Abstract
Assessment of the health risks resulting from exposure to ambient polycyclic aromatic hydrocarbons (PAH) is limited by a lack of environmental exposure data among the general population. This study characterized personal exposure and ambient concentrations of PAH in the Village of Waterfront South (WFS), an urban community with many mixed sources of air toxics in Camden, New Jersey, and CopeWood/Davis Streets (CDS), an urban reference area located ~1 mile east of WFS. A total of 54 and 53 participants were recruited from non-smoking households in WFS and CDS, respectively. In all, 24-h personal and ambient air samples were collected simultaneously in both areas on weekdays and weekends during summer and winter. The ambient PAH concentrations in WFS were either significantly higher than or comparable to those in CDS, indicating the significant impact of local sources on PAH pollution in WFS. Analysis of diagnostic ratios and correlation suggested that diesel truck traffic, municipal waste combustion and industrial combustion were the major sources in WFS. In such an area, ambient air pollution contributed significantly to personal PAH exposure, explaining 44–96% of variability in personal concentrations. This study provides valuable data for examining the impact of local ambient PAH pollution on personal exposure and therefore potential health risks associated with environmental PAH pollution.
Keywords: PAH, personal exposure, ambient measurement, association between personal and ambient levels
Introduction
Polycyclic aromatic hydrocarbons (PAH) are formed during the incomplete combustion of organic materials (e.g., coal, oil, biomass, and gasoline and diesel fuels) and are ubiquitous in the atmosphere. PAH compounds are one of the first pollutants to be identified as human carcinogens. Occupational exposure to PAH entails substantial risks that have been associated with adverse health effects, such as lung, skin, and bladder cancers (Armstrong et al., 1986; IARC, 1987; Steineck et al., 1989). However, health effects associated with environmental exposure to PAH in community settings remain unclear. Therefore, there is a need to characterize exposure of general population to ambient PAH so that potential health effects associated with PAH exposure can be examined.
Some studies have been conducted to characterize PAH exposure in community settings (Lioy et al., 1988; Chuang et al., 1999; Fiala et al., 2001; Georgiadis et al., 2001; Naumova et al., 2002; Fromme et al., 2004; Tonne et al., 2004; Ohura et al., 2005; Choi et al., 2008; Saborit et al., 2009). For example, Lioy et al. (1988) measured indoor concentrations of benzo(a)pyrene (BaP) in homes in Phillipsburg, New Jersey (NJ), and estimated personal exposure to BaP based on the indoor concentrations and the outdoor concentrations measured concurrently. The Relationship of Indoor, Outdoor and Personal Air study (RIOPA) monitored indoor and outdoor concentrations of PAH in Los Angeles, California; Elizabeth, NJ; and Houston, Texas. It was found that the outdoor concentrations in the three cities were significantly different, and the variability in the indoor concentrations was substantially greater than that in outdoors (Naumova et al., 2002). Tonne et al. (2004) measured personal exposure to PAH for pregnant minority women in New York City. They found particularly high exposure to pyrene (PY) among the pregnant women, compared with those previously reported. The above studies provide baseline data on community PAH air pollution and personal exposure to PAH.
However, data obtained from previous studies are not suitable for evaluating health effects caused by ambient PAH air pollution. This is because indoor sources are dominant of personal PAH exposure in most cases (Fiala et al., 2001; Georgiadis et al., 2001; Naumova et al., 2002; Tonne et al., 2004; Ohura et al., 2005; Saborit et al., 2009). As reported by Tonne et al. (2004), personal exposures of pregnant women to benzo(b)fluoranthene (BbFA), benzo(k)fluoranthene (BkFA), BaP, benzo(g,h,i)perylene and the sum of B2 PAH (PAH compounds that have been identified as probable human carcinogens by the USEPA Integrated Risk Information System) were not associated with the time spent outdoors but with indoor sources, that is, residential heating and indoor burning of incense. The results from the RIOPA study suggested that the concentrations of 3-ring PAH in residences were significantly affected by indoor sources (Naumova et al., 2002). In addition, many previous studies simultaneously measured PAH only in indoor and outdoor environments but not personal concentration (Lioy et al., 1988; Chuang et al., 1999; Fiala et al., 2001; Naumova et al., 2002; Fromme et al., 2004). It is not accurate to use the indoor and outdoor data to estimate personal concentration and assess the impact of ambient PAH air pollution on personal exposure, because personal exposure is expected to be strongly influenced by individual activities (Waldman et al., 1991; Tonne et al., 2004). Further, personal and concurrent community PAH air pollution data for the 16 USEPA priority PAH species are still scarce. The target PAH in previous studies are often limited to BaP and 6–8 carcinogenic species (Lioy et al., 1988; Georgiadis et al., 2001; Tonne et al., 2004; Choi et al., 2008; Saborit et al., 2009). Such knowledge background limits the ability to conduct an accurate assessment of the health risks resulting from exposure to PAH in ambient air, particularly for people living in areas with mixed emission sources. Many times the mixed sources of PAH are located in areas where minority and economically disadvantaged groups reside.
To advance our understanding of the impacts of ambient air pollution on personal PAH exposure and therefore potential health effects, we characterized personal exposure to PAH and other air contaminants, including PM2.5, VOCs and aldehydes in a “hot spot” of air pollution —the Village of Waterfront South (WFS) in Camden, NJ. This paper presents (1) the measurements of personal and ambient concentrations of PAH in WFS and in a reference area — the Copewood/Davis Streets (CDS); (2) the locational and temporal variations of personal and ambient concentrations of PAH; and (3) the contribution of local ambient PAH air pollution to personal exposure in both areas. The findings for the other air pollutants will be published separately.
Materials and methods
Study Areas
The communities of WFS and CDS have been previously introduced in detail by Zhu et al. (2008) and Wu et al. (2010). Briefly, WFS is located in South Camden, where many stationary (27 industrial and manufacturing facilities) and mobile (270–820 trucks/day) sources of air toxics have been identified by the New Jersey Department of Environmental Protection (NJDEP, 2005). In addition, east of WFS is Interstate 676 (I-676), one of the major commuting routes between NJ and Philadelphia, with a traffic volume of ~80,000 vehicles/day (NJDOT, 2006). Almost all WFS residents live within 200 m from at least one stationary source of air toxics or local roads (US Census, 2000). The CDS is located ~1 mile east of WFS but without a large number of mixed industrial facilities nearby (<1000 m). It is surrounded by NJ-168 on the west side (<100 m) with a traffic volume of ~25,000 vehicles/day, and by Haddon Avenue on the east side (<100 m) with a traffic volume of ~8000 vehicles/day (NJDOT, 2006).
The residents in WFS and CDS share similar characteristics in terms of ethnic makeup and socioeconomic status. In these two communities, the percentages of Black (58% in WFS and 64% in CDS) and Hispanic (27% in WFS and 15% in CDS) are higher than the corresponding state averages (12.9% and 12.5%, respectively). The household incomes are ~$22,000 in WFS and $25,000 in CDS, much lower than the state and national averages ($55,136 and $41,994, respectively, US Census, 2000).
Subjects Recruitment
To establish a subject recruitment program, a variety of activities and approaches were used in the study. Subjects were recruited with assistance of local community leaders and a local liaison. Recruitment was through direct contact with local residents, community meetings and events, fliers, advertisement in local newspapers, and word of mouth. Through above approaches, most subjects were successfully recruited in year 1 of the study, and the recruitment was completed within 2 years. A total of 107 subjects (recruitment goal was 100 subjects) from non-smoking households were recruited. Among them, 54 participants (37 adults and 17 children) were recruited from the WFS and 53 participants (34 adults and 19 children) from the CDS. Among the 71 adults, 48 participants were women and 23 participants were men. We like to note that the recruitment was not through a strict stratified randomization procedure. Nonetheless, the study cohort is representative for the entire neighborhood population except for gender. Females were over-sampled by 10–20% (Wu et al., 2010). This is because we intentionally recruited subjects being in the study areas for most of the day (>12 h) during the 24-h sampling period so that the impact of local air pollution on personal exposure can be examined. The consent forms, questionnaires and study protocols were approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey (UMDNJ) before the start of the study.
