Abstract
Objective
To investigate the protective effect of extracts prepared from avocado, walnut, flaxseed and Eruca sativa seeds in a rat model of kidney dysfunction induced by intraperitoneal cisplatin.
Methods
Ethanol and petroleum ether extracts mixture was prepared from each plant. Six groups of rats were conducted; control healthy, cisplatin group and four test groups where rats were given daily oral dose of each extract mixture before cisplatin injection. Different biochemical and cytogenetic parameters and kidney histopathology were determined. Acute toxicity was tested for the nutraceuticals. Total phenolic contents, fatty acids (FA) and unsaponifiable matter were assessed in the extracts.
Results
Walnut ethanol extract showed the highest content of total phenolic. FA analysis revealed that all the studied plants were rich in unsaturated FA. Gas-liquid chromatographic investigation of the unsaponifiable matter showed the presence of campesterol, stigmasterol and β-sitosterol in all the studied plants. Cisplatin treatment induced significant increase in plasma urea, creatinine and malondialdehyde along with significant reduction of plasma albumin, total protein, catalase and total antioxidant as well as reduction in creatinine clearance. Histopathological examination proved the induction of kidney dysfunction. Some sorts of chromosomal aberration and sperm-shape abnormalities were noticed after cisplatin treatment. Administration of extracts mixtures produced improvements in biochemical, histopathological and cytogenetic parameters.
Conclusions
Administration of the studied nutraceuticals proved to possess protective role against cisplatin-induced nephrotoxicity, chromosomal aberration and abnormal sperms. All studied nutraceuticals showed complete safety.
Keywords: Nutraceutical, Cisplatin, Kidney dysfunction, Rats, Oxidative stress
1. Introduction
Kidneys are dynamic organs and represent the major control system maintaining the body haemostasis; they are affected by many chemicals and drugs that may affect their function[1]. Changes in renal function are one of the most common manifestations of severe illness. Their importance is reflected in the routine physiological and biochemical monitoring of kidney function via urine output measures and blood laboratory measurements in critically ill patients[2]. Chronic kidney disease is associated with high morbidity and mortality[3]. Oxidative stress, an imbalance between the generation of reactive oxygen species (ROS) and antioxidant defense capacity of the body, is closely associated with the majority of chronic diseases[4],[5]. Elevated ROS can cause oxidative damages to many vital components of the cells thereby expose the body to susceptibility to different diseases[5]. Oxidative stress mediates a wide range of renal impairments, ranging from acute renal failure, obstructive nephropathy and glomerular damage to chronic renal failure associated with inflammation[6]–[9]. Increased levels of malondialdehyde and F2-isoprostanes, two products of lipid peroxidation, have been reported in various clinical settings associated with renal damage[10]. Oxidative stress may alter the structure and function of the glomerulus because of the effect of ROS on mesangial and endothelial cells[11]. The glomerulus is considerably more sensitive to oxidative injuries than other nephron parts[12]. Oxidative stress involved in inflammatory lesions caused by series of mediators, including cytokines and chemokines leading to leukocyte activation, production of ROS and increased glomerular damage[13]. Also, the molecules causing inflammation could be produced by the resident renal cells[14].
There is clear evidence that diet rich in fruits, vegetables, and some sorts of oil seeds and nuts are considered as chronic diseases preventive[15]–[18]. This is due to presence of different active constituents called nutraceuticals. Phytosterols, polyunsaturated fatty acids and phenolic compounds are important constituents of food (nutraceuticals) that have been proved previously to have health benefits such as antioxidant and anti-inflammatory effects, and can be utilized in form of nutraceuticals[19],[20]. The aim of the present study was preparation and evaluation of the protective effect of different plantś extract (nutraceuticals) towards kidney dysfunction. Total phenolic contents, fatty acids and unsaponifiable matter in the nutraceuticals were determined. Assessment of safety of nutraceuticals is among the aim of the present study.
2. Materials and methods
2.1. Plant materials
Avocado (Persea americana), walnut (Juglans regia L.), flaxseed (Linum usitatissimum L.) and Eruca sativa (E. sativa) seeds were purchased from local markets, Cairo, Egypt.
2.2. Main chemical
Cisplatin (platinol, 1 mg/mL) was purchased from Mayne Pharmaceuticals (Warwickshire, UK) to induce nephrotoxicity in rats according to the method of Prabhu et al[21]. The applied dose was 7.5 mg/kg rat body weight (intraperitoneal).
2.3. Animals
Male Sprague-Dawley rats weighing 140-160 g were used in the present study. Animals were obtained from animal house of National Research Centre, Cairo, Egypt. They were kept individually in metabolic stainless steel cages; water and food were given ad libitum all over the experiment. Male and female albino mice of 21-25 g body weight were used in acute toxicity test.
2.4. Methods
2.4.1. Preparation of plant materials
Fresh avocado (whole fruit including seed) was washed by tap water and cut into small pieces. Avocado, walnut, flaxseed and E. sativa seeds were dried separately in an air circulating oven at 40 °C till complete dryness, and then they were reduced into powder.
2.4.2. Preparation of plant extracts
The dried powder of all plants' were separately placed in a continuous extraction apparatus and subjected to extraction by petroleum ether (40-60 °C) then ethanol. The solvent of each extract was completely removed by evaporation under reduced pressure at a temperature not exceeding 40 °C. All extracts were kept in deepfreeze till used.
2.4.3. Determination of total phenolic contents in extracts
Total phenolics were determined in the ethanol extracts using Folin-Ciocalteu reagent[22]. Absorbance was measured at 765 nm using UVPC spectrophotometer. The total phenolic content was expressed as gallic acid equivalent (GAE) in grams per 100 g.
2.4.4. Assessment of fatty acids, hydrocarbons and phytosterols contents in the petroleum ether extracts (oils)
The unsaponifiable fraction and fatty acid methyl esters of the studied extracts were prepared according to Association of Official Analytical Chemists[23], and were subjected to gas-liquid chromatographic (GLC) analysis of fatty acids, hydrocarbons and phytosterols.
