Abstract
Background and Objectives
In the Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial, ultrafiltration (UF) removed volume more effectively than usual care (UC). Hypothetically, UF may be superior to UC due to increased sodium (Na) removal and less neurohormonal activation. We compared UF and UC in a randomized pilot trial of target weight guided therapy for acute decompensated heart failure (ADHF).
Subjects and Methods
Sixteen patients with ADHF were enrolled and target weights established prospectively, prior to randomization to UC or UF. UF patients did not receive diuretics and UC patients were all treated with a continuous furosemide drip. All urine and ultrafiltrate were collected and Na concentrations measured.
Results
Similar volumes were removed in UC and UF groups (110105 mL and 107415 mL, respectively) and the UF group also produced 45325 mL of urine. Na concentration was 138±6 meq/L in the ultrafiltrate, 85±73 meq/L in the UC group's urine, and 26±23 meq/L in the UF group's urine. Given the relevant associated volumes, total meq of the Na removed was similar (1168 in UC vs. 1216 in UF). The UF group produced isotonic ultrafiltrate and a higher volume of dilute urine than anticipated.
Conclusion
In a randomized pilot study of target weight guided therapy with UC or UF for ADHF, there were no differences in total volumes or Na removed, and lengths of hospital stays were similar. Isotonic fluid loss by UF was accompanied by the production of very dilute urine.
Keywords: Ultrafiltration, Heart failure, Diuretics, Sodium
Introduction
The primary therapeutic goal in acute decompensated heart failure (ADHF) is symptom relief.1,2,3) Most patients admitted to hospital for ADHF are in a volume overloaded state4) and require fluid removal for symptom relief. The standard of care revolves around the use of intravenous loop diuretics, sometimes supported by adjunctive therapy with vasodilators and inotropic agents.2) However, inhospital mortality as well as short-term outcomes remain problematic, with 30-day readmission rates of patients that have not improved significantly despite increased awareness over 20%.5) Loop diuretic therapy may have a direct deleterious effect on cardiac function,6) and the use of higher doses of loop diuretics may be associated with poorer outcomes,7) although the presence of a causal relationship is unproven. Nonetheless, ample imperatives exist to prompt an evaluation of novel strategies to remove volume in ADHF.
Extracorporeal ultrafiltration (UF) has been explored in the ADHF population for over two decades.8),9) With the development of the Aquadex system (CHF Solutions, Inc., Gambro), UF has become easier to use, requiring less intense nursing support.10) The Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) trial demonstrated that, when randomized to usual care (UC) or primary therapy with UF, UF removed more volume at 48 hours, although the degree of dyspnea did not differ.11) In addition, fewer short-term HF related readmissions were observed in the UF treated group. Subsequently, this therapy has achieved widespread use, and is included in the American Heart Association/American College of Cardiology practice guidelines as a viable therapy in diuretic resistant patients.12) Other putative benefits of UF include more effective sodium (Na) removal13) and less neurohormonal stimulation.14) For these reasons, UF may be a safer and more effective method of removing volume. However, if similar amounts of volume are removed, it is not known if UF provides more Na removal, shorter lengths of hospital stays, and less renal toxicity. We performed a randomized pilot study to evaluate these questions, under the hypothesis that total Na removed by UF is greater than that during UC when matched for volume loss.
Subjects and Methods
Patient selection
The protocol was approved by the Christ Hospital (Cincinnati, Ohio) Institutional Review Board. Patients admitted to the hospital for a primary diagnosis of ADHF were screened for the following inclusion criteria: left ventricular ejection fraction less than or equal to 35%, evidence of volume overload (ascites, jugular venous distension or edema), and New York Heart Association 3-4 functional class. Exclusion criteria included serum creatinine greater than 3.5 mg/dL, severe lung disease, resynchronization therapy within the past 3 months or planned for the following 3 months, life expectancy of less than 6 months (patients with malignancy were accepted as long as they satisfied this criterion), myocardial infarction within the past 3 months, percutaneous coronary intervention within the past 1 month, coronary artery bypass surgery within the past 3 months, inadequate venous access for UF, and hematocrit ≥45%.