Ambient and Personal Sampling
During the study, the ambient and personal samplings were performed concurrently for each participant on 4 separate days from January 2004 through July 2006: two in summer and two in winter; one weekday and one weekend during each season. The Sacred Heart Church and the NJDEP ambient monitoring site were selected as the fixed sampling sites for ambient PAH sampling in WFS and in CDS, respectively (Figure 1). These two sampling sites were within or close to residential areas and were void of nearby (<10 ft) emission sources of PAH. PAH in PM2.5 and in the gas phase were collected for 24 h by a SKC Leland Legacy personal sampler (SKC, Eighty-Four, PA, USA) containing one 37-mm Teflon filter and two polyurethane foams (PUF) connected in series downstream of the filter. For ambient sampling, the sampler was deployed on a 6-ft sampling rack equipped with a covered top to provide a barrier for the sampler from rain/snow. For personal sampling, the sampler was placed in a sampling backpack, and the sampling head was attached to the shoulder strap of the backpack to be close to the personal breathing zone. Before each sampling trip, the pump was calibrated and leak tested. The flow rate was set to be ~9 l/min for the ambient sampling and ~4 l/min for the personal sampling, and was measured in an average of 10 successive readings on a Dry-Cal meter (SKC) before and after each sampling period. For a valid sample, the change in flow rate during a sampling period should be <15% and the sample collection time should be longer than 20 h (~80% of the target duration). Among the 107 participants recruited in this study, 80 completed the 4 planned measurements, 6 completed 3 measurements, 12 completed 2 measurements, and 9 was sampled once. Relocation was the primary reason for discontinuation of participation. In all, 93% of planned personal measurements were made successfully.
Figure 1.
A map of the WFS and CDS neighborhoods and the location of the fixed sampling sites (star marks).
Sample Analysis
This study measured the concentrations of the 16 USEPA priority PAH, including naphthalene (NAP, 2-ring), acenaphthylene (ACEN, 3-ring), acenaphthene (ACE, 3-ring), fluorene (FLN, 3-ring), phenanthrene (PHE, 3-ring), anthracene (AN, 3-ring), fluoranthene (FL, 4-ring), PY (4-ring), benz(a)anthracene (BaA, 4-ring), chrysene (CHR, 4-ring), BbFA (5-ring), BkFA (5-ring), BaP (5-ring), indeno(1,2,3,-cd)pyrene (IP, 6-ring), dibenz(a,h)anthracene (DBahA, 5-ring), and benzo(g,h,i)perylene (BghiP, 6-ring). The PAH concentrations measured on the filter and PUF samples were summed for data analysis because the gas/ particle partitioning was not the primary interest of this study. The recoveries of PAH were obtained by measuring the surrogate standards (d8-naphthalene, d10-phenanthrene, d10-pyrene, and d12-benzo(a)pyrene) that were added to each sample before extraction. Briefly, the samples were Soxhlet extracted for 16 h. Then, the extracts were concentrated to 50–100 μl and analyzed after the addition of internal standards (d10-acenaphthene and d10-anthracene) by a Varian 3900 GC with a Saturn 2000MS ion trap detector (Varian, Walnut Creek, CA, USA). The analytical column used for PAH separation was a VF-5MS capillary column (30 m × 0.25 mm with film thickness of 0.25 μm). Detailed analytical condition of GC/MS can be found in Fan et al. (2006).
The overall precision was calculated as a coefficient of variation of the PAH concentrations in the duplicate ambient samples collected across the entire study period. The precision was 23.8–48.8% for the 16 target PAH species. The observed low precision for IP and BghiP (>40%) was due to their low concentrations, which were below the method detection limits (MDLs) in ~60% of the duplicate samples. The mean recovery was 63.3 ± 38.9% for d8-naphthalene, 88.2 ± 29.6% for d10-phenanthrene, 66.2 ± 28.4% for d10-pyrene, and 54.7±33.2% for d12-benzo(a)-pyrene. The final PAH concentrations in the samples were corrected with the recoveries of the surrogates. No PAH were detected in the solvent blanks or the lab blanks. The 5- and 6-ring PAH were not detected in either filter or PUF field blanks. A typical level of ~30 ng of NAP was detected in both filter and PUF field blanks, equivalent to ~2 ng/m3 of NAP for a sampling volume of 14.4 m3 (i.e., sampling rate at 10 l/min for 24 h), and <10 ng of the 3- and 4-ring PAH was detected on PUF field blanks, equivalent to ~0.7 ng/m3 for a sampling volume of 14.4 m3. The MDLs, which were defined as three times of the SD of the field blanks collected from the entire study, are presented in Table 1. For 5- and 6-ring PAH compounds, which were not detected in the field blanks, seven spiked samples were analyzed and the SD of the spiked samples was used to determine the MDLs.
Table 1.
Ambient concentrations and personal exposures of PAHa in WFS and CDS (ng/m3).
| MDL | WFS
|
CDS
|
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM | GSD | Median | Range | GM | GSD | Median | Range | |||
| Ambient concentration | ||||||||||
| NAP | 2.25 | 7.55 | 3.08 | 9.78 | 0.13–144 | 7.49 | 2.67 | 7.62 | 0.80–53.0 | 0.759 |
| ACEN | 0.15 | 1.40 | 4.05 | 1.78 | 0.12–51.0 | 0.99 | 4.47 | 1.20 | 0.07–11.0 | 0.002 |
| ACE | 0.44 | 2.95 | 2.53 | 3.03 | 0.27–190 | 2.64 | 2.26 | 2.71 | 0.44–36.7 | 0.183 |
| FLN | 2.03 | 7.33 | 2.51 | 8.38 | 0.31–200 | 6.59 | 2.39 | 6.12 | 0.25–43.4 | 0.226 |
| PHE | 1.84 | 8.95 | 3.18 | 9.14 | 0.12–111 | 8.14 | 2.64 | 7.47 | 0.95–49.4 | 0.345 |
| AN | 0.96 | 0.94 | 1.62 | 0.96 | 0.17–4.01 | 0.92 | 1.52 | 0.96 | 0.51–7.13 | 0.630 |
| FL | 1.91 | 1.67 | 2.40 | 1.91 | 0.12–17.1 | 1.54 | 2.54 | 1.79 | 0.17–14.9 | 0.224 |
| PY | 0.49 | 1.05 | 2.17 | 0.99 | 0.28–9.58 | 0.86 | 2.33 | 0.91 | 0.12–5.88 | 0.008 |
| BaA | 0.15 | 0.23 | 2.43 | 0.15 | 0.06–3.14 | 0.16 | 1.97 | 0.15 | 0.03–2.99 | 0.0004 |
| CHR | 0.54 | 0.50 | 1.80 | 0.54 | 0.07–4.46 | 0.40 | 1.77 | 0.42 | 0.06–1.86 | 0.0002 |
| BbFA | 0.05 | 0.17 | 3.58 | 0.11 | 0.04–2.43 | 0.13 | 3.04 | 0.08 | 0.03–1.28 | 0.029 |
| BkFA | 0.05 | 0.12 | 2.85 | 0.09 | 0.03–1.79 | 0.10 | 2.27 | 0.07 | 0.03–1.48 | 0.004 |
| BaP | 0.05 | 0.17 | 3.42 | 0.18 | 0.05–2.16 | 0.12 | 2.68 | 0.12 | 0.05–1.60 | <0.0001 |
| IP | 0.05 | 0.10 | 2.99 | 0.05 | 0.03–2.26 | 0.08 | 2.39 | 0.05 | 0.05–1.38 | 0.001 |
| BghiP | 0.05 | 0.07 | 2.06 | 0.05 | 0.03–1.59 | 0.06 | 1.44 | 0.05 | 0.03–0.30 | 0.010 |
| DBahA | 0.05 | 0.10 | 2.85 | 0.05 | 0.05–1.65 | 0.08 | 2.14 | 0.05 | 0.05–0.76 | 0.005 |
| Σ16-PAH | 45.6 | 1.87 | 44.3 | 8.31–512 | 39.6 | 1.75 | 42.4 | 6.82–108 | 0.011 | |
| Personal exposure | ||||||||||
| NAP | 2.25 | 22.5 | 3.69 | 22.3 | 0.89–2055 | 34.5 | 3.57 | 32.0 | 1.95–9374 | 0.011 |
| ACEN | 0.15 | 2.21 | 4.17 | 2.53 | 0.08–84.4 | 2.03 | 4.40 | 2.60 | 0.10–37.4 | 0.856 |
| ACE | 0.44 | 6.97 | 3.20 | 7.34 | 0.26–132 | 7.22 | 3.43 | 9.02 | 0.34–183 | 0.401 |
| FLN | 2.03 | 22.8 | 3.11 | 27.2 | 1.68–435 | 29.9 | 3.28 | 28.2 | 1.09–517 | 0.011 |
| PHE | 1.84 | 16.2 | 2.81 | 17.5 | 1.02–243 | 16.1 | 2.59 | 17.4 | 0.99–117 | 0.554 |
| AN | 0.96 | 1.53 | 1.86 | 1.30 | 0.52–16.5 | 1.74 | 2.01 | 1.64 | 0.54–18.2 | 0.036 |
| FL | 1.91 | 1.51 | 2.29 | 1.76 | 0.08–10.7 | 1.55 | 3.85 | 1.63 | 0.09–356,700 | 0.664 |
| PY | 0.49 | 0.97 | 2.08 | 0.95 | 0.12–7.38 | 1.03 | 3.99 | 0.88 | 0.13–407,100 | 0.402 |
| BaA | 0.15 | 0.20 | 2.12 | 0.15 | 0.04–13.9 | 0.20 | 2.44 | 0.15 | 0.06–29.7 | 0.869 |
| CHR | 0.54 | 0.47 | 1.77 | 0.54 | 0.03–8.46 | 0.53 | 1.84 | 0.54 | 0.06–10.7 | 0.054 |
| BbFA | 0.05 | 0.14 | 3.58 | 0.06 | 0.04–8.60 | 0.12 | 3.36 | 0.05 | 0.03–6.80 | 0.215 |
| BkFA | 0.05 | 0.10 | 2.58 | 0.05 | 0.04–2.69 | 0.10 | 2.80 | 0.05 | 0.04–10.8 | 0.859 |
| BaP | 0.05 | 0.13 | 3.66 | 0.05 | 0.05–8.59 | 0.13 | 3.66 | 0.05 | 0.04–6.79 | 0.624 |
| IP | 0.05 | 0.08 | 2.83 | 0.05 | 0.03–5.87 | 0.08 | 3.31 | 0.05 | 0.05–13.8 | 0.815 |
| BghiP | 0.05 | 0.07 | 2.46 | 0.05 | 0.03–9.02 | 0.07 | 2.84 | 0.05 | 0.04–42.4 | 0.729 |
| DBahA | 0.05 | 0.09 | 3.09 | 0.05 | 0.05–11.6 | 0.08 | 3.00 | 0.05 | 0.05–17.0 | 0.270 |
| Σ16-PAH | 99.3 | 2.51 | 98.2 | 9.54–2133 | 130 | 3.36 | 120 | 9.09–764,100 | 0.026 | |
The sum of PAH in gas and particle phases.
