Abstract
Development of simple processes to fractionate synthetic mixtures of single-wall carbon nanotubes (SWCNTs) into individual species is crucial to many applications. Existing methods for single-chirality SWCNT purification are cumbersome, often requiring multiple steps and different conditions for different species. Here, we report a method to achieve total fractionation of a synthetic SWCNT mixture by countercurrent chromatography, resulting in purification of many single-chirality SWCNT species in a single run. This method is based on tunable partition of sodium deoxycholate dispersed SWCNTs in polyethylene glycol / dextran aqueous two-phase system. By running the mobile phase with 0.02% of sodium deoxycholate and a gradient of sodium dodecyl sulfate from 0.1% to 0.7% (w/w), we observe clear diameter-dependent elution, with ~ 90% total recovery. Among all the fractions collected, a number of them are enriched in single-chirality (9,4), (7,5), (7,6), (8,3), (6,5) species, while most of the remaining ones contain no more than 2-3 major species. We also observe strong (n,m)-dependent elution peak width due to enantiomer-resolved partition. These results demonstrate CCC as an effective way to obtain high purity (n, m) species, and suggest the potential of CCC as an analytical tool for chirality distribution mapping of synthetic SWCNT mixtures.
Single-wall carbon nanotubes (SWCNT) are a family of cylindrical-shaped, single layer, and carbon-only macromolecules. Synthesis methods developed so far are incapable of producing SWCNTs of defined structures at significant scale, and so separation of synthetic mixtures of SWCNTs is both scientifically interesting and technologically important. The most challenging level of the separation problem is to purify each and every species of nanotube chirality from mixed population, preferably in a single run. Many efforts have been made towards the goal. It has been shown that specific ssDNA sequences can be used to obtain single-chirality species, one at a time, via ion-exchange chromatography.1,2 Liu et al have developed a multi-gel filtration3 plus multi-step temperature-controlled elution4 method to purify single-chirality species. Gohsh et al have also obtained near single-chirality species by non-linear density gradient ultracentrifugation (DGU).5 However, these methods in general are rather cumbersome, often requiring multiple steps and different conditions for different species. Simpler methods are thus highly desirable for both routine preparation of single-chirality SWCNTs and analytical mapping of chirality distribution in polydisperse populations.
Here, we demonstrate a robust chromatographic process to achieve total fractionation of a synthetic SWCNT mixture in a single run. This new methodology is based on the recent discovery of spontaneous, chirality-dependent partition of SWCNTs in polymer aqueous-two-phase (ATP) systems.6 It has been shown that the ATP partition method allows metal/semiconductor as well as diameter based separation of SWCNTs.6 Furthermore, multistep extraction based on the ATP partition has demonstrated the potential to purify single-chirality SWCNT species.7 A key finding of these previous works is that surfactant composition can be used to modulate SWCNT partition coefficients. These factors provide the foundation for the current work based on countercurrent chromatography (CCC) as both a preparative and analytical method.