Protocol
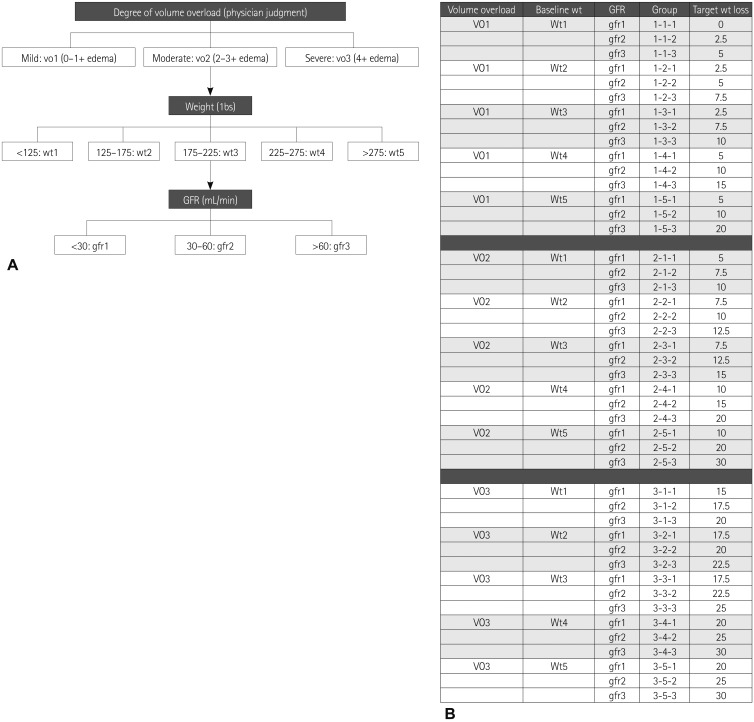
Each patient was evaluated and enrolled within 24 hours of admission. A target weight to be removed was prospectively established by the heart failure service in attendance (Drs. Chung or Menon) based on a review of the hospital chart, office chart, and patient/family interviews. If the target weight could not be established satisfactorily, it was determined using an algorithm designed for this study (Fig. 1).15) After the target weight was determined, the patients were then randomized to either UC or UF. All patients were placed on a 2-gram daily Na restricted diet. Although UC patients could be treated with any form of loop diuretic therapy as well as intravenous vasodilators or inotropes, all were given intravenous furosemide drips. No UF patients received loop diuretics after randomization. Patients were treated to target weight unless prevented by a clinical barrier, most typically, a rise in serum creatinine or the treating physician's judgment that euvolemia had been reached. After randomization, all urine and ultrafiltrate were collected and volumes recorded. Na concentrations from the first urine and ultrafiltrate samples of each day were measured, representing overnight collection. Weights were recorded each morning, after voiding, using the same scale and wearing similar clothing. Baseline as well as daily electrolytes and renal function were measured and brain natiuretic peptide levels were obtained at baseline and prior to discharge.
Fig. 1.
A: assigning a patient to a three-component group based on degree of volume overload (mild: 0.1+ edema, moderate: 2.3+ edema, severe: 4+ edema), admission weight, and admission GFR. B: based on patient group, estimated target weight to be removed is obtained from the table. GFR: glomerular filtration rate.
Ultrafiltration
Ultrafiltration was performed for each patient using the Aquadex 100 system (CHF Solutions, Inc., Gambro). Seven of the nine patients were treated through a 16-gauge two-chamber mid-line catheter placed by the intravenous team, and two patients required a central line inserted in the internal jugular vein. All UF patients received intravenous heparin to keep the activated partial thromboplastin time between 80-100 seconds.