Data Analysis
Descriptive analyses, Q–Q plot and Shapiro–Wilk test were performed to characterize the distributions of ambient and personal measurements of PAH. The analyses showed that the distributions of the data were skewed, and therefore natural log (Ln) transformation was applied to the original measurements.
Scatter plots of personal vs ambient air concentrations were completed to provide qualitative insight of the influence of outdoor sources on personal exposure level. Since the measurements obtained from our study are highly skewed to the right, Spearman’s rank correlations were used to measure the strength of the associations between personal and ambient concentrations of each compound. It can deal with the variables that are measured on wide interval scales so that the correlation will not be biased by “outliers”.
Given the possible inter-correlations among the data set collected from the repeated measurements, mixed effect model was performed to quantitatively estimate (1) locational differences (WFS vs CDS) in ambient and personal concentrations, and (2) associations between personal exposure and ambient air pollution. In the first set of analyses, the 23 factorial study design was used with location (WFS/CDS), day-of-the-week (weekday/weekend), and season (summer/winter) as predictors. Sampling date, subject ID, and season within a subject were treated as random effects. In the second set of analyses, the ambient concentration was used as a fixed effect to predict the corresponding personal exposure. Sampling date, subject ID, and season within a subject were considered random effects. Location (WFS and CDS), season (summer and winter), and day-of-the-week (weekday and weekend) were included as categorical variables to examine their effects on the association. The model yielded a slope and a P-value, and provided a predicted personal exposure concentration for each subject-measurement based on the parameters fit by the model. The coefficient of determination (R2) can be used to interpret how much of the variation in personal exposure could be explained by the ambient PAH level. An R2 was obtained by regressing the measured personal concentrations on the predicted values.
For the concentrations below MDL, half of the MDL was used as censored data so that the data set can be used for statistical analysis. MS Excel 2003 and SAS (version 9) programs were used for data analyses.
Results
Ambient and Personal Levels of PAH
As shown in Table 1, the ambient concentrations of individual PAH ranged from 0.03 to 200 ng/m3 in WFS and from 0.03 to 53.0 ng/m3 in CDS. The ambient concentration of the total PAH (Σ16-PAH) was 8.31–512 ng/m3 in WFS and 6.82–108 ng/m3 in CDS. The personal exposure to individual PAH had a wide range of 0.03–2055 ng/m3 in WFS and of 0.03–407,100 ng/m3 in CDS. Noticeably, three extremely high values were observed for personal exposure in CDS, that is, 9374 ng/m3 for NAP, 356,700 ng/m3 for FL, and 407,100 ng/m3 for PY. After exclusion of the extremes, the personal exposure to individual PAH ranged from 0.03 to 1060 ng/m3 in CDS. The personal exposure to Σ16-PAH was 9.54–2133 ng/m3 in WFS and 9.09–1203 ng/m3 in CDS (without the three extremes in CDS).
Among the 16 PAH measured, NAP, FLN, and PHE were the most abundant compounds, accounting for about 16–20% of the concentration of Σ16-PAH in the ambient samples and about 12–27% in the personal samples. IP, BghiP, and DBahA were species with the lowest concentrations in both the ambient and the personal samples. IP, BghiP and DBahA were not fully discussed in this paper because of >65% of the samples with concentrations lower than the MDLs.
Location and Temporal Variations of Ambient and Personal Levels of PAH
The ambient concentrations of ACEN, the 4- to 6-ring PAH and Σ16-PAH in WFS were significantly higher than those in CDS (Table 1, P<0.03), suggesting that there were local emission sources of PAH in WFS. For the 2- and 3-ring PAH and FL, the ambient concentrations in WFS were similar to those in CDS (Table 1, P>0.18).
Further comparisons were made to examine the effects of season (summer vs winter) and day-of-the-week (weekday vs weekend) on locational differences in ambient concentrations of PAH between WFS and CDS (Tables 2 and 3). For the 4- to 6-ring PAH (excluding FL), the locational differences in winter were observed to be greater than those in summer (Table 2). In winter, the ambient geometric means (GMs) of these compounds in WFS were 27–64% higher than those in CDS (P<0.03). However, in summer, the locational difference was marginally significant for CHR (P =0.047) and was insignificant (P>0.14) for other 4- to 6-ring PAH. Unlike the 4- to 6-ring PAH, ACEN and PHE presented greater location differences in ambient concentrations in summer than in winter (Table 2). In summer, the ambient GMs of ACEN and PHE in WFS were 54% and 30% higher than those in CDS respectively (P =0.017 for ACEN and 0.032 for PHE). In winter, the ambient GM of ACEN in WFS was 37% higher than that in CDS (P =0.032) and the ambient GM of PHE in WFS was not significantly different from that in CDS (P =0.498).
Table 2.
Comparison of ambient concentrations and personal exposures of PAHa between WFS and CDS in summer and winter (ng/m3).
