHERING
The goal of protein labeling for single-molecule fluorescence imaging is to achieve high efficiency, covalent attachment of a fluorophore with good photophysical properties onto the protein of interest without disrupting its function. Desirable properties include photostability (high photon yield prior to photobleaching), brightness (product of extinction coefficient and quantum yield), minimal blinking (transient interruptions in emission), and suitable spectral shape (narrow excitation and emission spectra, particularly for observation of multiple labels). Certain small-molecule fluorophores, including the cyanine dyes (GE Healthcare) (Levitus & Ranjit, 2011) and AlexaFluor dyes (Life Technologies), are often preferable to fluorescent proteins (e.g., GFP) because of their superior photophysical properties.
The photophysical properties of single-molecule fluorescence can often be improved by inclusion of certain agents in the imaging buffer, for example, an oxygen scavenging system such as glucose oxidase/catalase or protocatechuic acid/protocatechuate-3,4-dioxygenase (Crawford, Hoskins, Friedman, Gelles, & Moore, 2008) to improve photon yield, and reducing agents or triplet-state quenchers such as Trolox to reduce blinking and improve photostability (Ha & Tinnefeld, 2012). However, these agents can acidify samples (Shi, Lim, & Ha, 2010) and have adverse effects on protein function, so optimization is critical. In addition, each fluorophore should be tested for nonspecific accumulation on the microscope slide surface, which can be either spontaneous or photo-induced.
The most frequently used methods for fluorescent labeling of proteins employ maleimide- or succinimidyl ester derivatives of small-molecule dyes to target surface-exposed sulfhydryls (cysteine residues) or primary amines (lysine residues, protein N-terminus), respectively. Single-residue labeling techniques often require some protein engineering (such as substitution of endogenous cysteines) and additional purification steps to remove unreacted dye, and yet they can still result in nonspecific or inefficient labeling (Means & Feeney, 1971).
Chemical biology techniques for protein labeling that use tags such as SNAP (New England Biolabs), CLIP (New England Biolabs), Halo (Promega), DHFR (Jing & Cornish, 2013), sortagging (Popp, Antos, Grotenbreg, Spooner, & Ploegh, 2007), or ybbR (Yin et al., 2005) can combine the benefits of genetically encoded tags with those of small-molecule fluorophores. First, the labeling tag is engineered to be at one end of the target protein, along with any additionally desired purification tags such as 6His, GST, and MBP. Then, the labeling tag is reacted with any of a wide array of available small-molecule dyes conjugated to the small-molecule target of the labeling tag. The labeling reactions are highly specific and efficient and can be performed during purification so as to achieve effective clearance of unreacted dye conjugates. Recent development of hetero-bifunctional substrates for the SNAP tag has been particularly useful in designing novel TIRF experiments. These substrates link both a small-molecule fluorophore and a biotin moiety to the SNAP tag and can be used to tether a protein of interest to a microscope slide and also visualize its location (Smith, Padrick, et al., 2013).
Regardless of the labeling method used, investigators should take care to verify that the labeled protein displays the same activity as the unlabeled, wild-type protein. For ensemble activity assays, it is usually necessary to achieve high fractional labeling of the protein to ensure that activity is not confined to the unlabeled subpopulation.
3. LABELING AND SURFACE CONFINEMENT OF ACTIN FILAMENTS
For studying actin regulation by TIRF, filaments are fluorescently labeled and tethered or confined to a microscope slide surface. To label filaments, a mixture of unlabeled and fluorescently labeled (~10%) actin monomers is used. Higher percentage mixtures of labeled actin (>50%) can have undesirable effects on polymerization or actin-associated protein binding (Amann & Pollard, 2001; Kuhn & Pollard, 2005). A short wavelength fluorophore (excitable by a blue 488-nm laser), such as AlexaFluor488 or Oregon Green, is the best choice for labeling actin in multiwavelength experiments for a number of reasons. Despite the suboptimal photophysical properties typical of short wavelength fluorophores, filaments are readily visible at 10% labeling because they contain multiple subunits per optically resolvable length (~100 subunits). Furthermore, since the spectra of most dyes are broader on the short wavelength side than the long wavelength side of the excitation peak, filaments labeled with the shortest wavelength dye will be less excited by the longer wavelength lasers, used at higher power to image single molecules of labeled actin-associated proteins. For TIRF, cysteine-reactive probes (such as Oregon Green-maleimide) are usually preferable, because actin has a single reactive cysteine, and therefore labeling is specific. Cysteine-labeled actin yields polymerization rates nearly identical to those of unlabeled actin (Amann & Pollard, 2001; Kuhn & Pollard, 2005). An alternative is to use amine-reactive probes (such as AlexaFluor488 carboxylic acid, tetrafluorophenyl ester; AF488-TFPE), which offers the advantage of not interfering with binding of some actin-associated proteins that bind near the cysteine (Isambert et al., 1995; Kovar, Harris, Mahaffy, Higgs, & Pollard, 2006).
To confine filaments near the surface, enabling observation by TIRF, two recommended approaches are (a) inclusion of a crowding agent or (b) tethering of biotin-actin to a streptavidin surface. Crowding agent polymers such as methyl cellulose or dextran will force filaments against the slide surface due to entropic effects (Uyeda, Kron, & Spudich, 1990). These agents, used without filament tethering, are particularly recommended to visualize filament severing or debranching, since they allow the severed products to diffuse apart, increasing the chance of detecting such events (Gandhi et al., 2010). The amount of agent included needs to be optimized, as too much can induce filament bundling.
In some experiments, it is desirable to physically tether filaments to the surface, for example, so that they remain in the field of view under flow, allowing new ingredients to be introduced during reactions. A small amount of biotinylated actin (~1%) can be included, often in combination with a polymer crowding agent, to tether filaments to a streptavidin-coated surface. Firmly tethering filaments can also simplify quantitative image analysis, but potentially interferes with detection of some regulatory activities (e.g., bundling, severing, or debranching).