Statistics
Discrete data were compared using Fisher's exact t-test, while continuous variables were compared using the analysis of variance. Categorical variables were compared using chi-square. Any p<0.05 was considered to be significant. All data were analyzed using the R 2.9.2 statistical package. There was a proportion of patients with missing Na data (21 of 129, 16.3%). These data were replaced with imputed values, defined as the mean of the particular patient's other Na levels during hospitalization.
Results
A total of sixteen patients were enrolled (8 patients in each arm), with largely similar baseline characteristics, although some variables tended to differ due to the small sample sizes (Table 1). Prospectively established, pre-randomization target weights and actual achieved weight loss were as shown in Table 2. Table 3 shows the weight data for individual patients. Overall, the UC group was treated with a mean daily diuretic dose of 212 mg of furosemide per day (continuous intravenous drip) and achieved 80% of the target weight reduction, while the UF group achieved 88% of the target weight loss (p=not significant). In the UC group, 3 of 8 patients lost more weight than the target, and in the UF group, 4 of 8. Randomization and initiation of study therapies took place within 24 hours of presentation.
Table 1.
Baseline demographics
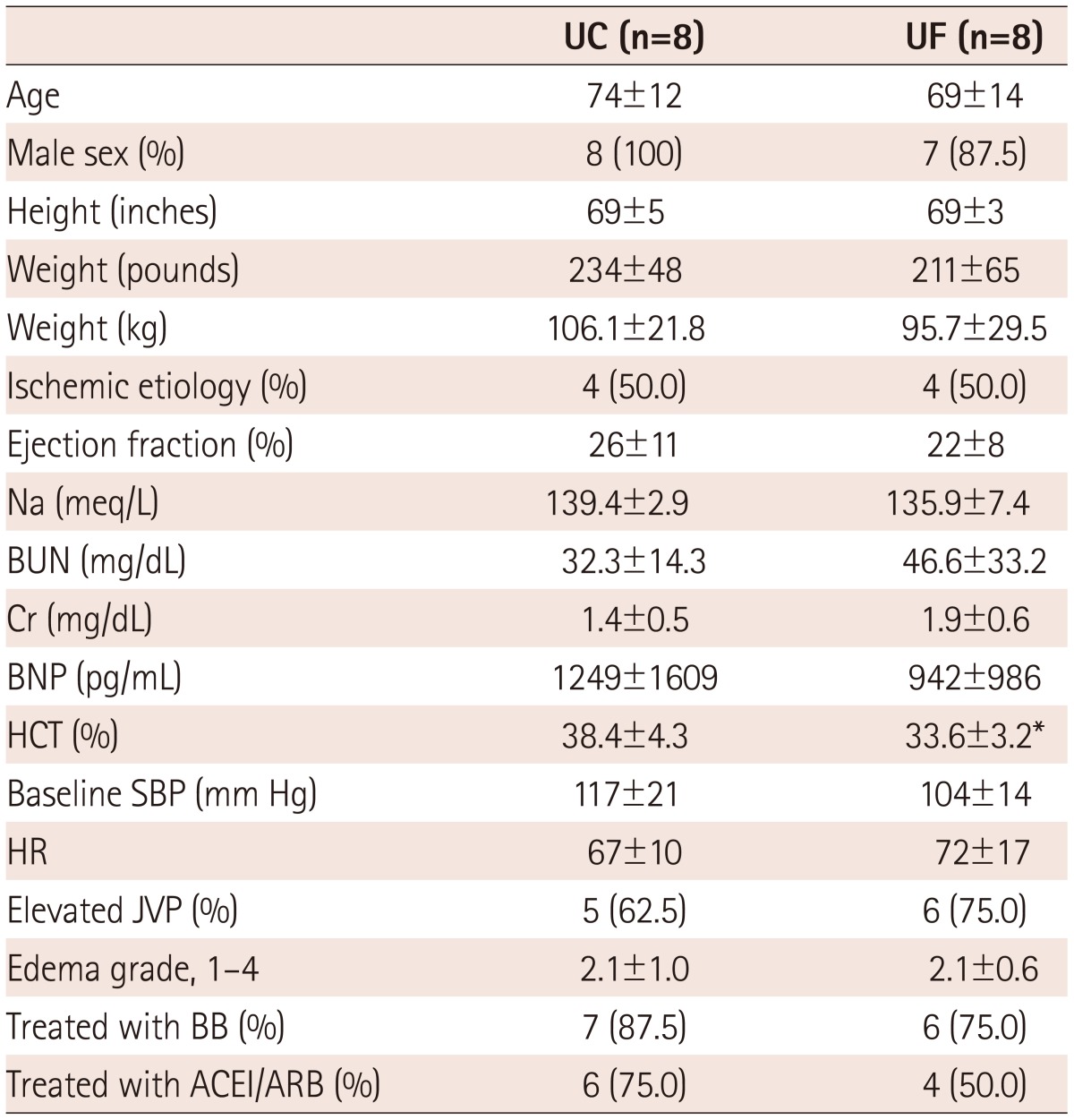
*p<0.05 vs. UC. UC: usual care, UF: ultrafiltration, Na: sodium, BUN: blood urea nitrogen, BNP: b-type naturiuretic peptide, Cr: Creatinine, HCT: hematocrit, SBP: systolic blood pressure, HR: heart rate, JVP: jugular venous pressure, BB: beta blocker, ACEI/ARB: angiotension converting enzyme inhibitor/angiotensin receptor blocker
Table 2.
Urine and ultrafiltrate production
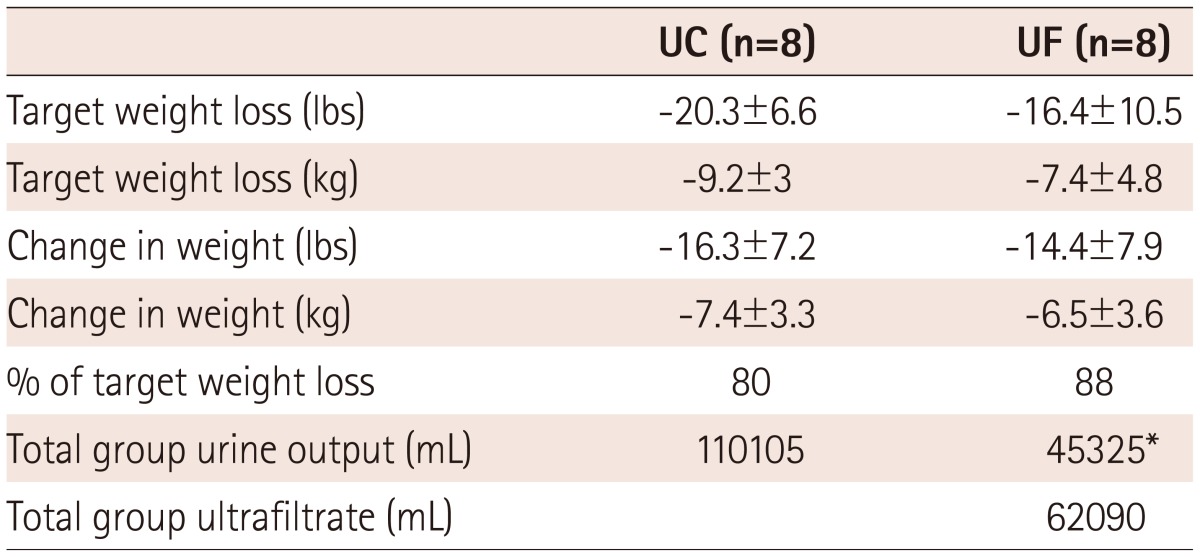
*p<0.05 vs. UC. UC: usual care, UF: ultrafiltration
Table 3.