EXPERIMENTAL SECTION
CCC Instrument Setup
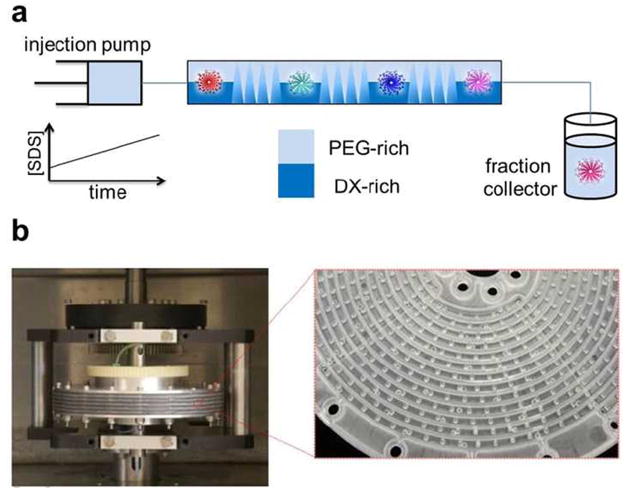
CCC, invented in the early 1970s,8,9 has evolved over the past forty years or so into a high-resolution, high-capacity separation method based on continuous liquid-liquid phase partition.10 It has been used for both natural and synthetic product separations.11 Separation of chiral molecules by CCC has also been demonstrated (ref), typically via the addition of chiral selector molecules in the stationary phase. CCC can be viewed simply as an automated, continuous liquid two-phase extraction method with many theoretical plates. The key component of a CCC setup is a column made of a long channel wound on a rotor, which undergoes the so-called planetary motion during a CCC run: the rotor rotates about its own axis while revolving around another axis. Due to the density difference of the two liquid phases, the centrifugal force generated by the planetary motion effectively pushes the two liquid phases in opposite directions, and at the same time creates both spatially and temporally oscillating patterns of mixing and settling for the two phases in the channel (Figure 1a). Typically, one of the phases is chosen to be the mobile phase, and is injected into the column with a pressure pump. The second phase, known as the stationary phase, is held in place by the centrifugal force. The composition of the mobile phase can be varied in a controlled fashion, similar to conventional liquid chromatography setups (Figure 1a), allowing either isocratic or gradient elution schemes. To overcome the sluggish mass transfer kinetics caused by high viscosity of polymer aqueous phases, in this work we have adopted a special mixer-settler disk assembly column structure (Figure 1b),12,13 in which the long channel is segmented by barricades at regular intervals to minimize laminar flow, and glass beads are added into one out of every four segmented regions along the channel to promote mixing.
Figure 1.
(a) Schematics for CCC separation of SWCNTs. (b) Mixer-settler CCC column structure used in this work. The column is made of multilayer disks mounted on a rotor. In each disk (made of polyethylene by injection molding), there are four interwoven channels (2.6 mm wide and 2 mm deep) and each channel (ca 1 m long) is divided into many 7 mm long sections by barricades. To further enhance the mixing of the two phases, a glass bead (1 mm diameter) is inserted in one out of every 4 sections.
Preparation of Aqueous Two-phase Systems
For this work we chose an ATP system composed of polyethylene glycol (PEG, MW 8,000 DA, Sigma-Aldrich, USA) and dextran (DX, MW 70,000 DA, Tokyo Chemical Industry CO., LTD, Japan), to match the conditions previously explored in the single-stage separations. PEG was added into deionized water to make a stock solution of 10% (wt.), DX was dissolved in deionized water to make a stock solution of 16% (wt.). Dissolution of both polymers was aided by heating and stirring.
We chose an SDS gradient in the mobile phase to elute nanotubes of different chirality. This elution scheme requires two ATP solvent systems with different SDS concentrations. To make the first (#1) system, 180 ml of the PEG stock solution and 288 ml of the DX stock solution were mixed with 0.936 ml of sodium deoxycholate (SDC, 10% wt.) to achieve a final concentration of SDC equal to 0.02%. To make the second (#2) system, 180 ml of the PEG stock solution and 288 ml of the DX stock solution were mixed, and 3.366 g of sodium dodecyl sulfate (SDS) and 0.936 ml of SDC (10% wt.) were added so that the concentrations of SDS and SDC are 0.7% and 0.02%, respectively. After thorough mixing by shaking, the two systems were allowed to settle at room temperature until the phases were totally separated. The lower phase of #1 system was used as the stationary phase of mixer-settler CCC, while the upper phases of both #1 and #2 systems were used as the mobile phase with different SDS concentrations.