4. SLIDE SURFACE TREATMENT
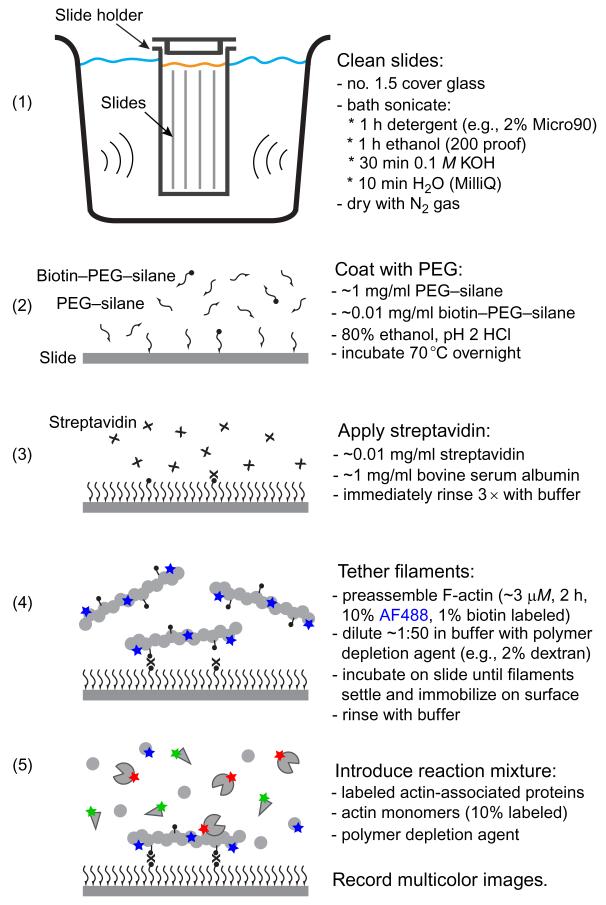
In experiments where one is observing the interactions of labeled actin-binding proteins with surface-confined filaments, it is critical to minimize nonspecific binding of the actin-binding proteins to the surface. This can be achieved in two complementary ways. First, the slide surfaces can be covalently modified with polymer chains such as polyethylene glycol (PEG), which interferes with the approach of proteins to the surface. Second, thesurface can be passivated by absorbing high concentrations (1 mg/ml) of a carrier protein such as bovine serum albumin to block nonspecific binding sites. A protocol for microscope slide surface preparation is included in Fig. 6.1. Once slides have been coated with PEG, they should be used within ~24 h or frozen at −80°C under nitrogen gas for long-term storage.Streptavidin should be applied immediately before the biotinylated protein to be tethered.
Figure 6.1.
Protocol for preparation of microscope slides and observing protein binding to surface-confined actin filaments. Thorough cleaning and passivation of slides, using PEG-silane (Laysan Bio, Arab, AL), is essential to reduce unwanted fluorescent signals and minimize nonspecific binding of proteins. Immobilization of filaments, using biotin-PEG-silane (Laysan Bio), streptavidin, and biotinylated actin, facilitates prolonged analysis of protein-filament interactions.
5. REQUIREMENTS FOR SINGLE-MOLECULE DETECTION
To detect the weak emission from single molecules, two conditions must be met. First, in order to observe fluorescently labeled single molecules interacting with a target (e.g., actin filament or other actin regulator), the target should be immobilized for at least the duration of a single-image frame acquisition. Immobilization is required so that the emitted photons from the single molecule are imaged as a discrete diffraction-limited spot and can be accomplished by tethering or confining the target filament or actin regulator as described above. The second requirement is that both the solution concentration of the fluorescent protein and the microscope optics must be optimized to ensure that the fluorescent spot is visible over the diffuse background of labeled molecules diffusing freely in the surrounding solution. The evanescent field created by a totally internally reflected excitation beam (in TIRF microscopy) at the surface of a microscope slide provides a means of exciting only the molecules within ~50–200 nm of the slide surface. This minimizes the background fluorescence from most of the molecules in solution. As a rule of thumb, the background will be low enough for good detection if the concentration of fluorescent molecules in the reaction is <50 nM; however, the precise limit depends on many factors, including the desired time resolution. Importantly, even proteins with weak binding (in the micromolar Kd range) can be studied very effectively at low nanomolar concentrations by TIRF; this is because even at low occupancy, the binding and dissociation kinetics (kon and koff) can be directly measured.