Individual weight changes
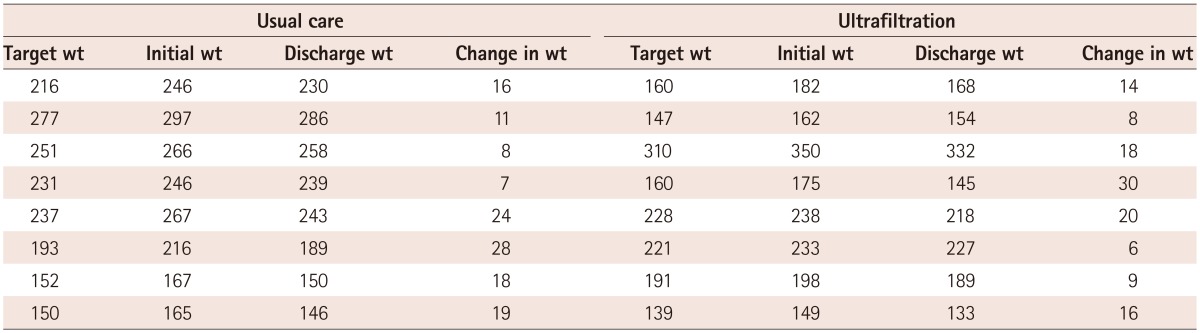
wt: weight in pounds
The amounts of urine and ultrafiltrate removed are also shown, and demonstrate similar amounts of total volume removal for both treatment groups. The mean ultrafiltrate rate was 162 mL/hour in the UF group. Mean Na concentrations in the urine and ultrafiltrate are shown in Table 4, and the absolute amounts of Na removed were calculated by considering the relative volumes. While Na concentrations were higher in the ultrafiltrate, the urine Na concentration was very low in UF patients, resulting in similar amounts of total Na removed per patient. The amount of Na removed per liter of volume (urine or ultrafiltrate or both), were similar in both groups.
Table 4.
Sodium concentrations and total sodium removed
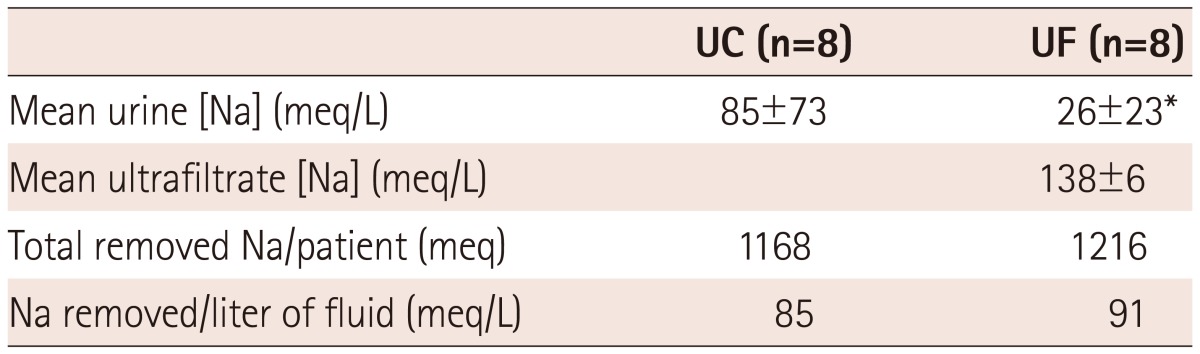
*p<0.05 vs. UC. UC: usual care, UF: ultrafiltration, Na: sodium
The mean change in creatinine between admission and discharge did not differ between the two groups, although the maximum observed change was higher in the UF group. Compared with admission levels, UF patients had a slightly lower creatinine level on discharge, while it was slightly higher at discharge among UC patients. Four patients in each group developed a transient rise in serum creatinine >0.3 mg/dL above the baseline value. Short-term readmissions and length of stay did not differ substantially (Table 5).