The unsaponifiable fraction was analyzed by GLC adopting the following conditions: column: 10% OV-101 packed column; stationary phase: chromosorb W-HP; detector temperature: 290 °C; injector temperature: 28 °C; carrier gas: N2; flow-rate 30 mL/min; air flow-rate: 300 mL/min; H2 flow-rate 30 mL/min; detector: flame ionization detector; chart speed: 0.5 cm/min; oven program: initial temperature, 70 °C; final temperature: 270 °C; programmed 4 °C/min. The process lasted for 35 min at 270 °C, total time, 85 min. Identification of hydrocarbons and phytosterols contents of the unsaponifiable matter was carried out by comparison of their retention times with co-injected authentic reference compounds. Quantification was based on peak area integration.
Analysis of the methyl ester by GLC was carried out according to the following conditions: stationary phase: 10% diethylene glycosuccinate packed column; oven temperature: 170 °C; detector temperature: 300 °C; injector temperature: 250 °C; carrier gas: N2; flow-rate: 30 mL/min; air flow-rate: 350 mL/min; H2 flow-rate: 350 mL/min; detector: flame ionization detector; chart speed: 2 cm/min. Identification of the fatty acid methyl ester was carried out by direct comparison of retention times of each of the separated compounds with authentic samples of the fatty acid methyl esters analyzed under the same conditions. Quantification was based on peak area integration.
2.4.5. Diets
A balanced diet composed of 10% casein, 10% corn oil, 23.5% sucrose, 47% maize starch, 5% fiber, 3.5% salt mixture[24], and 1% vitamin mixture[25] was prepared for feeding the rats all over the experimental period.
2.4.6. Preparation of nutraceuticals
Ethanol and petroleum ether extracts of each plant was mixed together in the ratio of their presence in the whole plant. Each plant extract mixture was dispersed separately in water using the same amount of gum acacia. For the control, the vehicle was prepared through dissolving the same amount of gum acacia in water.
2.5. Experimental procedures
Thirty-six male rats were divided into six groups, each comprised six rats. Four groups were served as the test groups. Rats of the test groups were given daily oral dose of nutraceuticals prepared from avocado, E. sativa, flaxseed or walnut as 250 mg/kg rat body weight. Two groups served as control where rats were given only daily equal amounts of the vehicle received by the test groups. All treatments were given by stomach tube. After 20 d of nutraceuticalś dosing, rats of all groups except one group of control (served as normal control) were received a single intraperitoneal dose of cisplatin 7.5 mg/kg rat body weight[21]. Rats were maintained on the balanced diet all over the experiment. On the Day 5 after cisplatin administration, 24 h urine was collected from rats and blood samples were obtained from fasted animals. Heparin was used as an anticoagulant and plasma was separated by centrifugation at 3 500 r/min for 10 min and stored at -20 °C till being analyzed. Plasma malondialdehyde (MDA) was determined according to Satoh as indicator of lipid peroxidation and oxidative stress[26]. Plasma total antioxidant capacity and catalase were assessed according to Perez-Gutierrez et al.[27], and Winyard et al.[28], respectively as an antioxidant biomarker. Plasma adiponectin as inflammatory biomarker was determined using Quantikine enzyme-linked immunosorbent kit (R&D Systems, Minneapolis, MN). Plasma creatinine[29], urea[30], total protein[31], and albumin were estimated as indicator of kidney function[32]. After blood sampling, all rats were injected intraperitoneally by 2 mL colchicine (0.2 g/100 mL saline). After 2 h of injection, bone marrow was separated for studying chromosomal aberration. Also the epididymides were excised for assessing sperm-shape abnormalities. Kidney was immediately removed for histopathological examination. Creatinine was determined in the collected 24 h urine for calculation of creatinine clearance[29]. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre, Cairo, Egypt, and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
2.5.1. Histopathological examination
Kidneys from rats of all the groups were fixed in 10% formaldehyde, dehydrated in graded alcohol and embedded in paraffin. Fine sections were obtained, mounted on glass slides and counterstained with hematoxylin and eosin for light microscopic analysis[33].
2.5.2. Assessment of cytogenetic parameters
For chromosomal aberrations, bone marrow metaphases were prepared following the method of Yosida and Amano[34], and stained with 7% Giemsa stain in phosphate buffer (pH 6.8). Then 100 well spread metaphases per animal were analyzed for chromosomal aberrations. The structural aberrations included gaps, breaks, fragments and deletions, numerical aberrations, tetraploidy and polyploidy.
For sperm shape abnormalities, the epididymides were excised and minced in isotonic sodium citrate solution (2.2%). Fixed in acetic acid:methanol (1:3) once a time. Smears were prepared and sperms were stained with Eosin Y[35]. At least 1 000 sperm per animal (5 000/group) were assessed for morphological abnormalities of the sperm shape.
Acute lethal toxicity test of nutraceuticals was carried out according to Goodman et al[36]. The 24 h mortality counts among equal sized groups of mice (eight animals/group) receiving progressively increasing oral dose levels of the different extracts (up to 12 g/kg mice body weight) were recorded.
2.6. Statistical analysis
The results of animal experiments were expressed as the mean±SE and they were analyzed statistically using the One-way analysis of variance ANOVA followed by Duncan's test. In all cases P<0.05 was used as the criterion of statistical significance. For statistical analysis of cytogenetic parameters Chi-square test (2×2 contingency table) was applied.
3. Results
Total phenolic content of the ethanol extracts of different plants under study were present as 37.44, 6.34, 5.46 and 47.42 g GAE/100 g ethanol extract in avocado, E. sativa, flaxseed and walnut, respectively.
Tables 1 and 2 shows the fatty acids and unsaponifiable matter in petroleum ether extract of the studied plants, respectively. Avocado oil showed the highest content of linoleic acid (66.00%) followed by walnut oil (46.6%) then flaxseed oil (11.8%) and E. sativa oil (0.84%). Flaxseed oil showed the highest content of linolenic acid (48.4%). Total saturated fatty acids ranged from 9.0% in walnut oil to 10.86% in avocado oil. The highest content of unsaturated fatty acid was present in walnut oil (74.9%) followed by flaxseed oil 73.8% then avocado oil (70.71%) and E. sativa oil (50.04%) (Table 1). GLC investigation of the unsaponifiable matter showed the presence of campesterol, stigmasterol and β-sitosterol in all oils under investigation. The highest content of campesterol was present in avocado oil as 7.90%. Walnut oil showed the highest content of β-sitosterol (6.10%). Total phytosterol was 8.50% in flaxseed oil, 9.30% in E. sativa oil 11.03% in walnut oil and 16.2% in avocado oil. Total hydrocarbon was more or less equal in all plants; it ranges from 5.02% in avocado oil to 15.28% in E. sativa oil.