6. MICROSCOPE CONSIDERATIONS
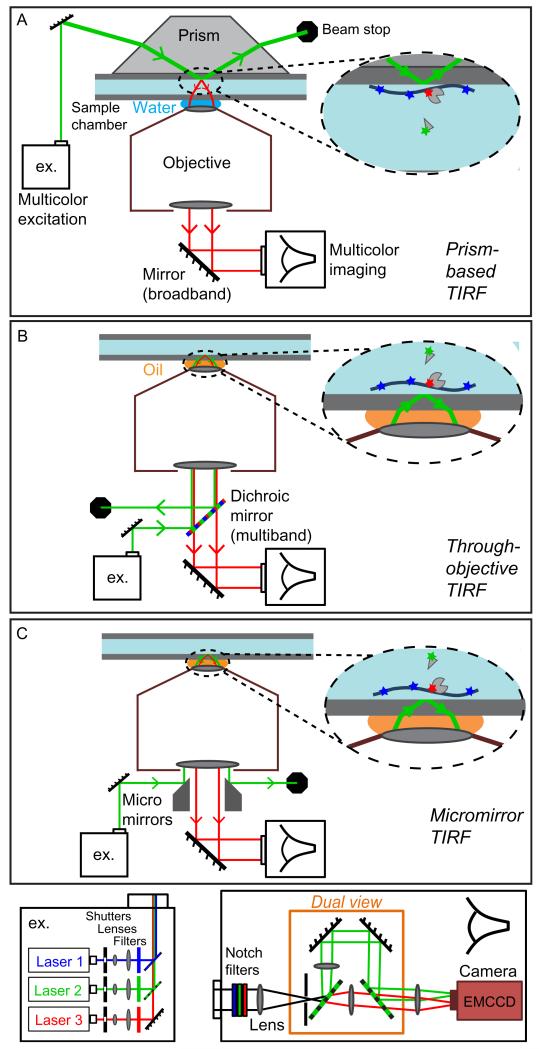
Many single-molecule experiments can be performed using commercially available TIRF microscopes, which use through-objective laser excitation. The newest of these commercial instruments have all of the necessary features for the most common single-molecule applications. However, home-built instruments (Friedman, Chung, & Gelles, 2006; Roy, Hohng, & Ha, 2008) can provide improved performance, for example, in experiments requiring rapid imaging of multiple fluors and/or the highest image quality. First-time microscope builders will find that a prism-based TIRF microscope (Fig. 6.2A) is easier to assemble and use than a through-objective TIRF microscope (Fig. 6.2B). However, prism-based TIRF involves imaging across the specimen chamber, which is not as sensitive as through-objective TIRF that images the chamber surface proximal to the objective (Axelrod, 2003).
Figure 6.2.
Schematics of various multicolor TIRF microscope designs. Prism-based TIRF (A) is in general simpler to set up than through-objective TIRF (B), but is less sensitive since imaging across the full thickness of sample chamber is required. Custom micromirror TIRF (C) further improves the broad spectrum sensitivity by spatially separating excitation (green) and emission (red) optical paths. In each case, excitation (ex.) can be generated by a combination of different color laser beams (bottom left module), and fluorescence emissions can be split into two (or more) color channels, focused on an EMCCD camera, for multicolor image sequence acquisition (bottom right module).
Microscope features to consider in any TIRF single-molecule imaging experiment are (1) a camera with high sensitivity (high quantum efficiency and low noise), for example, an electron multiplying charged coupled device (EMCCD) camera, (2) high-quality optical filter sets that maximize recovery of the emitted photons, and (3) a high numerical aperture (NA ≥1.4) oil-immersion objective (if through-objective TIRF is used). Microscope optics should be configured so that a diffraction-limited spot spans more than one pixel, allowing spots to be distinguished from noise transients, but a spot size that includes too many pixels can reduce the accuracy of spot detection.
A variation on through-objective TIRF, designed to optimize multicolor single-molecule fluorescence imaging, uses broadband micromirrors, rather than a dichroic mirror, to spatially instead of spectrally separate excitation from emission light (Friedman et al., 2006) (Fig. 6.2C). Multiwavelength imaging most commonly uses multiple colors of excitation lasers, for example, 488, 532, and 633 nm (blue, green, and red, respectively). Solid-state lasers are available at these and other wavelengths at powers in the range of 10–300 mW. The power needed depends on the microscope design since different optical systems vary in the efficiency with which they transfer laser power into the sample.
7. IMAGING ACTIN FILAMENTS AND SINGLE MOLECULES
Successful imaging depends on proper selection of laser power and image acquisition time. These two factors in turn depend on the timescale of the events to be monitored, the photostability of the fluors, and the degree to which the molecules under observation are immobilized or confined at the surface. If laser powers and acquisition times are too high, rapid photobleaching will occur, and if they are too low, the images will have low signal-to-noise ratios (i.e., poor quality).
TIRF reactions used to monitor actin filament growth typically contain 1 μM G-actin, which means that the barbed ends grow at ~10 subunits/s (~27 nm/s). At these rates, recording filament growth does not require image acquisition more than about once every 5–10 s, which helps to minimize photobleaching. A separate consideration from the time interval between image acquisitions is the duration of single-image acquisitions. If filaments are not rigidly attached, their ends can undergo rapid Brownian motion and will be blurred unless the individual frame acquisition time is reduced to <0.3 s. At this acquisition time, filaments grown from 10% labeled actin (incorporating ~38 dyes/μm) can still be imaged at low excitation powers, <1 mW for illumination of a ~50 μm diameter field of view. In contrast, much higher laser powers may be required to monitor labeled proteins transiently interacting with the filaments. For example, Arp2/3 complex binds filament sides with lifetimes as short as ~0.2 s, such that observation required 0.05 s/frame acquisition with ~5 mW excitation laser power (Smith, Padrick, et al., 2013). Higher laser powers can also be required for quantitative analysis of protein complex stoichiometry by stepwise photobleaching (Leake et al., 2006), and for the accurate measurements of dye photostability required for some kinds of kinetics analysis.
Another important consideration in designing multiwavelength single-molecule experiments is to ask whether truly simultaneous acquisition at multiple wavelengths is required. If the reaction dynamics are slow, it is usually sufficient to alternate between image records of the dye labels on filaments and those on associated proteins. However, faster reaction dynamics can make it desirable to capture simultaneous multi-channel fluorescence image sequences, particularly if more than one dye-labeled actin-associated protein is being visualized (Smith, Padrick, et al., 2013).
8. DUAL-COLOR TIRF IMAGING OF ACTIN-REGULATORY MECHANISMS
A dual-color experiment that monitors labeled actin-regulatory molecules interacting with labeled filaments provides a real-time record of filament association and dissociation events and the order of events in a mechanism. Analysis of these records can define critical aspects of a mechanism, for example, the time delays between association of an actin-regulatory protein with a filament and the event in which the filament state is altered (e.g., severing or branched nucleation). Furthermore, by counting the number of filament-binding events in a window of time and the number of those events that lead to the activity being monitored, one can quantify the efficiency of the actin-regulatory protein. We now discuss examples of such analyses.