Preparation of SWCNTs Dispersion and Starting Material Pretreatment
CoMoCAT SWCNTs (grade SG65i) were provided by SouthWest NanoTechnologies (Norman, OK). The SWCNTs were dispersed in SDC (1% wt.), following the procedure described in reference 6. In short, the dispersion procedure includes a sonication step, followed by two centrifugation steps for removal of non-nanotube contaminants, and a concentration step. To prepare the injection sample, another ATP system (#3) was made by mixing 4 ml of PEG stock solution and 2.5 ml of the DX stock solution. Right before CCC separation, 0.5 ml of SWCNT dispersion and 0.5 ml of sodium cholate (SC, 10%) were mixed with 4 ml of the PEG-rich upper phase and 0.5 ml of the DX-rich lower phase of the #3 system. The mixture was vortex-mixed vigorously then centrifuged at 1000 rpm for 5 min. The 0.5 ml lower phase containing SWCNTs with 0.09% SDC was taken out and mixed with 0.5 ml of PEG-rich upper phase of the #3 system to make the sample for injection.
Mixer-settler CCC Separation
Separation of SWCNTs was performed on a mixer-settler CCC instrument (CC Biotech LLC, Rockville, MD, instrument size ~ 30 cm × 40 cm × 60 cm, cost ~ $20,000) with a total capacity of 90 ml. First the assembly disks were filled with stationary phase (the lower phase of #1 system) using a HPLC pump (LC10 Av, Shimadzu Corporation, Kyoto, Japan). Then, the 1 ml SWCNT sample prepared by the above procedure was injected into the disks. After the injection, the binary mobile phase (the upper phases of #1 and #2 systems) was pumped into the disks from tail to head at a flow rate of 0.5 ml/min while the rotor was rotating at a rotational speed of 1000 rpm. A linear gradient of SDS concentration from 0.1% to 0.7% was achieved by adjusting the proportion of the upper phases of #1 and #2 systems over an 8 hr period. Fractions were collected at 8-minute intervals. The stationary phase retention after the solvent front was ≈ 62 %.
Spectroscopic Analysis
The UV-vis-NIR absorbance of the as-dispersed CoMoCAT sample and separated fractions were recorded using a Varian Cary 5000 spectrophotometer. Test samples were placed in a 10 mm path length microcuvette. For the collected CCC fractions, no dilution was made for the absorbance measurement, and the bottom phase of the #2 ATP system was used as reference to obtain baseline. Circular dichroism (CD) measurements were performed with a Jasco J-715 spectropolarimeter. The instrument was flushed with nitrogen and calibrated with a standard solution of ammonium l-10-camphorsulfonate. Samples were held in a 1 mm path length quartz cuvette, which was maintained by a thermostat at 20°C. Spectra were measured four times from 750 to 300 nm and averaged. Instrument settings: scan speed = 50 nm/min, time constant = 1 s, bandwidth = 1 nm and slit width = 500 μm.
Surfactant Replacement by Sodium Dodecylbenzenesulfonate
To avoid any possible induced circular dichroism by chiral molecules such as DX, SC and SDC, we used nonchiral surfactant sodium dodecylbenzenesulfonate (SDBS) to replace the cholate surfactants on the SWCNT surface prior to CD measurements. This was achieved by a sodium thiocyanate (NaSCN) precipitation method described as follows. First, NaSCN and SDC were added into CCC fractions so that the final concentrations of NaSCN and SDC were 0.5 M and 1% (wt.), respectively. The mixtures were incubated at 4°C for at least 24 hrs to precipitate the SWCNTs. The precipitate was collected by centrifugation and redispersed into 1% (wt.) SDBS. The NaSCN precipitation step was repeated one more time without addition of SDC, and the collected pellet was redispersed in 1% (wt.) SDBS. After two rounds of precipitation and redispersion, the SWCNTs were mostly coated by SDBS, as evidenced by the SWCNTs E11 spectral peak shifts.
