Abstract
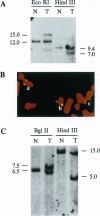
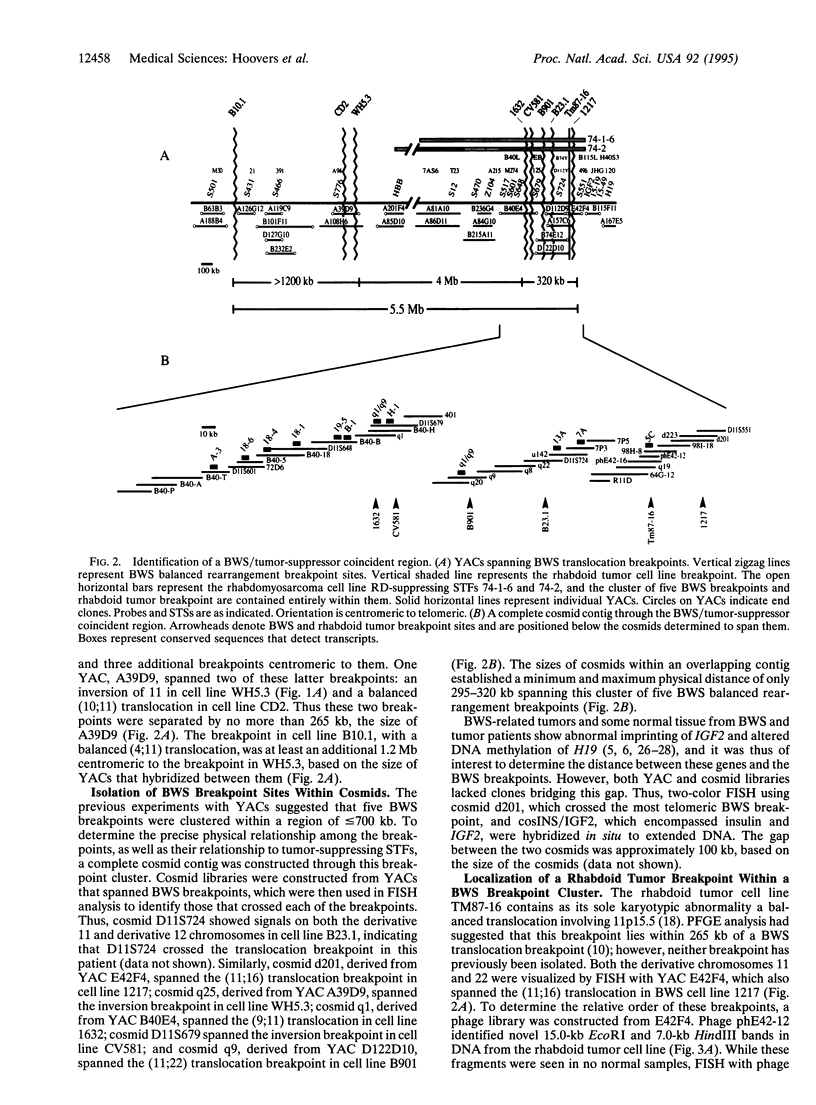
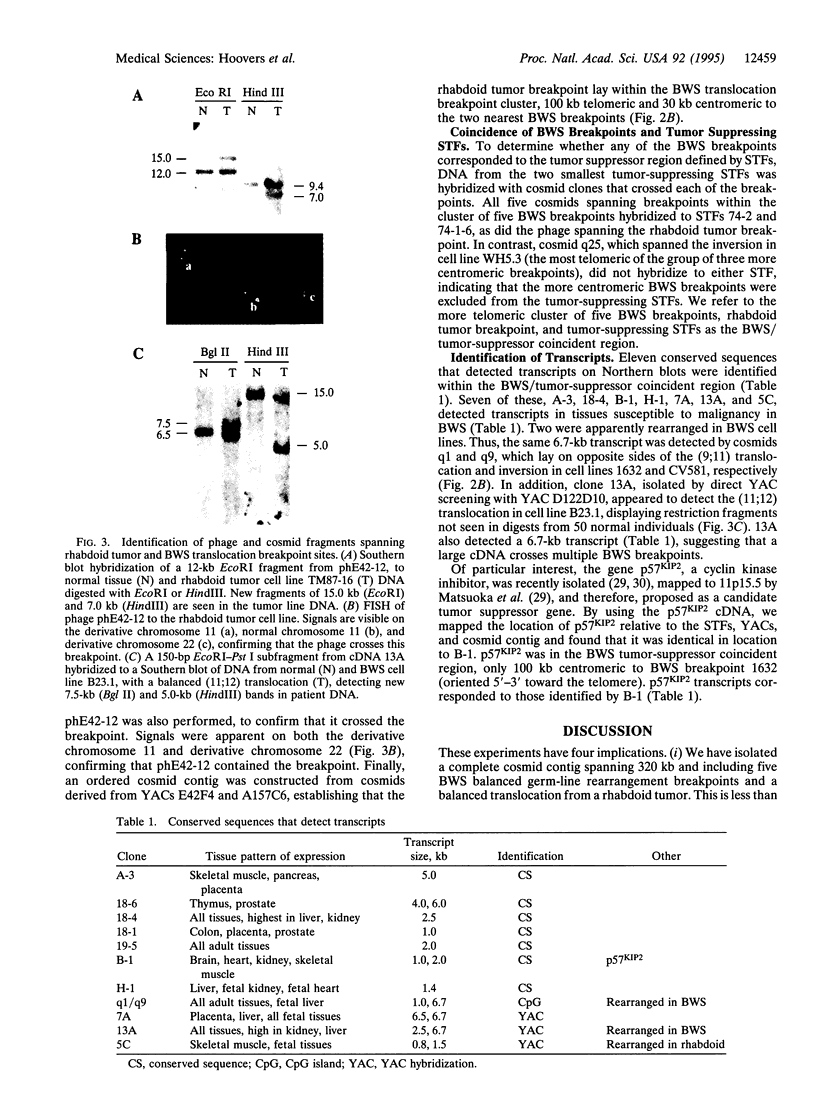
Beckwith-Wiedemann syndrome (BWS) involves fetal overgrowth and predisposition to a wide variety of embryonal tumors of childhood. We have previously found that BWS is genetically linked to 11p15 and that this same band shows loss of heterozygosity in the types of tumors to which children with BWS are susceptible. However, 11p15 contains > 20 megabases, and therefore, the BWS and tumor suppressor genes could be distinct. To determine the precise physical relationship between these loci, we isolated yeast artificial chromosomes, and cosmid libraries from them, within the region of loss of heterozygosity in embryonal tumors. Five germ-line balanced chromosomal rearrangement breakpoint sites from BWS patients, as well as a balanced chromosomal translocation breakpoint from a rhabdoid tumor, were isolated within a 295- to 320-kb cluster defined by a complete cosmid contig crossing these breakpoints. This breakpoint cluster terminated approximately 100 kb centromeric to the imprinted gene IGF2 and 100 kb telomeric to p57KIP2, an inhibitor of cyclin-dependent kinases, and was located within subchromosomal transferable fragments that suppressed the growth of embryonal tumor cells in genetic complementation experiments. We have identified 11 transcribed sequences in this BWS/tumor suppressor coincident region, one of which corresponded to p57KIP2. However, three additional BWS breakpoints were > 4 megabases centromeric to the other five breakpoints and were excluded from the tumor suppressor region defined by subchromosomal transferable fragments. Thus, multiple genetic loci define BWS and tumor suppression on 11p15.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bestor T. H., Chandler V. L., Feinberg A. P. Epigenetic effects in eukaryotic gene expression. Dev Genet. 1994;15(6):458–462. doi: 10.1002/dvg.1020150603. [DOI] [PubMed] [Google Scholar]
- Blanchard M. M., Taillon-Miller P., Nowotny P., Nowotny V. PCR buffer optimization with uniform temperature regimen to facilitate automation. PCR Methods Appl. 1993 Feb;2(3):234–240. doi: 10.1101/gr.2.3.234. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fidlerová H., Senger G., Kost M., Sanseau P., Sheer D. Two simple procedures for releasing chromatin from routinely fixed cells for fluorescence in situ hybridization. Cytogenet Cell Genet. 1994;65(3):203–205. doi: 10.1159/000133632. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Heding I. J., Ivens A. C., Wilson J., Strivens M., Gregory S., Hoovers J. M., Mannens M., Redeker B., Porteous D., van Heyningen V. The generation of ordered sets of cosmid DNA clones from human chromosome region 11p. Genomics. 1992 May;13(1):89–94. doi: 10.1016/0888-7543(92)90206-8. [DOI] [PubMed] [Google Scholar]