8.1. Actin branch formation by the Arp2/3 complex
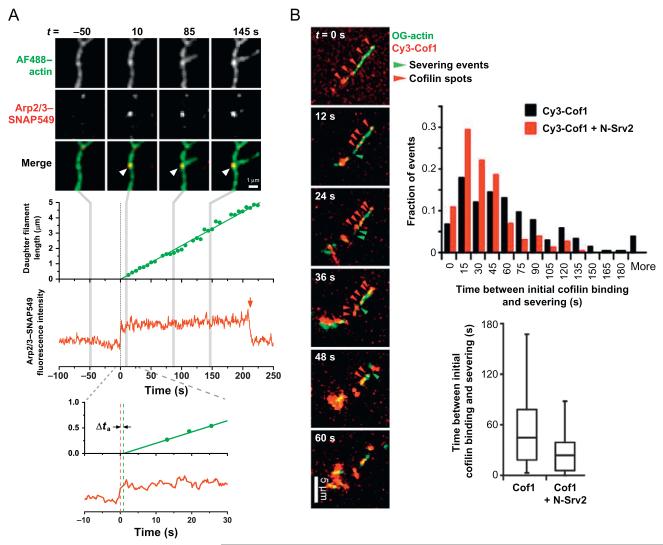
In a study that examined the mechanism of branched actin nucleation by Arp2/3 complex (Smith, Daugherty-Clarke, Goode, & Gelles, 2013), the delay between time of Arp2/3 complex association with the side of a pre-existing (mother) filament and the nucleation of a new (daughter) filament was directly observed (Fig. 6.3A). For these experiments, S. cerevisiae Arp2/3 complex was purified from a strain carrying an integrated SNAP-TEV-3HA tag at the C-terminus of the Arc18/ARPC3 subunit, and labeled with a benzyl guanine-derivatized Dyomics-549 dye (SNAP Surface 549; New England Biolabs). Actin was labeled with AF488-TFPE (10%) and biotin (1%), and unlabeled VCA was included to activate Arp2/3 complex. Using micromirror TIRF microscopy with alternating 488/532 nm laser excitation, Arp2/3-filament-binding events were detected by the appearance of an Arp2/3-SNAP549 fluorescence spot at locations where AF488-filament fluorescence was also observed. That the spots were single molecules was confirmed by single-step photobleaching of the SNAP549 dye. The time at which branched nucleation occurred was determined by tracking the elongation of the daughter filaments, measuring filament lengths, and extrapolating to zero filament length. The delay between filament side binding of Arp2/3 complex and daughter nucleation was found to be short (< ~5 s), and the efficiency of nucleation from Arp2/3-filament complexes was very low (<2%). These results provided valuable new insights into the kinetic mechanism of filament branch formation (Smith, Daugherty-Clarke, Goode, & Gelles, 2013).
Figure 6.3.
Dual-color TIRF studies of actin filaments and actin-associated proteins. (A) Two-color imaging of actin and individual Arp2/3 complexes showed a short activation time delay (Δta ~1 s) between binding of Arp2/3 complex to the side of a filament (at t=0) and nucleation of a new filament branch (Smith, Daugherty-Clarke, Goode, & Gelles, 2013). Single-molecule observation was confirmed by single-step photobleaching (red arrow) of the dye label on Arp2/3 complex incorporated into a stable branch junction. (B) Real-time observation of actin and cofilin during cooperative filament severing by Srv2/CAP and cofilin. Srv2 shortened the delay between cofilin binding and severing compared to controls without Srv2 (see histogram and whisker plot). Reproduced from Chaudhry et al. (2013).
8.2. Filament severing by cofilin and Srv2/CAP
In a different study, regulated actin filament disassembly by cofilin and Srv2/CAP was studied using dual-color imaging (Chaudhry et al., 2013). Here, cofilin (with amino acid substitutions T46C/C62A) was labeled with Cy3-maleimide, actin was labeled with Oregon Green 488 iodoacetamide (10%) and biotin (0.5%), and the effect of unlabeled Srv2/CAP on cofilin-mediated severing was tested. Cooperative binding of labeled cofilin on the surface-tethered filaments was visualized as spots of fluorescence using a commercial through-objective TIRF microscope (Nikon-Ti200) and interleaved two-color fluorescence image acquisition (Fig. 6.3B). The time delay between appearance of labeled cofilin and local severing events was recorded, which revealed that Srv2/CAP had no effect on rate of cofilin accumulation but substantially decreased the delay between cofilin binding and severing (Chaudhry et al., 2013). Since each severing event is likely induced by the cooperative binding of many cofilin molecules in a single spot (Suarez et al., 2011), more advanced single-molecule analysis (e.g., multistep photobleaching; Leake et al., 2006) could be used in the future to investigate the stoichiometry of protein complexes around sites of filament severing.
9. MEASURING KINETICS OF SINGLE-MOLECULE INTERACTIONS WITH ACTIN FILAMENTS
An analysis of single actin-regulatory molecules interacting with filaments can provide a deep quantitative understanding of the molecular mechanism. This analysis can help resolve the step-by-step rate constants and intermediate states along a pathway of activity, and identify steps at which regulation occurs. The unique challenge presented by optical studies of actin-associated proteins is that there are many potential binding sites per optically resolvable length of filament (~0.5 μm, or ~180 actin subunits). Therefore, to obtain reliable single actin-binding protein ~kinetics, experiments should be conducted under conditions of low occupancy (i.e., much less than one protein per 0.5 μm filament length). It is also important to accurately measure the fraction of protein that is labeled. This is required to estimate the true occupancy from the number of fluorescence spots per filament length. At low occupancy, the following method can be used to evaluate rate constants for association and dissociation of actin-associated proteins on the sides of filaments. The protocol is based on the assumption that protein molecules bind independently to sites on the filament; if there is evidence of cooperative binding, for example, as seen with cofilin (Fig. 6.3B), modifications to the method would be required.