| Summer
|
Winter
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WFS
|
CDS
|
P | WFS
|
CDS
|
P | |||||
| GM | Median | GM | Median | GM | Median | GM | Median | |||
| Ambient concentration | ||||||||||
| NAP | 3.88 | 3.90 | 3.89 | 3.95 | 0.942 | 13.8 | 16.0 | 13.1 | 14.4 | 0.726 |
| ACEN | 0.69 | 0.46 | 0.45 | 0.23 | 0.017 | 2.68 | 3.02 | 1.95 | 2.80 | 0.032 |
| ACE | 3.21 | 2.97 | 3.00 | 3.16 | 0.440 | 2.74 | 3.04 | 2.37 | 2.59 | 0.273 |
| FLN | 11.9 | 11.3 | 10.8 | 13.0 | 0.359 | 4.74 | 4.75 | 4.31 | 4.14 | 0.425 |
| PHE | 21.4 | 23.2 | 16.5 | 19.3 | 0.032 | 4.07 | 4.32 | 4.46 | 5.29 | 0.498 |
| AN | 1.13 | 0.97 | 1.08 | 0.96 | 0.576 | 0.80 | 0.96 | 0.80 | 0.89 | 0.892 |
| FL | 2.94 | 2.56 | 3.03 | 2.95 | 0.983 | 1.00 | 1.11 | 0.87 | 1.00 | 0.096 |
| PY | 1.46 | 1.22 | 1.28 | 1.28 | 0.142 | 0.79 | 0.74 | 0.61 | 0.51 | 0.022 |
| BaA | 0.17 | 0.15 | 0.14 | 0.15 | 0.231 | 0.29 | 0.21 | 0.18 | 0.15 | 0.0001 |
| CHR | 0.39 | 0.43 | 0.33 | 0.35 | 0.047 | 0.61 | 0.54 | 0.47 | 0.49 | 0.001 |
| BbFA | 0.07 | 0.05 | 0.07 | 0.05 | 0.834 | 0.36 | 0.38 | 0.22 | 0.23 | 0.005 |
| BkFA | 0.06 | 0.05 | 0.06 | 0.05 | 0.785 | 0.23 | 0.21 | 0.14 | 0.12 | 0.0002 |
| BaP | 0.07 | 0.05 | 0.06 | 0.05 | 0.214 | 0.39 | 0.40 | 0.24 | 0.25 | <0.0001 |
| IP | 0.06 | 0.05 | 0.05 | 0.05 | 0.246 | 0.15 | 0.09 | 0.12 | 0.06 | 0.001 |
| BghiP | 0.05 | 0.05 | 0.05 | 0.05 | 0.577 | 0.08 | 0.05 | 0.06 | 0.05 | 0.002 |
| DBahA | 0.06 | 0.05 | 0.05 | 0.05 | 0.288 | 0.15 | 0.13 | 0.11 | 0.08 | 0.005 |
| Σ16-PAH | 53.8 | 52.2 | 47.7 | 47.4 | 0.068 | 39.3 | 42.6 | 33.8 | 37.0 | 0.072 |
| Personal exposure | ||||||||||
| NAP | 26.8 | 24.0 | 42.3 | 35.6 | 0.035 | 18.4 | 20.0 | 29.2 | 29.9 | 0.075 |
| ACEN | 1.92 | 2.05 | 1.53 | 1.79 | 0.976 | 2.59 | 3.57 | 2.63 | 3.29 | 0.822 |
| ACE | 11.3 | 10.6 | 8.84 | 9.25 | 0.645 | 4.06 | 5.40 | 6.21 | 7.17 | 0.099 |
| FLN | 40.2 | 38.1 | 37.8 | 33.1 | 0.459 | 12.1 | 15.9 | 24.3 | 24.8 | 0.003 |
| PHE | 30.2 | 32.0 | 25.2 | 24.6 | 0.517 | 8.07 | 10.1 | 10.5 | 14.5 | 0.140 |
| AN | 2.11 | 2.02 | 2.27 | 2.26 | 0.258 | 1.08 | 0.96 | 1.37 | 1.13 | 0.042 |
| FL | 2.24 | 1.98 | 1.99 | 1.91 | 0.580 | 0.97 | 1.25 | 1.23 | 1.13 | 0.240 |
| PY | 1.36 | 1.26 | 1.37 | 1.43 | 0.783 | 0.67 | 0.60 | 0.80 | 0.56 | 0.363 |
| BaA | 0.21 | 0.15 | 0.22 | 0.15 | 0.490 | 0.19 | 0.15 | 0.18 | 0.15 | 0.365 |
| CHR | 0.44 | 0.53 | 0.55 | 0.54 | 0.014 | 0.50 | 0.54 | 0.52 | 0.54 | 0.699 |
| BbFA | 0.11 | 0.05 | 0.09 | 0.05 | 0.366 | 0.18 | 0.25 | 0.16 | 0.14 | 0.394 |
| BkFA | 0.08 | 0.05 | 0.08 | 0.05 | 0.411 | 0.12 | 0.09 | 0.12 | 0.09 | 0.580 |
| BaP | 0.06 | 0.05 | 0.08 | 0.05 | 0.269 | 0.27 | 0.33 | 0.20 | 0.20 | 0.076 |
| IP | 0.06 | 0.05 | 0.07 | 0.05 | 0.260 | 0.10 | 0.05 | 0.10 | 0.05 | 0.429 |
| BghiP | 0.06 | 0.05 | 0.06 | 0.05 | 0.899 | 0.07 | 0.05 | 0.08 | 0.05 | 0.719 |
| DBahA | 0.07 | 0.05 | 0.06 | 0.05 | 0.960 | 0.13 | 0.06 | 0.10 | 0.05 | 0.110 |
| Σ16-PAH | 149 | 144 | 159 | 139 | 0.363 | 63.2 | 69.6 | 109 | 93.8 | 0.013 |
The sum of PAH in gas and particle phases.
Table 3.
Comparison of ambient concentrations and personal exposures of PAHa between WFS and CDS on weekday and weekend (ng/m3).
| Weekday
|
Weekend
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WFS
|
CDS
|
P | WFS
|
CDS
|
P | |||||
| GM | Median | GM | Median | GM | Median | GM | Median | |||
| Ambient concentration | ||||||||||
| NAP | 8.45 | 10.6 | 8.40 | 8.54 | 0.831 | 6.63 | 7.80 | 6.47 | 6.47 | 0.826 |
| ACEN | 1.84 | 2.04 | 1.19 | 1.74 | 0.013 | 1.03 | 1.04 | 0.78 | 0.78 | 0.044 |
| ACE | 3.21 | 3.19 | 2.90 | 2.71 | 0.520 | 2.69 | 2.93 | 2.35 | 2.35 | 0.204 |
| FLN | 8.27 | 9.52 | 7.53 | 8.30 | 0.633 | 6.37 | 5.71 | 5.56 | 5.56 | 0.203 |
| PHE | 9.67 | 9.64 | 8.73 | 7.95 | 0.567 | 8.18 | 7.63 | 7.45 | 7.45 | 0.443 |
| AN | 1.00 | 0.96 | 0.97 | 0.96 | 0.823 | 0.89 | 0.92 | 0.85 | 0.85 | 0.640 |
| FL | 1.95 | 2.01 | 1.57 | 1.91 | 0.034 | 1.40 | 1.41 | 1.51 | 1.51 | 0.603 |
| PY | 1.32 | 1.18 | 0.85 | 0.87 | <0.0001 | 0.81 | 0.81 | 0.88 | 0.88 | 0.634 |
| BaA | 0.27 | 0.18 | 0.16 | 0.15 | <0.0001 | 0.18 | 0.15 | 0.16 | 0.16 | 0.319 |
| CHR | 0.57 | 0.54 | 0.41 | 0.45 | 0.0002 | 0.42 | 0.45 | 0.39 | 0.39 | 0.120 |
| BbFA | 0.18 | 0.12 | 0.15 | 0.16 | 0.222 | 0.15 | 0.10 | 0.11 | 0.11 | 0.055 |
| BkFA | 0.14 | 0.13 | 0.11 | 0.10 | 0.096 | 0.11 | 0.08 | 0.08 | 0.08 | 0.014 |
| BaP | 0.20 | 0.20 | 0.15 | 0.15 | 0.001 | 0.14 | 0.08 | 0.10 | 0.10 | 0.008 |
| IP | 0.11 | 0.05 | 0.09 | 0.05 | 0.005 | 0.08 | 0.05 | 0.07 | 0.07 | 0.090 |
| BghiP | 0.08 | 0.05 | 0.06 | 0.05 | 0.002 | 0.06 | 0.05 | 0.05 | 0.05 | 0.687 |
| DBahA | 0.10 | 0.05 | 0.08 | 0.05 | 0.053 | 0.10 | 0.05 | 0.07 | 0.07 | 0.044 |
| Σ16-PAH | 50.6 | 48.8 | 42.9 | 45.8 | 0.050 | 40.5 | 41.1 | 35.8 | 35.8 | 0.100 |
| Personal exposure | ||||||||||
| NAP | 20.7 | 19.4 | 35.0 | 31.1 | 0.013 | 24.4 | 26.6 | 34.0 | 35.6 | 0.039 |
| ACEN | 1.77 | 2.23 | 2.23 | 2.71 | 0.112 | 2.78 | 3.14 | 1.83 | 2.54 | 0.064 |
| ACE | 6.58 | 6.77 | 7.90 | 10.1 | 0.205 | 7.40 | 8.06 | 6.54 | 7.17 | 0.958 |
| FLN | 20.9 | 21.6 | 30.8 | 29.9 | 0.031 | 25.0 | 28.9 | 29.0 | 27.1 | 0.067 |
| PHE | 14.1 | 15.2 | 15.9 | 17.0 | 0.191 | 18.7 | 20.4 | 16.2 | 18.1 | 0.641 |
| AN | 1.48 | 1.26 | 1.75 | 1.65 | 0.052 | 1.60 | 1.32 | 1.73 | 1.32 | 0.111 |
| FL | 1.62 | 1.86 | 1.76 | 1.66 | 0.552 | 1.40 | 1.71 | 1.35 | 1.40 | 0.976 |
| PY | 1.02 | 0.99 | 1.19 | 1.00 | 0.263 | 0.93 | 0.93 | 0.88 | 0.66 | 0.967 |
| BaA | 0.20 | 0.15 | 0.19 | 0.15 | 0.891 | 0.20 | 0.15 | 0.20 | 0.15 | 0.700 |
| CHR | 0.54 | 0.54 | 0.54 | 0.54 | 0.289 | 0.41 | 0.46 | 0.53 | 0.54 | 0.069 |
| BbFA | 0.16 | 0.09 | 0.12 | 0.05 | 0.075 | 0.13 | 0.05 | 0.12 | 0.05 | 0.950 |
| BkFA | 0.09 | 0.05 | 0.10 | 0.05 | 0.551 | 0.10 | 0.05 | 0.09 | 0.05 | 0.728 |
| BaP | 0.13 | 0.05 | 0.14 | 0.05 | 0.807 | 0.13 | 0.05 | 0.11 | 0.05 | 0.323 |
| IP | 0.09 | 0.05 | 0.09 | 0.05 | 0.774 | 0.07 | 0.05 | 0.07 | 0.05 | 0.512 |
| BghiP | 0.07 | 0.05 | 0.06 | 0.05 | 0.937 | 0.06 | 0.05 | 0.07 | 0.05 | 0.539 |
| DBahA | 0.10 | 0.05 | 0.08 | 0.05 | 0.199 | 0.09 | 0.05 | 0.08 | 0.05 | 0.762 |
| Σ16-PAH | 92.9 | 90.3 | 143 | 128 | 0.010 | 107 | 111 | 117 | 113 | 0.310 |
The sum of PAH in gas and particle phases.