RESULTS AND DISCUSSION
Separation of SWCNTs on Mixer-settler CCC
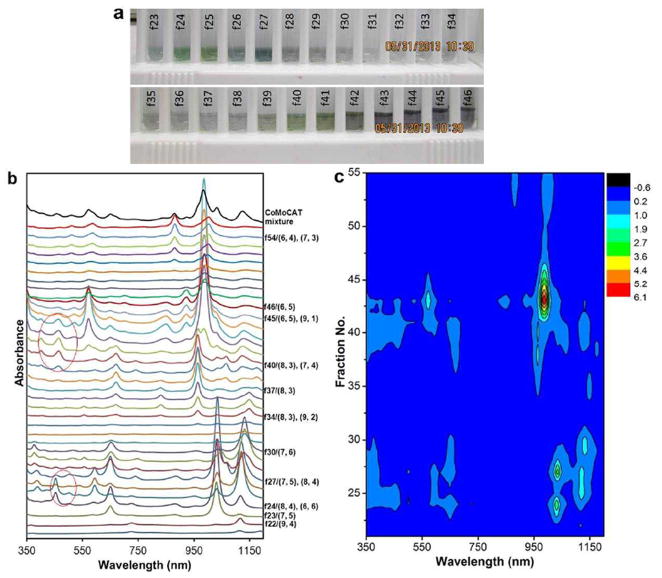
We have conducted multiple CCC runs with different sets of parameters. In a typical CCC run, ≈ 0.5 mg of CoMoCAT SWCNTs dispersed by SDC is mixed into 1 mL of PEG / DX two-phase solution, so that the final SDC concentration is 0.045% and SWCNTs are in the bottom phase. The mixture is then injected into the CCC column initially filled with the DX-rich stationary phase solution. Elution is achieved by running the mobile phase with an SDS gradient from 0.1 to 0.7% at a flow rate of 0.5 ml/min (Figure 1a). Figure 2a shows a photograph of the collected fractions containing eluted SWCNTs from a typical run. The optical absorption spectra from the injected mixture and the collected fractions are shown in Figure 2b. Based on the spectral measurement, fractions 22, 23, 30, 37 and 46 are enriched in single-chirality (9,4), (7,5), (7,6), (8,3) and (6,5), respectively. The remaining fractions contain no more than 2-3 major chirality species. It is possible to do iterative CCC runs to improve single-chirality purification. This will be explored in the future.
Figure 2.
(a) A photograph of collected fractions (f23 to f46) from a CCC run described in the text. (b) UV-vis-NIR absorption spectra of the collected fractions (f21 to f55). Spectra are offset for easy comparison. Red circles mark two regions of optical absorption from metallic (6, 6) and (7, 4), respectively. (c). Contour plot of the spectra shown in (b).
Elution Order
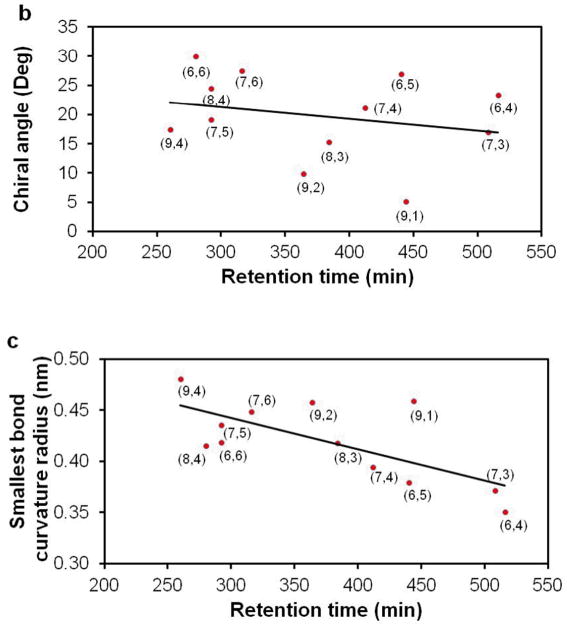
To elucidate the physical basis for the observed elution order, we have examined correlation between the elution time of a given (n,m) species and a number of its physical characteristics, including tube diameter, chiral angle, and smallest C-C bond curvature radius (Figure 3). We find that elution order correlates best with tube diameter. Figure 3a shows a near-linear relation between tube diameters and retention times. This observation is in qualitative agreement with our previous observation that addition of SDS generally brings SDC-dispersed SWCNTs from the bottom DX-rich phase to the top PEG-rich phase: larger diameter tubes