- Junien C. Beckwith-Wiedemann syndrome, tumourigenesis and imprinting. Curr Opin Genet Dev. 1992 Jun;2(3):431–438. doi: 10.1016/s0959-437x(05)80154-6. [DOI] [PubMed] [Google Scholar]
- Karnes P. S., Tran T. N., Cui M. Y., Bogenmann E., Shimada H., Ying K. L. Establishment of a rhabdoid tumor cell line with a specific chromosomal abnormality, 46,XY,t(11;22)(p15.5;q11.23). Cancer Genet Cytogenet. 1991 Oct 1;56(1):31–38. doi: 10.1016/0165-4608(91)90359-3. [DOI] [PubMed] [Google Scholar]
- Koi M., Johnson L. A., Kalikin L. M., Little P. F., Nakamura Y., Feinberg A. P. Tumor cell growth arrest caused by subchromosomal transferable DNA fragments from chromosome 11. Science. 1993 Apr 16;260(5106):361–364. doi: 10.1126/science.8469989. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Reynisdóttir I., Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995 Mar 15;9(6):639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Green E. D., Cremer T. Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics. 1992 Jul;13(3):826–828. doi: 10.1016/0888-7543(92)90160-t. [DOI] [PubMed] [Google Scholar]
- Mannens M., Hoovers J. M., Redeker E., Verjaal M., Feinberg A. P., Little P., Boavida M., Coad N., Steenman M., Bliek J. Parental imprinting of human chromosome region 11p15.3-pter involved in the Beckwith-Wiedemann syndrome and various human neoplasia. Eur J Hum Genet. 1994;2(1):3–23. doi: 10.1159/000472337. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Edwards M. C., Bai C., Parker S., Zhang P., Baldini A., Harper J. W., Elledge S. J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995 Mar 15;9(6):650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Norman A. M., Read A. P., Clayton-Smith J., Andrews T., Donnai D. Recurrent Wiedemann-Beckwith syndrome with inversion of chromosome (11)(p11.2p15.5). Am J Med Genet. 1992 Feb 15;42(4):638–641. doi: 10.1002/ajmg.1320420441. [DOI] [PubMed] [Google Scholar]
- Ogawa O., Eccles M. R., Szeto J., McNoe L. A., Yun K., Maw M. A., Smith P. J., Reeve A. E. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature. 1993 Apr 22;362(6422):749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Pueschel S. M., Padre-Mendoza T. Chromosome 11 and Beckwith-Wiedemann syndrome. J Pediatr. 1984 Mar;104(3):484–485. doi: 10.1016/s0022-3476(84)81127-0. [DOI] [PubMed] [Google Scholar]