Table 1. Fatty acids' content of the petroleum ether extracts of different studied plants (as percentage of total fatty acids).
| Fatty acids | Avocado oil | E. sativa seed oil | Flaxseed oil | Walnut oil |
| Capric, C10:0 | - | 8.00 | - | - |
| Lauric, C12:0 | - | 0.72 | - | - |
| Myristic, C14:0 | - | 0.65 | - | - |
| Palmitic, C16:0 | 10.20 | 0.73 | 5.3 | 7.0 |
| Palmitoleic, C16:1 | 2.90 | 12.70 | 13.6 | - |
| Stearic, C18:0 | 0.66 | - | 4.0 | 2.0 |
| Oleic, C18:1 | 1.00 | 33.10 | - | 22.2 |
| Linoleic, C18:2 | 66.00 | 0.84 | 11.8 | 46.6 |
| Linolenic, C18:3 | 0.81 | 3.40 | 48.4 | 6.1 |
| Total saturated fatty acids | 10.86 | 10.10 | 9.3 | 9.0 |
| Total unsaturated fatty acids | 70.71 | 50.04 | 73.8 | 74.9 |
Table 2. GLC analysis of unsaponifiable matter of the different petroleum ether extracts of the plants under study (as percentage of total unsaponifiable matter).
| Compound | Avocado oil | E. sativa seed oil | Flaxseed oil | Walnut oil | |
| Hydrocarbon | C15 | 0.03 | - | 0.09 | - |
| C16 | 0.01 | - | 0.17 | - | |
| C17 | 0.09 | 0.45 | 0.50 | 0.46 | |
| C18 | 0.70 | 1.71 | 1.43 | 3.78 | |
| C20 | 0.27 | 5.72 | 0.44 | 2.95 | |
| C21 | 0.39 | 0.65 | 0.29 | - | |
| C22 | 0.23 | 1.50 | 0.69 | 0.02 | |
| C23 | 0.40 | 0.63 | 0.64 | - | |
| C24 | - | - | 1.98 | - | |
| C27 | - | - | 1.84 | 1.47 | |
| C28 | 2.90 | 4.62 | 1.69 | 3.42 | |
| Total hydrocarbon | 5.02 | 15.28 | 9.76 | 12.10 | |
| Phytosterols | Campesterol | 7.90 | 3.10 | 3.20 | 2.25 |
| Stigmasterol | 3.60 | 1.30 | 1.90 | 2.68 | |
| β-Sitosterol | 4.70 | 4.90 | 3.40 | 6.10 | |
| Total phytosterols | 16.20 | 9.30 | 8.50 | 11.03 | |
The determined biochemical parameters of different experimental groups are shown in Table 3. Plasma levels of creatinine and urea as indicator of kidney function were significantly elevated in cisplatin control when compared with all groups. Administration of all studied nutraceuticals showed significant reduction in plasma levels of creatinine and urea compared to cisplatin control but still significantly higher than normal control except for avocado nutraceutical that showed non-significant change in creatinine compared to control normal. Plasma levels of total protein and albumin were reduced significantly in cisplatin control compared with control normal. Oral administration of different nutraceuticals showed significant elevation in plasma level of total protein and albumin. Total protein was comparable to control normal in case of E. sativa and flaxseed groups. MDA level as indicator of lipid peroxidation showed significant increase in cisplatin group compared with normal group. Plants' nutraceuticals administration showed significant reduction in MDA level with different degrees when compared with cisplatin control but the levels were still significantly higher than control normal group. Plasma levels of catalase and total antioxidant capacity as antioxidant indicator were reduced significantly in cisplatin control compared to control normal group. Administration of different nutraceuticals produced significant elevation of plasma catalase and total antioxidant capacity with different degrees. Catalase levels in the groups treated with flaxseed and walnut nutraceuticals were similar to that of normal rats. Plasma level of total antioxidant capacity of E. sativa extract group was also normalized. Plasma level of adiponectin was similar among all groups.
Table 3. Biochemical parameters of different experimental groups.
| Groups | Creatinine (mg/dL) | Urea (mg/dL) | Total protein (g/dL) | Albumin (g/dL) | Adiponectin (ng/mL) | Catalase (µ/mL) | TAC (mmol/L) | MDA (nmol/mL) |
| Normal control | 0.762±0.027a | 30.1000±1.4490a | 7.200±0.114a | 3.800±0.076a | 5 060.000±62.009a | 380.600±10.700a | 1.500±0.036a | 9.000±0.344a |
| Cisplatin control | 3.100±0.153b | 204.4000±5.6370b | 6.200±0.116b | 2.700±0.081b | 5 103.000±57.800a | 200.200±8.607b | 1.000±0.035b | 19.600±0.432b |
| Change (%) | 307 | 597 | -14 | -27 | 1 | -47 | -34 | 117 |
| Avocado extract | 0.972±0.097ac | 90.7000±3.9994c | 6.700±0.102c | 3.100±0.154c | 5 081.700±64.454a | 260.900±14.882c | 1.400±0.019c | 13.800±0.304c |
| Change (%) | -69 | -56 | 8 | 15 | - | 30 | 43 | -30 |
| E. sativa extract | 1.050±0.062c | 54.0000±2.9490c | 6.900±0.181ac | 3.300±0.042c | 5 078.300±39.776a | 285.600±18.128c | 1.500±0.033ac | 13.200±0.279c |
| Change (%) | -68 | -74 | 11 | 22 | - | 43 | 50 | 33 |
| Flaxseed extract | 1.200±0.080c | 78.9000±3.4580c | 7.100±0.059ac | 3.200±0.066c | 5 063.000±34.889a | 393.600±13.906ac | 1.400±0.020c | 13.900±0.320c |
| Change (%) | -61 | -61 | 15 | 19 | - | 97 | 40 | -29 |
| Walnut extract | 1.600±0.076c | 65.4000±4.0690c | 6.500±0.106ac | 3.200±0.055c | 5 086.700±52.883a | 348.400±18.909ac | 1.300±0.048c | 14.100±0.268c |
| Change (%) | -48 | -68 | 5 | 19 | - | 74 | 30 | -28 |
In column different letters means significant difference at 0.05 probabilities. Change (%) calculated in comparison to cisplatin control.