The locational differences in ambient concentrations of PAH between WFS and CDS were also affected by day-of-the-week. For ACEN and most of the 4- to 6-ring PAH, the locational differences in ambient concentrations were greater on weekdays than on weekends (Table 3). On weekdays, the ambient GMs of ACEN, FL, PY, BaA, CHR, IP and BghiP in WFS were 55%, 24%, 56%, 69%, 40%, 31%, and 35% higher than those in CDS, respectively (P<0.04). However, on weekends, the ambient GM of ACEN in WFS was only 32% higher than that in CDS (P =0.044), and the ambient GMs of the other six PAH species in WFS were not significantly different from those in CDS (P>0.09). It was noted that, for BaP, the ambient GM in WFS were significantly higher than that in CDS on both weekdays (P =0.001) and weekends (P =0.008).
Personal exposures showed similar levels in WFS and in CDS for the 16 measured PAH except for NAP, FLN, AN, and CHR (P>0.21, Table 1). The GMs of personal exposures to these PAH were 0.07–16.2 ng/m3 in WFS, which were comparable to those of 0.07–16.1 ng/m3 in CDS. For NAP, FLN, AN, and CHR, the GMs of personal exposures in WFS were significantly lower or marginally lower than those in CDS by 12–35% (P<0.054, Table 1). Furthermore, for FLN and AN, the locational difference in personal exposure was greater in winter (P<0.05) than in summer (P>0.25, Table 2), and was greater on weekdays (P<0.05) than on weekends (P>0.06, Table 3). However, for CHR, the locational differences were greater in summer (P =0.014) and on weekends (P =0.069) than in winter (P =0.699) and on weekdays (P =0.289), respectively (Tables 2 and 3). It was also noted that the GM of personal exposure to BaP in WFS was found 35% higher (P =0.076) than that in CDS during the winter.
Association between Personal and Ambient Levels of PAH
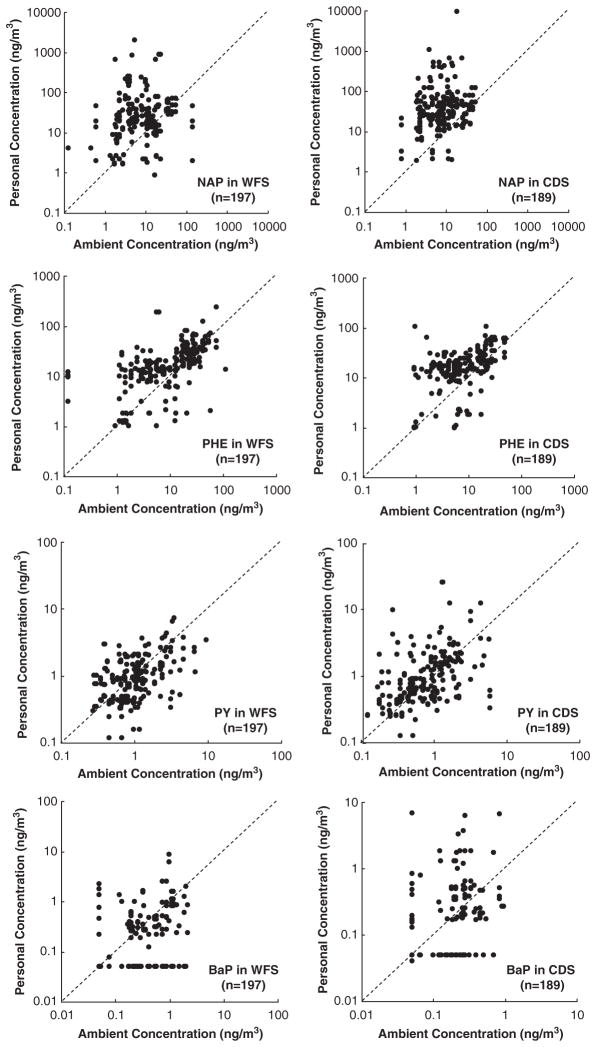
The scatter plots of personal vs ambient concentrations for the PAH species at the two locations were examined visually against the 1:1 line. Generally, the data of the 4- to 6-ring PAH were close to the 1:1 line in WFS and CDS. However, the data of the 2- and 3-ring PAH tended to scatter above the 1:1 line (Figure 2).
Figure 2.
Comparison between personal exposure and ambient concentration for naphthalene, phenanthrene, pyrene, and benzo(a)pyrene in CopeWood/Davis Street (CDS) and Waterfront South (WFS).
The Spearman correlation coefficients (Spearman R) are summarized in Table 4. For most of the PAH, statistically significant correlations were determined between personal and ambient levels, with the Spearman R ranging from 0.41 to 0.70 in WFS (P<0.002) and from 0.30 to 0.60 in CDS (P<0.025). However, poor correlations were observed for NAP, BaA, and BghiP in WFS and for AN and BghiP in CDS, with the spearman R <0.20 (P>0.16).
Table 4.
Spearman correlation coefficient (R) between personal exposure and ambient concentration.
| Location
|
Season
|
Day-of-the-week
|
||||
|---|---|---|---|---|---|---|
| WFS | CDS | Summer | Winter | Weekday | Weekend | |
| NAP | 0.14 | 0.30* | 0.24 | 0.42* | 0.13 | 0.30* |
| ACEN | 0.47* | 0.36* | 0.29* | 0.40* | 0.42* | 0.46* |
| ACE | 0.45* | 0.37* | 0.50* | 0.25 | 0.34* | 0.47* |
| FLN | 0.62* | 0.40* | 0.39* | 0.33* | 0.44* | 0.57* |
| PHE | 0.64* | 0.58* | 0.29* | 0.28* | 0.49* | 0.76* |
| AN | 0.43* | 0.19 | 0.30* | −0.39* | 0.34* | 0.23 |
| FL | 0.57* | 0.55* | 0.07 | 0.53* | 0.46* | 0.65* |
| PY | 0.53* | 0.46* | 0.32* | 0.19 | 0.47* | 0.52* |
| BaA | 0.18 | 0.30* | 0.30* | 0.30* | 0.30* | 0.19 |
| CHR | 0.46* | 0.43* | 0.54* | 0.27* | 0.42* | 0.42* |
| BbFA | 0.41* | 0.60* | 0.35* | 0.45* | 0.53* | 0.47* |
| BkFA | 0.44* | 0.48* | 0.01 | 0.66* | 0.51* | 0.44* |
| BaP | 0.50* | 0.58* | 0.26* | 0.34* | 0.48* | 0.58* |
| IP | 0.70* | 0.42* | 0.50* | 0.54* | 0.57* | 0.60* |
| BghiP | 0.17 | 0.14 | 0.18 | 0.10 | 0.29* | 0.07 |
| DBahA | 0.51* | 0.38* | 0.41* | 0.22 | 0.50* | 0.40* |
| Σ16-PAH | 0.41* | 0.42* | 0.35* | 0.32* | 0.28* | 0.57* |
P<0.05.