- Rainier S., Dobry C. J., Feinberg A. P. Loss of imprinting in hepatoblastoma. Cancer Res. 1995 May 1;55(9):1836–1838. [PubMed] [Google Scholar]
- Rainier S., Johnson L. A., Dobry C. J., Ping A. J., Grundy P. E., Feinberg A. P. Relaxation of imprinted genes in human cancer. Nature. 1993 Apr 22;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- Redeker E., Hoovers J. M., Alders M., van Moorsel C. J., Ivens A. C., Gregory S., Kalikin L., Bliek J., de Galan L., van den Bogaard R. An integrated physical map of 210 markers assigned to the short arm of human chromosome 11. Genomics. 1994 Jun;21(3):538–550. doi: 10.1006/geno.1994.1312. [DOI] [PubMed] [Google Scholar]
- Sait S. N., Nowak N. J., Singh-Kahlon P., Weksberg R., Squire J., Shows T. B., Higgins M. J. Localization of Beckwith-Wiedemann and rhabdoid tumor chromosome rearrangements to a defined interval in chromosome band 11p15.5. Genes Chromosomes Cancer. 1994 Oct;11(2):97–105. doi: 10.1002/gcc.2870110206. [DOI] [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Tommerup N., Brandt C. A., Pedersen S., Bolund L., Kamper J. Sex dependent transmission of Beckwith-Wiedemann syndrome associated with a reciprocal translocation t(9;11)(p11.2;p15.5). J Med Genet. 1993 Nov;30(11):958–961. doi: 10.1136/jmg.30.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R., Shen D. R., Fei Y. L., Song Q. L., Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993 Oct;5(2):143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Teshima I., Williams B. R., Greenberg C. R., Pueschel S. M., Chernos J. E., Fowlow S. B., Hoyme E., Anderson I. J., Whiteman D. A. Molecular characterization of cytogenetic alterations associated with the Beckwith-Wiedemann syndrome (BWS) phenotype refines the localization and suggests the gene for BWS is imprinted. Hum Mol Genet. 1993 May;2(5):549–556. doi: 10.1093/hmg/2.5.549. [DOI] [PubMed] [Google Scholar]
- Zhan S., Shapiro D. N., Helman L. J. Activation of an imprinted allele of the insulin-like growth factor II gene implicated in rhabdomyosarcoma. J Clin Invest. 1994 Jul;94(1):445–448. doi: 10.1172/JCI117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shields T., Crenshaw T., Hao Y., Moulton T., Tycko B. Imprinting of human H19: allele-specific CpG methylation, loss of the active allele in Wilms tumor, and potential for somatic allele switching. Am J Hum Genet. 1993 Jul;53(1):113–124. [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]