Creatinine clearance of different experimental groups is present in Table 4. Creatinine clearance was reduced significantly in cisplatin group compared to normal group. Administration of avocado and flaxseed showed significant increase in creatinine clearance compared with both cisplatin group and normal rats while treatment with E. sativa and walnut produced significant increase in creatinine clearance compared to cisplatin control and which was comparable to normal control.
Table 4. Creatinine clearance of different experimental groups.
| Groups | Creatinine clearance (mL/min) |
| Normal control | 0.069±0.004a |
| Cisplatin control | 0.047±0.004b |
| Change (%) | -32 |
| Avocado extract | 0.104±0.013c |
| Change (%) | 121 |
| Eruca sativa extract | 0.078±0.005ac |
| Change (%) | 66 |
| Flaxseed extract | 0.088±0.004c |
| Change (%) | 87 |
| Walnut extract | 0.074±0.006ac |
| Change (%) | 57 |
In column different letters means significant difference at 0.05 probability. Change (%) calculated in comparison to cisplatin control.
3.1. Cytogenetic Study
The antigenotoxic effect of different plant nutraceuticals against the genotoxic effect of cisplatin was assessed through studying chromosomal aberration in bone marrow cells and sperm shape abnormalities.
Table 5 illustrates number and percentage of different types of chromosomal aberration in different experimental groups. The percentage induced aberrations in cisplatin control group was found to be statistically highly significant P≤0.001 in including gaps and P≤0.01 after excluding gaps compared to normal control. Breaks and fragments recorded 5.4% of structural aberrations to be the highly affected one while numerical aberrations reached 1.2%, which is a marker of carcinogenic effect.
Table 5. Number and percentage of different types of chromosomal aberration in bone marrow in different experimental groups.
| Groups | TCA |
No. and percentage (%) of metaphases |
|||||
| Including gaps | Excluding gaps | Chromatide gap | Break and fragment | Deletion | More than one aberration | Polyploidy | |
| Normal control | 3.40±0.25 | 1.80±0.20 | 8 (1.6) | 4 (0.8) | 2 (0.4) | - | 3 (0.6) |
| Cisplatin control | 13.20±0.97c | 9.20±1.06b | 20 (4.0) | 27 (5.4) | 6 (1.2) | 7 (1.4) | 6 (1.2) |
| Avocado extract | 8.00±0.73bd | 5.40±0.38ad | 13 (2.6) | 15 (3.0) | 4 (0.8) | 3 (0.6) | 5 (1.0) |
| E. sativa extract | 6.60±0.25ad | 4.60±0.51ad | 10 (2.0) | 13 (2.6) | 3 (0.6) | 3 (0.6) | 4 (0.8) |
| Flaxseed extract | 7.40±0.39ad | 4.60±0.25ad | 14 (2.8) | 10 (2.0) | 7 (1.4) | 4 (0.8) | 2 (0.4) |
| Walnut extract | 6.80±0.60bd | 4.20±0.38ad | 13 (2.6) | 11 (2.2) | 3 (0.6) | 2 (0.4) | 5 (1.0) |
Values of TCA are expressed as mean±SE. When all groups were compared with normal control, asignificant at 0.05 level, bsignificant at 0.01 level, csignificant at 0.001 level. When test groups were compared with cisplatin control: dsignificant at P<0.001. TCA: total chromosomal aberrations.
Pre-treatment of rats with E. sativa, flaxseed, walnut and avocado nutraceuticals significantly decreased the aberration percentage to be 4.6%, 4.6%, 4.2% and 5.4%, respectively, after excluding gaps compared to control cisplatin. Also, significant reduction was noticed in percentage including gaps on administration of different nutraceutical compared to cisplatin control. The previous improvement did not reach that of the control. Table 6 demonstrates the different types of sperm shape abnormalities. Cisplatin induced highly significant percentage of abnormal sperms, reached 7.29% in cisplatin control group compared to normal control. Pre-treatment with nutraceuticals inhibited this percentage significantly, the efficiency of nutraceuticals was in the following order, flax seed>avocado>E. sativa>walnut.
Table 6. Number and mean percentage of different types of sperm shape abnormalities in rat sperms in different experimental groups.
| Groups | No. of scored sperms | Abnormal sperms |
No. and percentage (%) of different types of abnormal sperms |
||||||
| No. | Percentage (%) | Amorphous | straight | Banana shape | Without hook | Coiled tail | Double head | ||
| Normal control | 5 040 | 151 | 2.92±0.13 | 32 (0.63) | 72 (1.43) | 26 (0.52) | 21 (0.42) | - | - |
| Cisplatin control | 5 143 | 375 | 7.29±0.21b | 63 (1.22) | 142 (2.76) | 86 (1.67) | 53 (1.03) | 23 (0.45) | 8 (0.16) |
| Avocado extract | 5 118 | 212 | 4.15±0.13c | 42 (0.82) | 82 (1.60) | 40 (0.78) | 23 (0.45) | 21 (0.41) | 49 (0.08) |
| E. sativa extract | 5 089 | 239 | 4.70±0.27c | 33 (0.65) | 88 (1.73) | 59 (1.16) | 44 (0.86) | 13 (0.26) | 2 (0.04) |
| Flaxseed extract | 5 044 | 194 | 3.85±0.23c | 33 (0.65) | 69 (1.37) | 53 (1.05) | 34 (0.67) | 3 (0.06) | 2 (0.04) |
| Walnut extract | 5 070 | 255 | 5.03±0.21ac | 41 (0.81) | 102 (2.01) | 58 (1.14) | 46 (0.91) | 2 (0.04) | 6 (0.12) |
All groups were compared with normal control. a: significant at 0.05 level, b: significant at 0.01 level. When test groups were compared with cisplatin control, c: significant at P<0.001.