Table 5.
Outcomes
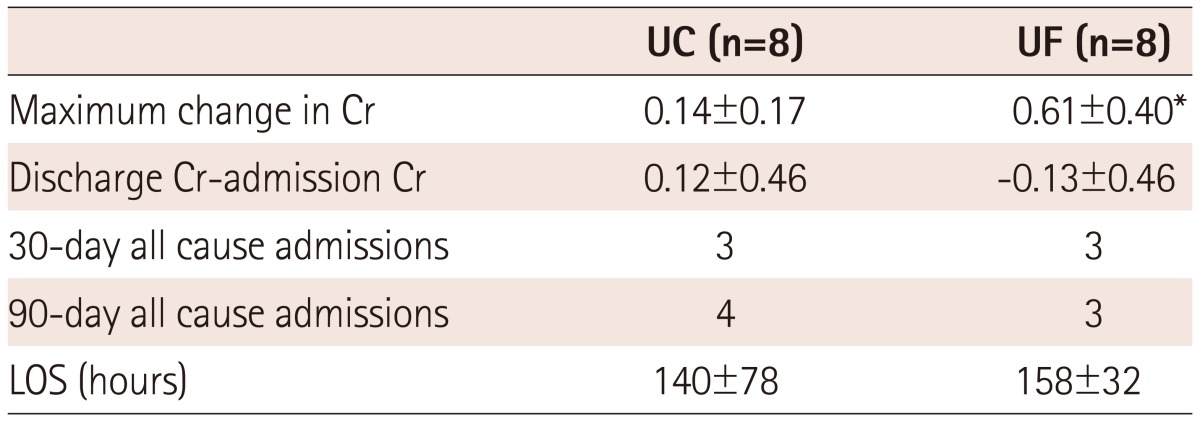
*p<0.05 vs. UC. UC: usual care, UF: ultrafiltration, Cr: serum creatinine, LOS: length of stay
Discussion
In this pilot study of target weight driven therapy utilizing a prospective randomized design, UC with loop diuretic infusion compared with UF yielded similar results with regard to volumes removed, total Na removed, and length of hospital stay. These data, particularly in the context of similar amounts of total Na removed, raise questions regarding one of the major rationales for UF use: that the higher concentration of Na in the ultrafiltrate is associated with greater amounts of Na removed, and consequent volume benefits.
The finding that a similar amount of volume is removed by each treatment may appear to be at odds with prior studies that demonstrated greater volume removal following UF compared with UC (e.g., UNLOAD). In UNLOAD, volume differences were captured at 48 hours, whereas in the present study, therapy was continued for longer, perhaps allowing for early differences to resolve. Furthermore, in the current study, target weight was prospectively established, and treatment was aimed at achieving that target, in comparison to the UNLOAD trial, where conventional clinical judgment guided the therapy. These differences in study design may explain the disparate findings regarding volumes removed in each study. In addition, short-term readmission rates were lower following UF in UNLOAD, whereas readmission rates were similar in the current study. However, the small sample size precludes any definitive commentary in relation to readmission rates. There are other data that potentially support observations made in the present study. In a small substudy of UNLOAD, Rogers et al.16) demonstrated that treatment with either UF or loop diuretic therapy for 48 hours in hospitalized ADHF patients had similar effects on renal blood flow, glomerular filtration rate, and total volume removed.
The Na concentration in the ultrafiltrate has been recognized to be similar to that of plasma and much higher than that found in diuretic induced urine.13) Given the assumed parallel movement of fluid and Na, it has been hypothesized that UF, by removing more Na, is the preferred method for the treatment of ADHF.11) However, the present study is the first in which these issues have been evaluated in a clinical setting. We observed that the urine produced by patients undergoing UF therapy rapidly becomes diluted, so that when the Na concentrations of both urine and ultrafiltrate are measured, the total amount of Na removed by the two treatment methods is similar. This observation does not negate the premise that more effective Na removal may benefit HF patients. However, the need to develop adjunctive therapies during UF to, in part, overcome renal adaptive mechanisms to produce dilute urine is evident. Total Na removal with UF, if combined with the induction of more concentrated urine, should exceed that achievable with UF or diuretic therapy alone, and may yield a more effective treatment regime for ADHF.