Step 1
Record dual-color fluorescence image sequences of actin-associated proteins interacting with actin filaments immobilized on a microscope slide (Fig. 6.1). Movies should be recorded for long enough time to observe at least one binding event in each resolvable segment of filament, and at a frame rate and laser power that allows observation of most binding events for at least three consecutive frames.
Step 2
Track the positions of all individual actin-associated protein fluorescent spots in the image sequence. Although this can be done manually, we instead use an automated method to identify spots (Crocker & Grier, 1996), which makes the analysis far less laborious. Use of the algorithm requires adjustment of several parameters to optimize detection and tracking, including an intensity threshold, a nominal spot size, and a maximum displacement for tracking a spot between consecutive frames. The displacement tolerance is particularly important when the spots are moving, for example, for motor proteins, for proteins that diffuse on filaments, or when filaments are weakly tethered to the slide. For particularly noisy recordings, it is advantageous to use a dual threshold analysis, where analysis with a high-intensity threshold identifies binding events and analysis with a low-intensity threshold identifies dissociation events (Friedman, Mumm, & Gelles, 2013), minimizing false positives and negatives, respectively.
Step 3
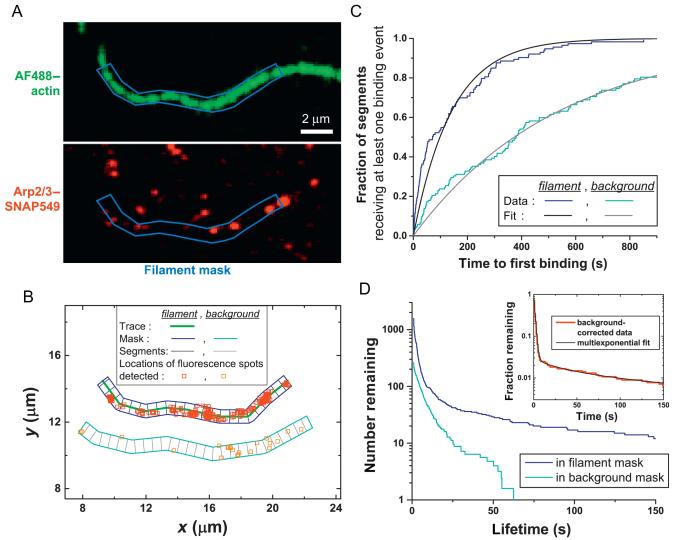
In the filament fluorescence image sequence, trace the contour of a filament. In Matlab (MathWorks, Natick, MA), this can be accomplished using the improfile function. Then create a mask of fixed width (w) along the contour of the filament (Fig. 6.4A). Adjust the width so that filament movements are enclosed by the mask boundary throughout the course of the observation.
Figure 6.4.
Analysis of the kinetics of a protein binding to and dissociating from filaments. (A) Dual-color observations of surface-tethered actin filaments and Arp2/3 complex were analyzed by first tracing a section of filament and generating a mask of uniform width (blue). (B) Fluorescence spots corresponding to single Arp2/3 complexes were tracked over a ~15-min recording. Spots that appeared in the filament mask (red squares) and in control masks covering regions of the field of view that did not contain filament (orange squares in cyan background mask) were retained for analysis. (C) Cumulative distribution of times of first appearance of Arp2/3 complex in each segment of filament and background masks in B. Single-exponential fits are shown (smooth curves), yielding the binding rate constants. (D) Dissociation rate constants were analyzed by generating survival plots of Arp2/3 complexes in filament and background masks. A specific Arp2/3-filament lifetime distribution was generated (inset: red line) by subtracting the background survival plot from the filament survival plot. Multi-exponential fit (black curve) to the background-corrected data reveals that multiple bound states of the Arp2/3-filament complex exist. Adapted from Smith, Daugherty-Clarke, Goode, and Gelles (2013).
Step 4
Select the subset of individual actin-associated protein “tracks” identified in Step 2 that appear within the filament mask constructed in Step 3 (Fig. 6.4B). Manually inspect tracks to eliminate spots that are a single pixel or are large and asymmetrical (presumed to not represent bona fide single molecules). To measure association rates, divide the filament mask into segments of equal length, and tabulate the times at which a protein is first observed in each segment. The smaller the segment length, the more data are obtained from a single filament, but the segment length should not be smaller than the optical resolution. To measure dissociation rates, record the duration of all tracks that fall within a filament mask. Steps 3 and 4 should be repeated as needed with additional filaments in order to obtain sufficient data. Approximately 100 filament segments or more are needed to obtain precise association rates, but at least 1000 bound protein observations may be required to provide sufficient data to identify rare bound states within the population.
Step 5
As a control, measure the kinetics of interactions of the actin-associated protein with the microscope slide. To accomplish this, repeat Steps 3 and 4 for similarly shaped and sized regions of the recorded field of view that do not contain filaments (Fig. 6.4B).