Prediction of Personal PAH Exposure by Ambient Concentration
As shown in Table 5, the ambient concentrations of most of the PAH species were significant predictors of the corresponding personal exposure (P<0.04). A 1-Ln-unit increase in the ambient concentration was generally associated with a significant increase of 28–62% in personal exposure in WFS and 30–73% increase in CDS. The R2s for the PAH ranged from 0.44 to 0.96 in WFS and from 0.25 to 0.98 in CDS, indicating that the ambient concentration greatly contributed to personal exposure to PAH. A comparison between CDS and WFS showed that the R2s in WFS were 4–61% higher than those in CDS for the 2- and 3-ring PAH, and they were 25–37% lower than those in CDS for the 4- to 6-ring PAH, such as FL, PY, CHR, BkFA, and BaP. The contribution from ambient concentration to personal exposure were not statistically significant for BghiP in WFS (P =0.155) and AN, IP, BghiP, and DBahA in CDS (P>0.11).
Table 5.
Prediction of personal exposure based on ambient concentration using a mixed model.
| WFS
|
CDS
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | P | R2 | Slope | Intercept | P | R2 | |
| NAP | 0.28 | 2.53 | 0.037 | 0.96 | 0.38 | 2.86 | 0.056 | 0.87 |
| ACEN | 0.41 | 0.77 | 0.005 | 0.75 | 0.30 | 0.82 | 0.025 | 0.67 |
| ACE | 0.52 | 1.29 | 0.003 | 0.80 | 0.56 | 1.52 | 0.018 | 0.50 |
| FLN | 0.62 | 1.82 | 0.001 | 0.81 | 0.63 | 2.18 | 0.004 | 0.77 |
| PHE | 0.51 | 1.61 | 0.0002 | 0.81 | 0.47 | 1.78 | 0.003 | 0.73 |
| AN | 0.62 | 0.40 | 0.002 | 0.90 | 0.39 | 0.63 | 0.109 | 0.91 |
| FL | 0.47 | 0.18 | 0.001 | 0.64 | 0.53 | 0.37 | 0.004 | 0.88 |
| PY | 0.46 | −0.06 | 0.001 | 0.71 | 0.58 | 0.37 | 0.004 | 0.98 |
| BaA | 0.32 | −1.12 | 0.005 | 0.88 | 0.67 | −0.39 | 0.007 | 0.86 |
| CHR | 0.48 | −0.38 | 0.001 | 0.66 | 0.69 | 0.00 | 0.008 | 0.88 |
| BbFA | 0.31 | −1.35 | 0.012 | 0.44 | 0.67 | −0.71 | 0.002 | 0.36 |
| BkFA | 0.43 | −1.39 | 0.002 | 0.48 | 0.54 | −1.02 | 0.021 | 0.76 |
| BaP | 0.47 | −1.25 | 0.002 | 0.67 | 0.73 | −0.43 | 0.002 | 0.94 |
| IP | 0.50 | −1.37 | 0.0004 | 0.71 | 0.31 | −1.61 | 0.183 | 1.00 |
| BghiP | 0.17 | −2.23 | 0.155 | 0.74 | 0.75 | −0.53 | 0.110 | 0.25 |
| DBahA | 0.31 | −1.66 | 0.016 | 0.62 | 0.23 | −1.93 | 0.183 | 0.45 |
| Σ16-PAH | 0.58 | 2.37 | 0.003 | 0.91 | 0.61 | 2.74 | 0.026 | 0.95 |
We like to note that it was impossible to entirely control for environmental tobacco smoke (ETS) exposure in WFS and CDS where many smokers and smoking households were found. To assess the confounding effect of ETS on the relationship between personal and ambient concentrations of PAH, nicotine, an indicator of ETS, was measured in 234 personal air samples collected in this study. Nicotine was found in a subset of PAH samples collected from both areas, with the personal concentration of nicotine significantly higher in CDS (the average of 0.66 μg/m3) than in WFS (the average of 0.22 μg/m3). Although the P-value increased from 0.0003–0.0447 to 0.0010–0.0489 when modeling with nicotine measurements in the regression analysis, the personal and ambient PAH relationship remained significant.
Discussion
A “Hot Spot” of Air Pollution
It was hypothesized that WFS was a “hot spot” of PAH air pollution, that is, an area with many sources of PAH. The hypothesis is supported by the observations, which showed that the ambient concentrations of PAH in WFS were higher than those in CDS. The observations were consistent with the source information reported for WFS (NJDEP, 2005). According to the NJDEP, the sources of PAH found in WFS include a local industrial facility dealing with municipal waste combustion, as well as diesel trucks traveling through and idling in this area to serve local industrial and commercial facilities. Thus, emissions from both local industry and diesel trucks would result in the elevated levels of PAH in WFS. Furthermore, the locational difference (WFS vs CDS) was observed to be greater on weekdays than on weekends. This again suggests that emissions from industrial operation and traffic volume on weekdays have a significant impact on PAH pollution in WFS.
To further examine the hypothesis, the ambient concentrations of PAH in WFS were compared with those in other urban areas across the United States (Mitra and Ray, 1995; Fraser et al., 1998; Eisenreich et al., 2001; Park et al., 2001; Naumova et al., 2002). It was found that the concentrations in WFS were higher than those found in big cities, such as Los Angeles, and in highly developed industrial cities with mixed industrial, commercial and traffic sources, such as Houston. For example, the nation-wide ambient concentration of BaP ranged from non-detectable level to 1.0 ng/m3 with the GM of 0.025–0.14 ng/m3 and the mean of 0.050–0.27 ng/m3 in previous studies (Mitra and Ray, 1995; Fraser et al., 1998; Eisenreich et al., 2001; Park et al., 2001; Naumova et al., 2002). The reported GM and mean were exceeded by 21% and 33% in WFS, respectively. The upper end of the nation-wide level range (1.0 ng/m3) was even exceeded in 9% of the ambient samples collected in WFS (Table 1). Currently, the USEPA lists PAH as a hazardous air pollutant under the 1990 Clean Air Act and emissions of PAH are controlled. The ambient air quality for PAH has not so far been regulated. In the United Kingdom, the government’s Expert Panel on Air Quality Standards has suggested a different approach to control PAH air pollution by recommending an annual average standard of 0.25 ng/m3 for BaP in ambient air. The average ambient concentration of BaP observed in WFS (0.36 ng/m3) was higher than the standard value. For the sum of BbFA and BkFA, two carcinogenic PAH species, the GM and the mean in WFS were 0.29 and 0.61 ng/m3. These concentrations were higher than those reported in other studies, which were 0.15–0.20 ng/m3 and 0.14–0.55 ng/m3, respectively (Fraser et al., 1998; Eisenreich et al., 2001; Park et al., 2001; Naumova et al., 2002). For PHE, an abundant PAH compound in this study, the GM and the mean in WFS were 8.95 and 15.5 ng/m3, respectively, which were comparable to those reported in Los Angeles, Seabrook, and Jersey City (Eisenreich et al., 2001; Park et al., 2001; Naumova et al., 2002). The comparison above demonstrated that WFS was an area with high levels of PAH, and there are significant local sources of PAH air pollution.
Major Sources of PAH in WFS and CDS
To identify the major sources of PAH in WFS and CDS, the commonly used diagnostic ratios of PAH (Dickhut et al., 2000; Muendo et al., 2006; Hong et al., 2007; Motelay-Massei et al., 2007; Kim and Young, 2009) and correlation between PAH and other air pollutants (VOCs and PM2.5) measured in this study were examined. As shown in Table 6, the ratios of PHE/(PHE +AN), FL/(FL +PY), and BaA/(BaA +CHR) in WFS and CDS were similar to that reported for municipal solid waste combustion (0.88, Nammari et al., 2004), diesel exhaust (0.6–0.7, Sicre et al., 1987), and oil combustion (0.32, Muendo et al., 2006), respectively. The medians of BbFA/BkFA and BaA/CHR in WFS and CDS were similar to those reported for automobile exhaust (i.e., a ratio of 1.26 and 0.53, respectively), but much lower than those for coke/coal combustion (i.e., a ratio of 3.70 and 1.11, respectively) (Dickhut et al., 2000; Kim and Young, 2009). Li and Kamens (1993) reported that the ratio of BaA/BaP was 0.5–3.7 for gasoline exhaust, 0.9–1.7 for diesel exhaust, and 1.0–1.5 for wood combustion. In WFS and CDS, the ratio of BaA/BaP was in the typical range of 0.5–3.7 for >80% of the samples, suggesting that the major source was gasoline exhaust. The ratio of BaP/(BaP +CHR) in WFS and CDS was close to the value of 0.33 reported for an urban area where catalyst equipped gasoline automobiles were predominant sources (Gogou et al., 1996). On the basis of the above analysis, there were multiple sources of PAH, that is, diesel exhaust, gasoline exhaust, waste combustion, and oil combustion, in WFS and CDS. The specific sources of each area were further identified by correlation analysis below.