3.2. Histopathology
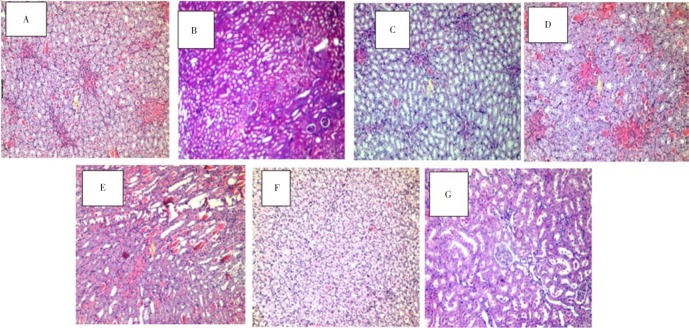
Photomicrographs of kidney sections from various treatment groups are shown in Figure 1. Histopathological examination of sections from rat kidney treated with cisplatin (control rats given cisplatin without nutraceuticals) (Figure 1E and 1F) showed severe and generalized tubular epithelial cell necrosis associated with diffuse tubular lumina (hyalinized casts), diffuse intratubular ducts, foci of fibrosis and congested vessels. The normal control rats were free from any significant pathological changes (Figure 1G).
Figure 1. Section of rat kidney of different groups (×400).
A: avocado nutraceutical+cisplatin; B: E. sativa nutraceutical+cisplatin; C: flaxseed nutraceutical+cisplatin; D: walnut nutraceutical+cisplatin; E, F: cisplatin control; G: normal control.
Administration of avocado nutraceutical afforded some protection against the nephro-toxic effect of cisplatin as compared with the unprotected treated control where rats showed only focal casts in about 30% of the medullary renal tubules, with congested vessels and scattered foci of fibrosis (Figure 1A). E. sativa extract provided complete protection against the nephrotoxic effect of the cisplatin in 70% of the rats while only 30% of them showed focal effect in the form of few casts in some tubules (Figure 1B). Flaxseed nutraceutical produced good protection aginst the nephrotoxic effect of the cisplatin with almost complete protection at histopatholgical level in about 50% of the rats. The changes are noted in half of the cases in the form of few casts inside the medullary tubules (Figure 1C). Nutraceuticals prepared from walnut exerted partially protective effect against the nephrotoxicity of cisplatin and the kidneys were only focally affected in the form of intratubular castsin in some medullary tubules (Figure 1D).
The acute lethal toxicity test revealed that all studied nutraceuticals were very safe up to the highest studied dose (12 g/kg mice body weight) which corresponds to 93 g/70 kg man body weight for human when the dose of mice was extrapolated to corresponding estimates in human adopting interspecies dosage conversion scheme[37]. This reflects the highest safety of the bioactive extracts.
4. Discussion
Induction of kidney dysfunction in experimental animals is important for studying new therapeutic agents including nutraceuticals that may possess therapeutic or protective effect towards kidney dysfunction. Experimental impairment of kidney function is induced through treatment by specific chemical or drugs or through surgical means. In the present research, kidney dysfunction was induced in rats by injection of cisplatin according to Prabhu et al[21]. Cisplatin accumulates in the renal tubular cells approximately five times its extracellular concentration[38]. Consequently, the kidney is considered to be the primary target organ for cisplatin toxicity[1],[39]. The mechanism for cisplatin nephrotoxicity may involve decreased protein synthesis, membrane peroxidation, mitochondrial dysfunction, and/or DNA injury and thereby cause tubular injury. Cytochrome P450, a group of heme proteins, may serve as a significant source of catalytic iron in cisplatin-induced nephrotoxicity[40]. Cisplatin nephrotoxicity has also been demonstrated to be mediated by DNAaseI a highly reactive renal endonuclease[38],[41]. The nephrotoxicity of the drug may also be due to complex metabolic pathways that activates the drug to become a potent kidney toxin[38],[40]. Cisplatin is known to accumulate in the mitochondria of renal epithelial cells and induces ROS in these cells via decreasing the activity of antioxidant enzymes. Reactive oxygen molecules can trigger several apoptotic mechanisms activated by cisplatin. Cisplatin induces apoptotic process by initiating the cytochrome C release and then generating superoxide[42].
Searching new natural agents that might improve renal dysfunction is considered as an important issue nowadays due to increased renal dysfunction cases in Egypt. Natural agents that possess antioxidant, anti-inflammatory and hypotensive effects are expected to possess a renal protective effect.
In the present study, increased creatinine and urea in plasma along with decreased plasma albumin, plasma total protein and creatinine clearance were noticed on treatment with cisplatin. The serum albumin concentration may be directly altered, due to increased loss of albumin through damaged glomeruli in case of renal failure[43]. These results agreed with previous researches[44],[45]. In the present study, pretreatment with different nutraceuticals provided a significant protection towards kidney dysfunction reflected in the significant reduction of the levels of plasma creatinine and urea and the increase in plasma albumin, plasma total protein and creatinine clearance.
Significant increase in lipid peroxides represented by malondialdehyde along with significant reduction of the antioxidant catalase enzymes and total antioxidant capacity reflected an elevated oxidative stress on cisplatin treatment in the present study. A significant decline in antioxidant enzymes and increase in free radicals in experimental models as well as in subjects is typical during cisplatin treatment[46]. Signs of injury including the decrease in kidney glutathione levels and increase in MDA levels in addition to decreased activities of kidney superoxide dismutase, catalase, glutathione peroxidase and glutathione s-transferase enzymes are a proof of the oxidative stress caused by cisplatin treatment and have been previously reported in a number of studies[1],[44],[47].
Thiobarbituric acid reactive substances are produced by lipid peroxidation and are considered as indicators of oxidative stress[48]. Lipid peroxidation is ascribed to a free radical-mediated chain reaction that damages cell membranes and inhibition of this process by different nutraceuticals in the present study is mainly attributed to their high ability of scavenging free radicals. Free radicals induced by cisplatin can evoke extensive tissue damage, reacting with macromolecules, such as membrane lipids, proteins and nucleic acids[49],[50]. Such elevated oxidative stress was reflected in the significant reduction in total antioxidant capacity and catalase noticed in rats treated with cisplatin in the present study. Administration of different nutraceuticals produced elevation of total antioxidant capacity and catalase thereby reducing free radicals which may be reflected in improvement of kidney function through reduction of tissue damage and reduction of inflammatory process. This effect can be noticed by the improvement of histopathological changes noticed in the present study. Also reduction in free radicals due to administration of nutraceuticals resulted in reduction of chromosomal aberration and inhibition of percentage of abnormal sperms appeared on treatment with cisplatin.