Step 6
Generate a binding curve by plotting the fraction of filament segments that received at least one binding event (Step 4) versus time (Fig. 6.4C), and fit to an exponential to obtain the apparent first-order association rate constant. Apply the same analysis to slide surface binding data (Step 5) and calculate the corrected rate constant by subtracting the rate of binding to regions of the slide that do not contain filament from regions that do (assuming homogeneous binding in each case). If binding is consistently inhomogeneous across filament or background masks, the binding curves will not fit to a single exponential function with an amplitude equal to one, and background correction is more complicated (Smith, Daugherty-Clarke, Goode, & Gelles, 2013). Finally, divide the binding rate by the number of potential binding sites in each segment of filament (even if sites overlap; typically one per actin subunit) and by the labeled protein concentration (i.e., the product of the total protein concentration and the labeling fraction) to calculate the second-order binding rate constant per site. Measuring the first binding event in each segment is more accurate than measuring the overall frequency of binding to the filament since it (1) avoids overcounting of binding events due to dye blinking, (2) minimizes errors caused by occupation of sites by photobleached molecules, (3) circumvents the problem of detecting multiple proteins binding to multiple sites within the spatial resolution of the microscope, and (4) is sensitive to heterogeneities in the filament, at least on length scales longer than the microscope resolution.
Step 7
To analyze the kinetics of dissociation of actin-associated proteins from filaments, make a semi-logarithmic plot of the number of proteins remaining as a function of time since each first appeared (i.e., fraction with lifetimes greater than time t as a function of t; Fig. 6.4D). Correct these survival curves for interactions with the microscope slide at each time by subtracting the number remaining in background masks from the number remaining in filament masks. The result is a specific protein-filament complex survival plot (Fig. 6.4D, inset), the logarithm of which decreases linearly with time when there is a single-filament-bound state of the protein. Nonlinear log-survival curves (as in the example shown in Fig. 6.4D) are evidence for the presence of multiple bound states. The points on the survival curve are not independent measurements and thus the data should be fit using maximum likelihood methods (Colquhoun & Sigworth, 1983) instead of by conventional least-squares methods. In measurements of protein dissociation kinetics by single-molecule fluorescence, it is essential that the disappearance of the observed fluorescence spots be from protein dissociation and not photobleaching of the attached dye. Verify this by repeating experiments over a ~3-fold or greater range of laser powers and checking that the dissociation rates measured at different powers do not differ significantly. Alternatively, the photobleaching effect can be corrected by fitting measured rates to a linear function of laser power. The intercept of this fit at zero laser power identifies the true dissociation rate.
The results of the above stepwise analysis should yield accurate association and dissociation rate constants for interactions between actin-associated proteins and filaments, although some mathematical modeling may be required to relate observed rates to rate constants of individual reaction steps. These data, in combination with results from single-molecule activity analysis (e.g., Fig. 6.3) and bulk assays, can then be used to test hypothesized reaction mechanisms (e.g., Smith, Daugherty-Clarke, Goode, & Gelles, 2013).
10. COORDINATION OF MULTIPLE ACTIN-REGULATORY FACTORS FROM MULTICOLOR IMAGING
Many cellular processes are controlled by multiple actin-associated proteins working in concert. Thus, it is often desirable to directly observe multiple factors acting simultaneously to regulate actin filament dynamics. This was accomplished in two recent studies that used triple-color fluorescence analysis to visualize how different pairs of actin nucleation factors cooperate in assembling either unbranched or branched actin filaments (Breitsprecher et al., 2012; Smith, Padrick, et al., 2013) (Fig. 6.5). Both studies addressed key questions relating to the stoichiometry and order of assembly of transient prenucleation complexes, as well as the fate of the assembly factors following nucleation, all of which could be directly visualized in real time using single-molecule TIRF microscopy.
Figure 6.5.
Directly observing the coordination of multiple factors regulating actin filament dynamics. (A) Three-color image sequence (left) showing the coordinated action of APC (blue) and the formin mDia1 (red) in nucleation and elongation of an actin filament (green). Prior to filament elongation, APC and formin colocalized (asterisk), but during elongation APC remained with the filament pointed end whereas formin processively tracked the growing barbed end, defining the “rocket launcher” mechanism shown (cartoon below). (B) Three-color imaging (top) of single molecules of dimeric VCA (green) and Arp2/3 complex (red) binding the side of a preexisting mother filament and generating anew daughter filament(forming anactinbranch; blue)(Smith, Padrick, etal.,2013). Fluorescence intensity traces and daughter length records (middle) reveal that Arp2/3 complex and VCA appear on filament sides as a complex, but VCA departs rapidly prior to initiation of daughter filament elongation, indicating that VCA release from the nascent branch may trigger branch formation (simplified kinetic scheme, below). Panel (A): Reproduced from Breitsprecher et al. (2012). Panel (B): Portions modified from Smith, et al. (2013).
10.1. Cooperative filament assembly by APC and mDia1
In the first example (Breitsprecher et al., 2012), APC and the formin mDia1 were visualized by generating SNAP-tag fusions and labeling them with SNAP647 and SNAP549, respectively. Actin was labeled with Oregon Green-maleimide on 10% of monomers, and reactions included unlabeled capping protein (CapZ) and profilin (Fig. 6.5A). The combination of APC and mDia1 produced rapid formation of actin filaments despite the presence of inhibitory effects of profilin on nucleation and of CapZ on elongation. Using single-molecule analysis, it was shown that the mechanism of nucleation involves the interaction of an APC dimer with an mDia1 dimer and actin monomers. Upon formation of the filament, the APC-mDia1 complex dissociated, leaving the APC dimer stably associated with the filament near the nucleation site and the formin dimer moving away on the growing barbed end of the filament (Fig. 6.5A). This “rocket launcher” mechanism of unbranched filament assembly may also be used by other pairs of assembly factors, for example, Spire-formin and Bud6-formin.