Table 6.
Diagnostic ratios of PAH species.
| WFS
|
CDS
|
|||
|---|---|---|---|---|
| Mean±SD | Median | Mean±SD | Median | |
| PHE/(PHE+AN) | 0.87±0.13 | 0.91 | 0.87±0.13 | 0.89 |
| FL/(FL+PY) | 0.61±0.13 | 0.64 | 0.63±0.15 | 0.65 |
| BaA/(BaA+CHR) | 0.32±0.13 | 0.29 | 0.30±0.11 | 0.27 |
| BbFA/BkFA | 2.06±3.62 | 1.00 | 2.40±4.55 | 1.00 |
| BaA/CHR | 0.59±0.70 | 0.41 | 0.51±0.63 | 0.37 |
| BaA/BaP | 2.75±6.71 | 1.46 | 2.63±6.49 | 1.31 |
| BaP/(BaP+CHR) | 0.29±0.19 | 0.30 | 0.27±0.17 | 0.27 |
In WFS, significant correlations of Σ16-PAH with MTBE and o-xylene (P<0.05) were observed. The major sources of MTBE and o-xylene were found to be a metal processing company, a sewage treatment facility and a car scrapping facility in WFS in our spatial variation study (Zhu et al., 2008). Thus, the correlation between PAH and those VOCs suggested that those facilities in WFS were likely contributors of PAH air pollution as well. In addition, FL and PY were found to be significantly correlated with benzene (P<0.05) in WFS. Therefore, the car scrapping facility and on-road traffic, which were suggested as the major sources of benzene in WFS (Zhu et al., 2008), may also contribute to FL and PYair pollution. Differently, toluene, ethylbenzene, and m,p-xylenes were insignificantly correlated with PAH (P>0.05). The major emission sources of these species included an industrial paint shop and a local recycling plant. Emission of PAH from those facilities may not be significant.
In CDS, we observed significant or marginally significant correlations (P<0.05 and 0.08, respectively) of Σ16-PAH with MTBE, benzene, toluene, and xylenes. The results suggested that the on-road traffic, which was identified as the major source of those VOCs (Zhu et al., 2008), was the primary contributor to PAH pollution in CDS. Additionally, emissions transported from local industrial and commercial facilities in WFS, that is, the sewage treatment plant, a food processing factory, and the car scrapping facility, may contribute partially to the PAH levels in CDS because those facilities were found to contribute to the four VOCs in CDS remarkably. Ethylbenzene in CDS was contributed by a recycling plant. Thus, it was unsurprising to observe an insignificant correlation between Σ16-PAH and ethylbenzene (P>0.05).
The measured PAH were poorly correlated with PM2.5 in both WFS and CDS (P>0.23), suggesting different major sources for PAH and PM2.5. It is well known that PAH are generated by primary combustion sources, that is, incomplete combustion or pyrolysis of fossil fuels, or more generally, carbonaceous materials (Baek et al., 1991; ATSDR, 1995), while PM2.5 has both primary and secondary origins (NJDEP, 2005).
Personal Exposure and Its Relationship with Ambient Air Pollution
Personal exposure measured in this study were found to be comparable to or even higher than those reported in polluted urban areas in the United States, such as New York City and Phillipsburg (Lioy et al., 1988; Waldman et al., 1991; Buckley et al., 1995; Sisovic et al., 1996; Zmirou et al., 2000; Tonne et al., 2004). If the difference in sample collection was considered, that is, PM2.5 collected in this study but PM10 in some previous studies, the excess of personal concentrations in this study over those in previous studies would be more remarkable than the data appear to be. Our results indicated that PAH exposure for the residents in WFS and CDS may be of concern. The Spearman correlation analysis and the mixed effect modeling results (Tables 4 and 5) showed that the elevated personal exposure to PAH was greatly contributed by the neighborhood PAH air pollution in both WFS and CDS. Furthermore, the chemical profiles of PAH in the personal samples were similar to those in the ambient samples (correlation coefficient of 0.6–1.0 on 97% of the sampling days and >0.9 on 65% of the sampling days). The similarity provided more evidence on the impact of ambient PAH air pollution on personal exposure. The strong personal–ambient relationship could be explained as follows. First, the observed ambient concentrations of PAH were high in WFS and CDS when compared with the nation-wide levels. Local residents tended to spend more time outdoors compared with the US general population (Wu et al., 2010). As personal exposure is proportional to the concentrations of air pollutants in microenvironments along with the time spent in those microenvironments, personal exposure to ambient PAH for the study cohorts were expected to be elevated. Second, given the low employment rate in WFS and CDS, the WFS and CDS cohorts spent the majority of their time in the neighborhoods where the measurements were taken (18.2–23.6 h) (Wu et al., 2010). The unique time-location pattern of the study cohorts increased their chances of exposure to local ambient air pollution. Third, most households had no household air conditioners. Instead, open windows and/or exhaust fans are common ways for air ventilation, which would result in high air exchange rate, especially in summer. Thus, ambient air pollution contributed greatly to indoor levels of PAH in these areas and therefore the personal exposures that occurred indoors.
In conclusion, the study simultaneously characterized ambient and personal concentrations of the 16 US EPA priority PAH in WFS and an urban reference area, CDS, in different seasons and on different day-of-the-week. The results show that WFS has significantly higher ambient concentrations of 4- to 6-ring PAH and ACEN than CDS, and higher ambient concentrations of most of 5-ring PAH (BaP, BbFA, and BkFA) than those polluted urban areas reported in the United States. The elevation is more noticeable in winter when unfavorable diffusion condition and high emission from “cold” start of diesel vehicles occur, and on weekdays when high traffic volume and much industrial operation are observed. On the basis of diagnostic ratios of PAH and significant correlations between PAH and VOCs measured at community scale, the major sources of PAH in WFS were identified, that is, local traffic and several industrial facilities located in and around the neighborhood (a sewage treatment facility, a car scrapping facility, a metal processing company, and a food processing factory). Such information can aid in developing effective controlling strategies to reduce air pollution in local areas.
Personal concentrations of PAH in WFS, where there are many known local emission sources, are elevated as hypothesized in the study. Analysis of personal and ambient association reveals that variation of personal PAH exposure was dominated by local ambient PAH air pollution. This is expected given the high ambient PAH concentrations, long time spent outdoors by the study groups, and high ventilation rate of their houses (Wu et al., 2010). The influences of time-location and housing characteristics on the observed personal exposure will be quantitatively examined in future work. In addition, the unavoidable ETS exposure in such community did not change the significant association between personal and ambient concentrations of PAH in the study areas, re-affirming the strong impact of local air pollution of PAH on personal exposure in study areas. However, PAH in indoor air was not measured in this study, and thus the contributions of indoor sources are not readily estimated.
The database provided by the study can improve the ability to conduct a subsequent study on health effects associated with exposure to ambient PAH, which remains unclear so far. Additionally, the study shows that hot spots of PAH air pollution offers the potential to investigate health in groups that are more similar to the general population than occupational workers and many of which are more susceptible to air pollution.