Nutraceuticals studied in the present research were prepared from avocado, walnut, flaxseed and E. sativa seeds. Avocado is a good source of bioactive compounds such as unsaturated fatty acids, vitamin E and sterols[51], and has been shown to possess antioxidants, anti-inflammatory and hypotensive effect[52],[53]. Walnut is a good source of essential fatty acids (linoleic acid), tocopherols, tocotrienols, phytosterols, tannins and other polyphenols[54]. Most phenolic compounds commonly identified in walnut seeds are phenolic acids, namely gallic, ellagic, syringic, 5-O-caffeoylquinic, caffeic, p-coumaric, ferulic and sinapic acids, and tannins, such as glansrins A, B and C, casuarinin and stenophyllarin[55],[56], thereby it is expected to possess antioxidant and anti-inflammatory effect[57]. Flaxseed has been reported to have antioxidant effect[58], and beneficial effect towards certain autoimmune kidney disease. Dietary flaxseed and flaxseed oil attenuated the decline in renal function and reduced glomerular injury[59]. It has also been shown that treatment with flaxseed oil caused a significant improvement in histopathological picture of the kidney as well as the kidney function and antioxidant status, against lead acetate-induced renal toxicity[60]. E. sativa seeds have been reported to possess a potent antioxidant, anti-inflammatory and renal protective activity in rats[61],[62]. The renal protective effect of the studied nutraceuticals might be due to presence of phenolic compounds, polyunsaturated fatty acids, and phytosterols as shown from phytochemical analysis in the present study. Phenolic compounds possess several therapeutic properties including antioxidant and anti-inflammatory activity thereby they are free radicals scavengers, singlet oxygen quenchers, and metal chelators, in addition, a direct relationship between total phenolic content and antioxidant activity has been reported[63],[64]. Phytosterols have been reported to possess antioxidant and anti-inflammatory activity[65]. Previous studies showed polyunsaturated fatty acids to possess anti-inflammatory activity[66].
Cisplatin induced chromosomal aberration and sperm shape abnormalities might be due to increased oxidative stress during cisplatin treatment. Reduction of such chromosomal aberration and sperm shape abnormalities on nutraceutical administration might be ascribed to their antioxidant activity due to presence of the previously mentioned bioactive compounds especially phenolic compounds. Many of the phenolic compounds have properties including antioxidant, anti-mutagenic, anti-carcinogenic and anti-inflammatory effects that might potentially be beneficial in protecting the stability of the genome[67]. A significant cancer risk reduction by avocado extract, E. sativa and walnut extract has been reported which might be related to prevention of DNA damage[68]–[70].
Cisplatin-induced oxidative stress might be responsible for the induced kidney dysfunction reflected in biochemical parameters and histopathological changes. Cisplatin also resulted in chromosomal aberration and increased percentage of abnormal sperms. Administration of the studied nutraceuticals proved to possess protective role against cisplatin-induced nephrotoxicity, chromosomal aberration and abnormal sperms. All studied nutraceuticals showed complete safety.
Acknowledgments
The authors thank the National Research Centre, Cairo, Egypt for financing this work. The present research is a part of a project entitled “Prevention and Management of Some Chronic Diseases (Renal and hepatic) by Nutraceuticals and Functional Foods” (2010-2013). This paper is granted and totally funded by National Research Centre, Cairo, Egypt (Ninth plan of National Research Centre) (Grant No. 9130103).
Comments
Background
Kidneys are vital organs in the body. They have an important role in body hemostasis. So natural agents that may guard against renal dysfunction are needed.
Research frontiers
The present research studied kidney protection by different plant food extracts through assessing different biomarkers of renal functions and examining the histopathology of the kidney.
Related reports
Renal dysfunction rat model were reported using cisplatin or by high adenine phosphate diet. These models were used to evaluate new agents that may protect from renal dysfunction.
Innovations and breakthroughs
Avocado, walnut, flaxseed and E. sativa are rich in antioxidant and anti-inflammatory bioactive constituents. In the present study, the others utilized these previous activities to evaluate their protective effect towards kidneys.
Applications
This study recommends the use of avocado, walnut, flaxseed and E. sativa as renal protective natural agent.
Peer review
This is an important research that showed the possible protection from kidney dysfunction by administration of avocado, walnut, flaxseed and E. sativa extracts.