10.2. Coordination of Arp2/3 complex and its activator in actin branch formation
In the second example (Smith, Padrick, et al., 2013), branched actin filament nucleation was visualized using Arp2/3 complex (SNAP tagged and dye labeled), a dimeric VCA activator (N-terminally linked with bis-maleimide-Cy3), and actin (10% AlexaFluor488 labeled), and by surface tethering either the Arp2/3 complex or preassembled filaments. Simultaneous high-speed recording of individual Arp2/3 complex and VCA dimers (50 ms/frame; dual view), interleaved with time-lapse actin imaging (every ~10 s), was essential for this study. VCA dimers were observed to bind tethered Arp2/3 complex with high affinity when not associated with an actin filament, but did not bind Arp2/3 complex following branch formation. Correspondingly, when filaments were tethered, Arp2/3 complex was most often associated with VCA dimers upon binding to filament and Arp2/3–VCA complexes rapidly dissociated from filament as a unit without initiating filament formation. However, in cases where branch formation was observed, VCA dimers rapidly released from Arp2/3-filament complexes prior to initiation of new filament elongation (Fig. 6.5B). This suggested that WASp family proteins serve a dual role in both promoting the assembly of an actin nucleus for branch formation and in limiting new filament growth, such that VCA release from the nascent branch is required to trigger elongation.
Both of the studies described above (Fig. 6.5) contributed significant advances to our understanding of the mechanisms of reconstituted collaborative actin assembly. The quantitative kinetic and stoichiometric data obtained should prove invaluable in building systems-level models of cytoskeletal function. We expect that future studies will be similarly successful in elucidating the processes of actin disassembly orchestrated by multiple factors.
ACKNOWLEDGMENTS
Preparation of this article was funded in part by National Institutes of Health grants R01GM098143 (to J. G.), GM063691 (to B. L. G.), and GM098143 (to J. G. and B. L. G.), as well as National Science Foundation grant DMR-MRSEC-0820492 (to J. G. and B. L. G.). The authors thank Dennis Breitsprecher, Julian Eskin, Larry Friedman, Silvia Jansen, and Casey Ydenberg for comments on the manuscript.
REFERENCES
- Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15009–15013. doi: 10.1073/pnas.211556398. http://dx.doi.org/10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Methods in Enzymology. 2003;361:1–33. doi: 10.1016/s0076-6879(03)61003-7. [DOI] [PubMed] [Google Scholar]
- Beltzner CC, Pollard TD. Pathway of actin filament branch formation by Arp2/3 complex. The Journal of Biological Chemistry. 2008;283(11):7135–7144. doi: 10.1074/jbc.M705894200. http://dx.doi.org/10.1074/jbc.M705894200. [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD, Mullins RD. Interactions of ADF/cofilin, Arp2/3 complex, capping protein and profilin in remodeling of branched actin filament networks. Current Biology. 2000;10(20):1273–1282. doi: 10.1016/s0960-9822(00)00749-1. [DOI] [PubMed] [Google Scholar]
- Boettner DR, Friesen H, Andrews B, Lemmon SK. Clathrin light chain directs endocytosis by influencing the binding of the yeast Hip1R homologue, Sla2, to F-actin. Molecular Biology of the Cell. 2011;22(19):3699–3714. doi: 10.1091/mbc.E11-07-0628. http://dx.doi.org/10.1091/mbc.E11-07-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 2012;336(6085):1164–1168. doi: 10.1126/science.1218062. http://dx.doi.org/10.1126/science.1218062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008;134(5):828–842. doi: 10.1016/j.cell.2008.06.054. http://dx.doi.org/10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Beltzner CC, Pollard TD. Cofilin dissociates Arp2/3 complex and branches from actin filaments. Current Biology. 2009;19(7):537–545. doi: 10.1016/j.cub.2009.02.060. http://dx.doi.org/10.1016/j.cub.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Breitsprecher D, Little K, Sharov G, Sokolova O, Goode BL. Srv2/cyclase-associated protein forms hexameric shurikens that directly catalyze actin filament severing by cofilin. Molecular Biology of the Cell. 2013;24(1):31–41. doi: 10.1091/mbc.E12-08-0589. http://dx.doi.org/10.1091/mbc.E12-08-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Single-channel recording. Plenum; New York: 1983. Fitting and statistical analysis of single-channel records; pp. 191–263. [Google Scholar]
- Crawford DJ, Hoskins AA, Friedman LJ, Gelles J, Moore MJ. Visualizing the splicing of single pre-mRNA molecules in whole cell extract. RNA. 2008;14(1):170–179. doi: 10.1261/rna.794808. http://dx.doi.org/10.1261/rna.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker JC, Grier DG. Methods of digital video microscopy for colloidal studies. Journal of Colloid and Interface Science. 1996;179(1):298–310. http://dx.doi.org/10.1006/jcis.1996.0217. [Google Scholar]
- Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Current Opinion in Cell Biology. 2011;23(1):4–13. doi: 10.1016/j.ceb.2010.10.007. http://dx.doi.org/10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LJ, Chung J, Gelles J. Viewing dynamic assembly of molecular complexes by multi-wavelength single-molecule fluorescence. Biophysical Journal. 2006;91(3):1023–1031. doi: 10.1529/biophysj.106.084004. http://dx.doi.org/10.1529/biophysj.106.084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LJ, Mumm JP, Gelles J. RNA polymerase approaches its promoter without long-range sliding along DNA. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):9740–9745. doi: 10.1073/pnas.1300221110. http://dx.doi.org/10.1073/pnas.1300221110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara I, Remmert K, Hammer JA., 3rd Direct observation of the uncapping of capping protein-capped actin filaments by CARMIL homology domain 3. The Journal of Biological Chemistry. 2010;285(4):2707–2720. doi: 10.1074/jbc.M109.031203. http://dx.doi.org/10.1074/jbc.M109.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Smith BA, Bovellan M, Paavilainen V, Daugherty-Clarke K, Gelles J, et al. GMF is a cofilin homolog that binds Arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Current Biology. 