Acknowledgments
This study was supported by the Health Effects Institute (HEI Agreement Number: 4703-RFA03-1/03-15). Drs. Fan and Lioy are also supported in part by the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease, Grant No. NIEHS P30ES005022. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the funding agencies. The authors thank all the participants for their cooperation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Armstrong BG, Tremblay CG, Cyr D, Thériault GP. Estimating the relationship between exposure to tar volatiles and the incidence of bladder cancer in aluminum smelter workers. Scand J Work Environ Health. 1986;12:486–493. doi: 10.5271/sjweh.2109. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Polycyclic Aromatic Hydrocarbons. U.S. Department of Health and Human Services; Atlanta, GA: 1995. [PubMed] [Google Scholar]
- Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A review of atmospheric polycyclic aromatic hydrocarbons: sources, fate and behavior. Water Air Soil Pollution. 1991;60:279–300. [Google Scholar]
- Buckley TJ, Waldman JM, Dhara R, Greenberg A, Ouyang Z, Lioy PJ. An assessment of a urinary biomarker for total human environmental exposure to benzo[a]pyrene. Int Arch Occup Environ Health. 1995;67:257–266. doi: 10.1007/BF00409408. [DOI] [PubMed] [Google Scholar]
- Choi H, Perera F, Pac A, Wang L, Flak E, Mroz E, et al. Estimating individual-level exposure to airborne polycyclic aromatic hydrocarbons throughout the gestational period based on personal, indoor, and outdoor monitoring. Environ Health Perspect. 2008;116:1509–1518. doi: 10.1289/ehp.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Callahan PJ, Lyu CW, Wilson NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9:85–98. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzayus KM, Walker SE, et al. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ Sci Technol. 2000;34:4635–4640. [Google Scholar]
- Eisenreich SJ, Brunciak PA, Gigliotti C, Totten L, Nelson ED, Dachs J, et al. The Atmosphere as a Source and Sink of PCBs and PAHs in the NY-NJ Harbor Estuary. NJ DEP; Trenton, NJ: 2001. [Google Scholar]
- Fan Z, Jung KH, Lioy PJ. Development of a passive sampler to measure personal exposure to gaseous PAHs in community settings. Environ Sci Technol. 2006;40:6051–6057. doi: 10.1021/es060474j. [DOI] [PubMed] [Google Scholar]
- Fiala Z, Vyskocil A, Krajak V, Viau C, Ettlerova E, Bukac J, et al. Environmental exposure of small children to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 2001;74:411–420. doi: 10.1007/s004200100239. [DOI] [PubMed] [Google Scholar]
- Fraser MP, Cass GR, Simoneit BRT, Rasmussen RA. Air quality model evaluation data for organics. 5. C6-C22 nonpolar and semipolar aromatic compounds. Environ Sci Technol. 1998;32:1760–1770. [Google Scholar]
- Fromme H, Lahrz T, Piloty M, Gebhardt H, Oddoy A, Rüden H. Polycyclic aromatic hydrocarbons inside and outside of apartments in an urban area. Sci Total Environ. 2004;326:143–149. doi: 10.1016/j.scitotenv.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Georgiadis P, Stoikidou M, Topinka J, Kaila S, Gioka M, Katsouyanni K, et al. Personal exposures to PM2.5 and polycyclic aromatic hydrocarbons and their relationship to environmental tobacco smoke at two locations in Greece. J Expo Anal Environ Epidemiol. 2001;11:169–183. doi: 10.1038/sj.jea.7500156. [DOI] [PubMed] [Google Scholar]
- Gogou A, Stratigakis N, Kanakidou M, Stephanou E. Organic aerosols in eastern Mediterranean: components source reconciliation by using molecular markers and atmospheric back trajectories. Organic Geochem. 1996;25:79–96. [Google Scholar]
- Hong H, Yin H, Wang X, Ye C. Seasonal variation of PM10-bound PAHs in the atmosphere of Xiamen, China. Atmospheric Res. 2007;85:429–441. [Google Scholar]
- IARC (International Agency for Research on Cancer) Evaluation of the carcinogenic risk of chemicals to humans: overall evaluations of carcinogenicity. IARC Monogr Carc Risk Chem Hum. 1987;(Suppl 7):40–74. [Google Scholar]
- Kim D, Young TM. Significance of indirect deposition on wintertime PAH concentrations in an urban Northern California Creek. Environ Engineering Sci. 2009;26:269–277. doi: 10.1089/ees.2007.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CK, Kamens RM. The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling. Atmospheric Environ Part A-General Topics. 1993;27:523–532. [Google Scholar]
- Lioy PL, Waldman JM, Greenberg A, Harkov R, Pietarinen C. The Total Human Environmental Exposure Study (THEES) to benzo(a)pyrene: comparison of the inhalation and food pathways. Arch Environ Health. 1988;43:304–312. doi: 10.1080/00039896.1988.10545954. [DOI] [PubMed] [Google Scholar]
- Mitra S, Ray B. Patterns and sources of polycyclic aromatic hydrocarbons and their derivatives in indoor air. Atmospheric Environ. 1995;29:3345–3356. [Google Scholar]
- Motelay-Massei A, Ollivon D, Garban B, Tiphagne-Larcher K, Zimmerlin I, Chevreuil M. PAHs in the bulk atmospheric deposition of the Seine river basin: source identification and apportionment by ratios, multivariate statistical techniques and scanning electron microscopy. Chemosphere. 2007;67:312–321. doi: 10.1016/j.chemosphere.2006.09.074. [DOI] [PubMed] [Google Scholar]
- Muendo M, Hanai Y, Kameda Y, Masunaga S. Polycyclic aromatic hydrocarbons in urban air: concentration levels, patterns, and source analysis in Nairobi, Kenya. Environ Forensics. 2006;7:147–157. [Google Scholar]
- Nammari DR, Hogland W, Marques M, Nimmermark S, Moutavtchi V. Emissions from a controlled fire in municipal solid waste bales. Waste Manage. 2004;24:9–18. doi: 10.1016/j.wasman.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Naumova YY, Eisenreich SJ, Turpin BJ, Weisel CP, Morandi MT, Colome SD, et al. Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the U. S. Environ Sci Technol. 2002;36:2552–2559. doi: 10.1021/es015727h. [DOI] [PubMed] [Google Scholar]
- New Jersey Department of Environmental Protection (NJDEP) Camden Waterfront South Air Toxics Pilot Project-Final Report. Trenton, NJ: 2005. [Google Scholar]
- New Jersey Department of Transportation (NJDOT) Roadway Information and Traffic Counts. 2006 http://www.state.nj.us/transportation/refdata/roadway/traffic.shtm.
- Ohura T, Noda T, Amagai T, Fusaya M. Prediction of personal exposure to PM2.5 and carcinogenic polycyclic aromatic hydrocarbons by their concentrations in residential microenvironments. Environ Sci Technol. 2005;39:5592–5599. doi: 10.1021/es050571x. [DOI] [PubMed] [Google Scholar]
- Park J-S, Wade TL, Sweet S. Atmospheric distribution of polycyclic aromatic hydrocarbons and deposition to Galveston Bay, Texas, USA. Atmospheric Environ. 2001;35:3241–3249. [Google Scholar]
- Saborit JMD, Aquilina NJ, Meddings C, Baker S, Vardoulakis S, Harrison RM. Measurement of personal exposure to volatile organic compounds and particle associated PAH in three UK regions. Environ Sci Technol. 2009;43:4582–4588. doi: 10.1021/es9005042. [DOI] [PubMed] [Google Scholar]
- Sicre MA, Marty JC, Saliot A, Aparicio X, Grilmat J, Albaiges J. Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin. Atmospheric Environ. 1987;21:2247–2259. [Google Scholar]
- Sisovic A, Fugas M, Sega K. Assessment of human inhalation exposure to polycyclic aromatic hydrocarbons. J Expo Anal Environ Epidemiol. 1996;6:439–447. [PubMed] [Google Scholar]
- Steineck G, Plato N, Alfredsson L, Norell SE. Industry-related urothelial carcinogens: application of a job-exposure matrix to census data. Am J Ind Med. 1989;16:209–224. doi: 10.1002/ajim.4700160212. [DOI] [PubMed] [Google Scholar]
- Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect. 2004;112:754–759. doi: 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000 http://www.census.gov.
- Waldman JM, Lioy PL, Greenberg A, Butler JP. Analysis of human exposure to benzo[a]pyrene via inhalation and food ingestion in the Total Human Environmental Exposure Study (THEES) J Expo Anal Environ Epidemiol. 1991;1:193–225. [PubMed] [Google Scholar]
- Wu X, Fan Z, Ohman-Strickland P. Time-location patterns of a population living in an air pollution hotspot. J Environ Public Health. 2010;2010:1–10. doi: 10.1155/2010/625461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmirou D, Masclet P, Boudet C, Dor F, Déchenaux J. Personal exposure to atmospheric polycyclic aromatic hydrocarbons in a general adult population and lung cancer risk assessment. J Occup Environ Med. 2000;42:121–126. doi: 10.1097/00043764-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Zhu X, Fan Z, Wu X, Meng Q, Wang S-W, Tang X, et al. Spatial variation of volatile organic compounds in a “Hot Spot” for air pollution. Atmospheric Environ. 2008;42:7329–7338. doi: 10.1016/j.atmosenv.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]