Footnotes
Foundation Project: Supported by National Research Centre, Cairo, Egypt (Ninth plan of National Research Centre) (Grant No. 9130103).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Maliakel DM, Kagiya TV, Nair CK. Prevention of cisplatin-induced nephrotoxicity by glucosides of ascorbic acid and alpha-tocopherol. Exp Toxicol Pathol. 2008;60(6):521–527. doi: 10.1016/j.etp.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins R. New biomarkers of acute kidney injury and the cardio-renal syndrome. Korean J Lab Med. 2011;31:72–80. doi: 10.3343/kjlm.2011.31.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen CP, Matsushita K, Coresh J, Iseki K, Islam M, Katz R, et al. Relative risks of chronic kidney disease for mortality and end-stage renal diseaseacross races are similar. Kidney Int. 2014 doi: 10.1038/ki.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC, et al. Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J. 2014;35:1172–1177. doi: 10.1093/eurheartj/ehu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact. 2008;172:176–184. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari S, Galanello R. Renal aspects of thalassaemia a changing paradigm. Eur J Haematol. 2012;89(3):187–197. doi: 10.1111/j.1600-0609.2012.01819.x. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. doi: 10.1371/journal.pone.0067474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S, Kim CS, Lee J, Suk Kim J, Kim J. Effect of regular exercise on the histochemical changes of d-galactose-induced oxidative renal injury in high-fat diet-fed rats. Acta Histochem Cytochem. 2013;46(4):111–119. doi: 10.1267/ahc.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conner TA, McQuade C, Olp J, Pai AB. Effect of intravenous vitamin C on cytokine activation and oxidative stress in end-stage renal disease patients receiving intravenous iron sucrose. Biometals. 2012;25(5):961–969. doi: 10.1007/s10534-012-9562-6. [DOI] [PubMed] [Google Scholar]
- 10.Günal SY, Ustündağ B, Günal Aİ. The assessment of oxidative stress on patients with chronic renal failure at different stages and on dialysis patients receiving different hypertensive treatment. Indian J Clin Biochem. 2013;28(4):390–395. doi: 10.1007/s12291-013-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav AK, Kumar V, Jha V. Heat shock proteins 60 and 70 specific proinflammatory and cytotoxic response of CD4+CD28null cells in chronic kidney disease. Mediators Inflamm. 2013 doi: 10.1155/2013/384807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi X, Nickeleit V, James LR, Maeda N. α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. J Diabetes Complications. 2011;25(3):193–201. doi: 10.1016/j.jdiacomp.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Wu Y, Xu Y, Sun L, Zhang X. Human umbilical mesenchymal stem cells attenuate the progression of focal segmental glomerulosclerosis. Am J Med Sci. 2013;346(6):486–493. doi: 10.1097/MAJ.0b013e3182831777. [DOI] [PubMed] [Google Scholar]
- 14.Lin Q, Chen Y, Lv J, Zhang H, Tang J, Gunaratnam L, et al. Kidney injury molecule-1 expression in IgA nephropathy and its correlation with hypoxia and tubulointerstitial inflammation. Am J Physiol Renal Physiol. 2014;306:F885–F895. doi: 10.1152/ajprenal.00331.2013. [DOI] [PubMed] [Google Scholar]
- 15.Piscopo S. The Mediterranean diet as a nutrition education, health promotion and disease prevention tool. Public Health Nutr. 2009;12:1648–1655. doi: 10.1017/S1368980009990504. [DOI] [PubMed] [Google Scholar]
- 16.Luciano RL. Acute kidney injury from cherry concentrate in a patient with CKD. Am J Kidney Dis. 2014;63(3):503–505. doi: 10.1053/j.ajkd.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Sun J, Petrova K, Yang X, Greenhaw J, Salminen WF, et al. Metabolomics evaluation of the effects of green tea extract on acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 2013;62:707–721. doi: 10.1016/j.fct.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68:571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep. 2009;8:206–213. doi: 10.1249/JSR.0b013e3181ae8959. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum CC, O'Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16:32–40. [PubMed] [Google Scholar]
- 21.Prabhu VV, Kannan N, Guruvayoorappan C. 1,2-Diazole prevents cisplatin-induced nephrotoxicity in experimental rats. Pharmacol Rep. 2013;65(4):980–990. doi: 10.1016/s1734-1140(13)71079-x. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 23.Association of Official Analytical Chemists . Official methods of analysis of AOAC international. 17th ed. Gaithersburg, MD, USA: Association of Analytical Communities; 2000. [Google Scholar]
- 24.Briggs GM, Williams MA. A new mineral mixture for experimental rat diets and evaluation of other mineral mixtures. Fed Proc. 1963;22:261–266. [Google Scholar]
- 25.Morcos SR. The effect of protein value of the diet on the neurological manifestations produced in rats by beta-immodipropionitrile. Br J Nutr. 1967;21:269–274. doi: 10.1079/bjn19670029. [DOI] [PubMed] [Google Scholar]
- 26.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Gutierrez RM, Muñiz-Ramirez A, Gomez YG, Ramírez EB. Antihyperglycemic, antihyperlipidemic and antiglycation effects of Byrsonima crassifolia fruit and seed in normal and streptozotocin-induced diabetic rats. Plant Foods Hum Nutr. 2010;65(4):350–357. doi: 10.1007/s11130-010-0181-5. [DOI] [PubMed] [Google Scholar]
- 28.Winyard PG, Ryan B, Eggleton P, Nissim A, Taylor E, Lo Faro ML, et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem Soc Trans. 2011;39(5):1226–1232. doi: 10.1042/BST0391226. [DOI] [PubMed] [Google Scholar]
- 29.Houot O. Interpretation of clinical laboratory tests. Seal Beach : Biomedical publications; 1985. [Google Scholar]
- 30.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rheinhold JG. Total protein, albumin and globulin in standard methods of clinical chemistry. New York: Academic Press Inc; 1953. p. 88. [Google Scholar]
- 32.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green.1971. Clin Chim Acta. 1997;258:21–30. doi: 10.1016/s0009-8981(96)06447-9. [DOI] [PubMed] [Google Scholar]
- 33.Ekor M, Emerole GO, Farombi EO. Phenolic extract of soybean (Glycine max) attenuates cisplatin-induced nephrotoxicity in rats. Food Chem Toxicol. 2010;48:1005–1012. doi: 10.1016/j.fct.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Yosida TH, Amano K. Autosomal polymorphism in laboratory bred and wild Norway rat, Rattus norvegicus. Chromosoma. 1965;16:658–667. doi: 10.1007/BF00285115. [DOI] [PubMed] [Google Scholar]
- 35.Wyrobek AJ, Bruce WR. The induction of sperm-shape abnormalities in mice and humans. In: Hollaender A, de Serres FJ, editors. Chemical mutagens: principles and methods for their detection. New York: Plenum Press; 1978. pp. 255–285. [Google Scholar]
- 36.Goodman AG, Goodman LS, Gilman A. Goodman and Gilman's: the pharmacological basis of therapeutics. 6th ed. New York: Macmillan; 1980. pp. 1602–1615. [Google Scholar]