2010;20(9):861–867. doi: 10.1016/j.cub.2010.03.026. http://dx.doi.org/10.1016/j.cub.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T, Tinnefeld P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annual Review of Physical Chemistry. 2012;63:595–617. doi: 10.1146/annurev-physchem-032210-103340. http://dx.doi.org/10.1146/annurev-physchem-032210-103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund CM, Welch MD. Pathogens and polymers: Microbe-host interactions illuminate the cytoskeleton. The Journal of Cell Biology. 2011;195(1):7–17. doi: 10.1083/jcb.201103148. http://dx.doi.org/10.1083/jcb.201103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SD, Mullins RD. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. The Journal of Cell Biology. 2010;191(3):571–584. doi: 10.1083/jcb.201003014. http://dx.doi.org/10.1083/jcb.201003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isambert H, Venier P, Maggs AC, Fattoum A, Kassab R, Pantaloni D, et al. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. The Journal of Biological Chemistry. 1995;270(19):11437–11444. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- Jing C, Cornish VW. A fluorogenic TMP-tag for high signal-to-background intracellular live cell imaging. ACS Chemical Biology. 2013;8:1704–1712. doi: 10.1021/cb300657r. http://dx.doi.org/10.1021/cb300657r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124(2):423–435. doi: 10.1016/j.cell.2005.11.038. http://dx.doi.org/10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophysical Journal. 2005;88(2):1387–1402. doi: 10.1529/biophysj.104.047399. http://dx.doi.org/10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake MC, Chandler JH, Wadhams GH, Bai F, Berry RM, Armitage JP. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature. 2006;443(7109):355–358. doi: 10.1038/nature05135. http://dx.doi.org/10.1038/nature05135. [DOI] [PubMed] [Google Scholar]
- Levitus M, Ranjit S. Cyanine dyes in biophysical research: The photophysics of polymethine fluorescent dyes in biomolecular environments. Quarterly Reviews of Biophysics. 2011;44(1):123–151. doi: 10.1017/S0033583510000247. http://dx.doi.org/10.1017/S0033583510000247. [DOI] [PubMed] [Google Scholar]
- Martin AC, Welch, M D, Drubin DG. Arp2/3 ATP hydrolysis-catalysed branch dissociation is critical for endocytic force generation. Nature Cell Biology. 2006;8(8):826–833. doi: 10.1038/ncb1443. http://dx.doi.org/10.1038/ncb1443. [DOI] [PubMed] [Google Scholar]
- Means GE, Feeney RE. Chemical modification of proteins. Holden-Day, Inc.; San Francisco: 1971. [Google Scholar]
- Mizuno H, Higashida C, Yuan Y, Ishizaki T, Narumiya S, Watanabe N. Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science (New York, NY) 2011;331(6013):80–83. doi: 10.1126/science.1197692. http://dx.doi.org/10.1126/science.1197692. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Wollman R, Marshall WF. Quantitative modeling in cell biology: What is it good for? Developmental Cell. 2006;11(3):279–287. doi: 10.1016/j.devcel.2006.08.004. http://dx.doi.org/10.1016/j.devcel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science (New York, NY) 2009;326(5957):1208–1212. doi: 10.1126/science.1175862. http://dx.doi.org/10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Sortagging: A versatile method for protein labeling. Nature Chemical Biology. 2007;3(11):707–708. doi: 10.1038/nchembio.2007.31. http://dx.doi.org/10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145(7):1012–1022. doi: 10.1016/j.cell.2011.06.010. http://dx.doi.org/10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nature Methods. 2008;5(6):507–516. doi: 10.1038/nmeth.1208. http://dx.doi.org/10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaus TE, Taylor EW, Borisy GG. Self-organization of actin filament orientation in the dendritic-nucleation/array-treadmilling model. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(17):7086–7091. doi: 10.1073/pnas.0701943104. http://dx.doi.org/10.1073/pnas.0701943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Lim J, Ha T. Acidification of the oxygen scavenging system in single-molecule fluorescence studies: In situ sensing with a ratiometric dual-emission probe. Analytical Chemistry. 2010;82(14):6132–6138. doi: 10.1021/ac1008749. http://dx.doi.org/10.1021/ac1008749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripala AD, Welch MD. SnapShot: Actin regulators I. Cell. 2007;128(3):626. doi: 10.1016/j.cell.2007.02.001. http://dx.doi.org/10.1016/j.cell.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Smith BA, Daugherty-Clarke K, Goode BL, Gelles J. Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(4):1285–1290. doi: 10.1073/pnas.1211164110. http://dx.doi.org/10.1073/pnas.1211164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BA, Padrick SB, Doolittle LK, Daugherty-Clarke K, Corrêa IR, Jr., Xu MQ, et al. Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. eLife. 2013;2:e01008. doi: 10.7554/eLife.01008. http://dx.doi.org/10.7554/eLife.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Roland J, Boujemaa-Paterski R, Kang H, McCullough BR, Reymann AC, et al. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Current Biology. 2011;21(10):862–868. doi: 10.1016/j.cub.2011.03.064. http://dx.doi.org/10.1016/j.cub.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda TQ, Kron SJ, Spudich JA. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. Journal of Molecular Biology. 1990;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. http://dx.doi.org/10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Drubin DG. Clathrin-mediated endocytosis in budding yeast. Trends in Cell Biology. 2012;22(1):1–13. doi: 10.1016/j.tcb.2011.09.001. http://dx.doi.org/10.1016/j.tcb.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T, Nakase M, Nishiyama K, Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- Yin J, Straight PD, McLoughlin SM, Zhou Z, Lin AJ, Golan DE, et al. Genetically encoded short peptide tag for versatile protein labeling by Sfp phosphopantetheinyl transferase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):15815–15820. doi: 10.1073/pnas.0507705102. [DOI] [PMC free article] [PubMed] [Google Scholar]