It was previously thought that the more effective removal of volume by UF might reduce the length of stay for hospitalized HF patients.10) In the current study, similar volumes were removed using the prospective target weight guided approach and, not surprisingly, lengths of stay were similar. Given the cost of UF, as well as the attendant risks of venous access and heparinization, strategies must be further developed to improve the efficiency with which UF is delivered. In the UNLOAD trial, during the 48-hour "treatment time", UF was actually running for only 12.3 hours (26% efficiency). Clinically, the most common barriers to more efficient UF utilization include the establishment and maintenance of venous access, filter clotting, delays in decision-making to resume therapy, hemodynamic and renal changes associated with therapy that are not rapidly addressed, and a lack of a clear automated therapeutic protocol.
Limitations
The major limitation of the current study is the small sample size. Thus, it must be interpreted as a preliminary pilot study to evaluate the physiologic effects of UF in a clinical setting. Urine and ultrafiltrate Na concentrations were not obtained with each sample, but rather, once a day, from the overnight Foley bag collection. The logistics of collecting every sample to measure Na concentration and measuring/recording all volumes would be challenging, and might optimally be performed in a metabolic study unit. Another option would be to perform 24-hour urine and ultrafiltrate collections each day. Furthermore, pre-treatment urine Na concentrations were not measured, and it was therefore not possible to demonstrate a drop in urine Na concentration with the initiation of UF. It is noteworthy, however, that most of these subjects were randomized after an initial dose of intravenous diuretic was administered in the emergency room. Hence, the first obtainable urine sample would have represented a "post-diuretic" sample. Finally, 16.3% of Na was missing and required imputation (the mean of the Na levels from other samples from the patient). Nevertheless, even the exclusion of days with missing Na levels does not significantly alter the results obtained. These limitations will be addressed in a future study design.
In conclusions, in a randomized pilot study of target weight guided therapy with UF or UC, no differences in total volume or Na removed or in length of hospital stay were observed. Isotonic fluid loss with UF was accompanied by the production of very dilute urine.
Acknowledgments
This study was supported by a grant from CHF Solutions, Inc.
References
- 1.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 2.Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 3.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 6.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44:1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 7.Neuberg GW, Miller AB, O'Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 8.Fauchald P, Forfang K, Amlie J. An evaluation of ultrafiltration as treatment of therapy-resistant cardiac edema. Acta Med Scand. 1986;219:47–52. doi: 10.1111/j.0954-6820.1986.tb03274.x. [DOI] [PubMed] [Google Scholar]
- 9.Rimondini A, Cipolla CM, Della Bella P, et al. Hemofiltration as short-term treatment for refractory congestive heart failure. Am J Med. 1987;83:43–48. doi: 10.1016/0002-9343(87)90495-5. [DOI] [PubMed] [Google Scholar]
- 10.Costanzo MR, Saltzberg M, O'Sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047–2051. doi: 10.1016/j.jacc.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 11.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 13.Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47:1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 14.Agostoni P, Marenzi G, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: failure of furosemide to provide the same result. Am J Med. 1994;96:191–199. doi: 10.1016/0002-9343(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien TM, Menon S, Stephens T, Mazur W, Chung ES. Algorithm-based assessment of target weight removal in acute decompensated heart failure. Congest Heart Fail. 2012;18:43–46. doi: 10.1111/j.1751-7133.2011.00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Rogers HL, Marshall J, Bock J, et al. A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail. 2008;14:1–5. doi: 10.1016/j.cardfail.2007.09.007. [DOI] [PubMed] [Google Scholar]