- 37.Paget P, Barnes T. Evaluation of drug activities pharmacometrics. New York: Academic Press; 1964. pp. 135–140. [Google Scholar]
- 38.Hussein A, Ahmed AA, Shouman SA, Sharawy S. Ameliorating effect of DL-α-lipoic acid against cisplatin-induced nephrotoxicity and cardiotoxicity in experimental animals. Drug Discov Ther. 2012;6(3):147–156. [PubMed] [Google Scholar]
- 39.Shalby AB, Assaf N, Ahmed HH. Possible mechanisms for N-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicol Mech Methods. 2011;21(7):538–546. doi: 10.3109/15376516.2011.568985. [DOI] [PubMed] [Google Scholar]
- 40.Peres LA, da Cunha AD., Jr Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol. 2013;35(4):332–340. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 41.Hodeify R, Megyesi J, Tarcsafalvi A, Safirstein RL, Price PM. Protection of cisplatin cytotoxicity by an inactive cyclin-dependent kinase. Am J Physiol Renal Physiol. 2010;299(1):F112–F120. doi: 10.1152/ajprenal.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naqshbandi A, Rizwan S, Khan MW, Khan F. Dietary flaxseed oil supplementation ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Hum Exp Toxicol. 2013;32(4):385–394. doi: 10.1177/0960327112438929. [DOI] [PubMed] [Google Scholar]
- 43.Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 2009;47(7):1480–1484. doi: 10.1016/j.fct.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Atasayar S, Gürer-Orhan H, Orhan H, Gürel B, Girgin G, Ozgüneş H. Preventive effect of aminoguanidine compared to vitamin E and C on cisplatin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2009;61(1):23–32. doi: 10.1016/j.etp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Saad AA, Youssef MI, El-Shennawy LK. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47(7):1499–1506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 46.Longo V, Gervasi PG, Lubrano V. Cisplatin induced toxicity in rat tissues: the protective effect of Lisosan G. Food Chem Toxicol. 2011;49(1):233–237. doi: 10.1016/j.fct.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boghdady NA. Antioxidant and antiapoptotic effects of proanthocyanidin and ginkgo biloba extract against doxorubicin-induced cardiac injury in rats. Cell Biochem Funct. 2013;31(4):344–351. doi: 10.1002/cbf.2907. [DOI] [PubMed] [Google Scholar]
- 49.Sindhu ER, Kuttan R. Carotenoid lutein protects the kidney against cisplatin-induced acute renal failure. J Environ Pathol Toxicol Oncol. 2013;32(1):21–28. doi: 10.1615/jenvironpatholtoxicoloncol.2013004933. [DOI] [PubMed] [Google Scholar]
- 50.Nicolson GL, Conklin KA. Molecular replacement in cancer therapy: reversing cancer metabolic and mitochondrial dysfunction, fatigue and the adverse effects of cancer therapy. Cancer Genomics Proteomics. 2006;3:159–168. [PubMed] [Google Scholar]
- 51.Plaza L, Sánchez-Moreno C, de Pascual-Teresa S, de Ancos B, Cano MP. Fatty acids, sterols, and antioxidant activity in minimally processed avocados during refrigerated storage. J Agric Food Chem. 2009;57:3204–3209. doi: 10.1021/jf900541r. [DOI] [PubMed] [Google Scholar]
- 52.Gorinstein S, Haruenkit R, Poovarodom S, Vearasilp S, Ruamsuke P, Namiesnik J, et al. Some analytical assays for the determination of bioactivity of exotic fruits. Phytochem Anal. 2010;21:355–362. doi: 10.1002/pca.1207. [DOI] [PubMed] [Google Scholar]
- 53.Heinecke LF, Grzanna MW, Au AY, Mochal CA, Rashmir-Raven A, Frondoza CG. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthritis Cartilage. 2010;18:220–227. doi: 10.1016/j.joca.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Pereira JA, Oliveira I, Sousa A, Ferreira I, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Pribis P, Bailey RN, Russell AA, Kilsby MA, Hernandez M, Craig WJ, et al. Effects of walnut consumption on cognitive performance in young adults. Br J Nutr. 2011;107(9):1393–1401. doi: 10.1017/S0007114511004302. [DOI] [PubMed] [Google Scholar]
- 56.Bolling BW, Chen CY, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24(2):244–275. doi: 10.1017/S095442241100014X. [DOI] [PubMed] [Google Scholar]
- 57.Muthaiyah B, Essa MM, Chauhan V, Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011;36:2096–2103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upadhyay R, Mohan Rao LJ. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit Rev Food Sci Nutr. 2013;53(9):968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- 59.Caligiuri SP, Love K, Winter T, Gauthier J, Taylor CG, Blydt-Hansen T, et al. Dietary linoleic acid and α-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J Nutr. 2013;143(9):1421–1431. doi: 10.3945/jn.113.177360. [DOI] [PubMed] [Google Scholar]
- 60.Abdel Moneima AE, Dkhila MA, Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater. 2011;194:250–255. doi: 10.1016/j.jhazmat.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 61.Sarwar AM, Kaur G, Jabbar Z, Javed K, Athar M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem Toxicol. 2007;45:910–920. doi: 10.1016/j.fct.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Yehuda H, Khatib S, Sussan I, Musa R, Vaya J, Tamir S. Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (MTBI) isolated from rocket (Eruca sativa) seeds. Biofactors. 2009;35:295–305. doi: 10.1002/biof.32. [DOI] [PubMed] [Google Scholar]
- 63.Costa RM, Magalhães AS, Pereira JA, Andrade PB, Valentão P, Carvalho M, et al. Evaluation of free radical scavenging and antihemolyticactivities of Cydonia oblonga leaf: a comparative study with green tea (Camellia sinensis) Food Chem Toxicol. 2009;47:860–865. doi: 10.1016/j.fct.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Liu R, Halim L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Sci Technol. 2009;42:1–8. [Google Scholar]
- 65.Dreher ML. Pistachio nuts: composition and potential health benefits. Nutr Rev. 2012;70(4):234–240. doi: 10.1111/j.1753-4887.2011.00467.x. [DOI] [PubMed] [Google Scholar]
- 66.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 67.Xie C, Wang C, Wang X, Yang X. Two modified RNA extraction methods compatible with transcript profiling and gene expression analysis for cotton roots. Prep Biochem Biotechnol. 2013;43(5):500–511. doi: 10.1080/10826068.2012.759967. [DOI] [PubMed] [Google Scholar]
- 68.Jackson MD, Walker SP, Simpson-Smith CM, Lindsay CM, Smith G, McFarlane-Anderson N, et al. Associations of whole-blood fatty acids and dietary intakes with prostate cancer in Jamaica. Cancer Causes Control. 2012;23(1):23–33. doi: 10.1007/s10552-011-9850-4. [DOI] [PubMed] [Google Scholar]
- 69.Lamy E, Schröder J, Paulus S, Brenk P, Stahl T, Mersch-Sundermann V. Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food Chem Toxicol. 2008;46:2415–2421. doi: 10.1016/j.fct.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 70.Aithal BK, Kumar SM, Rao BN, Udupa N, Rao BS. Juglone, a naphthoquinone from walnut exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol Int. 2009;33:1039–1049. doi: 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]