first, followed by smaller diameter tubes.7 This observation is also consistent with our proposal that smaller diameter tubes are generally more hydrophilic than larger diameter tubes, and as such are more likely to be retained in the stationary DX-rich phase. At the molecular level, it is not clear how SDS interacts with SDC-dispersed SWCNT and brings SWCNTs from the bottom phase to the top phase. It is possible that the SDS sequesters SDC by forming SDS/SDC mixed micelles,14 leading to reduced coverage of SWCNT by SDC and increased hydrophobicity. Alternatively, competition by SDS and SDC molecules for SWCNT surface sites lead to concentration dependent surface coverage. (add new ref: Subbaiyan et al. (ACS Nano, 8, 1619 (2014)) More work is needed to test these possibilities.
Figure 3.
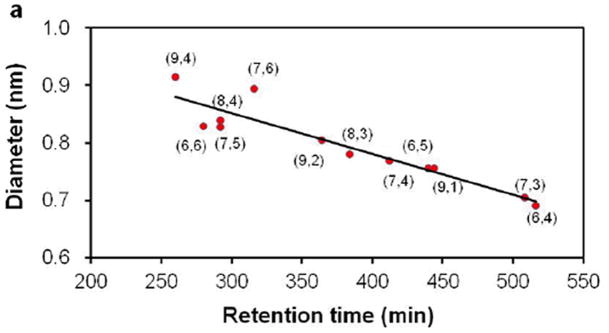
Correlation of retention time with (a) nanotube diameter (least squares fitting R2 = 0.8654); (b) chiral angle (R2 =0.0599); and (c) smallest bond curvature radius (R2 = 0.4793) 3.
Enantiomer Separation
Looking in more detail at Figure 2b, it is clear that not only the elution time, but also the breadth of the elution time window, i.e. the elution peak width, is strongly dependent on tube chirality. To make this point clear, we plot in Figure 4 E11 absorption intensities vs. fraction number for a few representative (n,m) species. The (7,5) species, for example, shows a clear bimodal elution profile: it first appears in fraction 23, reaches its maximum concentration in fraction 24, then deceases in concentration in fraction 25, and then again increases to reach a 2nd maximum in fraction 27 before total disappearance. Similarly, (8,3) and (6,5) also show broad, albeit not as clearly resolved bimodal elution profiles. In contrast, other species such as (6,6) and (7,6) show narrower, single-peaked, elution profiles. We infer from this observation that the bimodal elution profile is most likely due to the enantiomer separation through SWCNT interaction with the chiral SDC surfactant or /and the chiral dextran polymer. Similar observations have been reported for the DGU separation process.5,15
Figure 4.
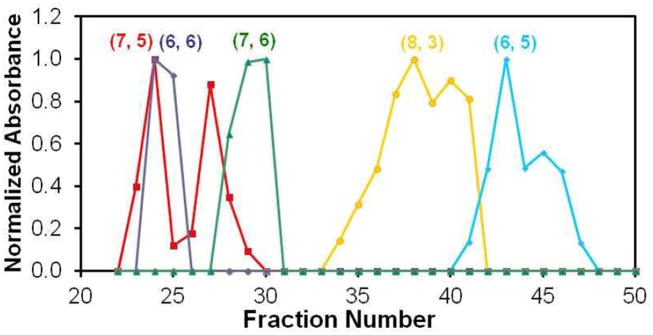
Chirality dependent elution peak width
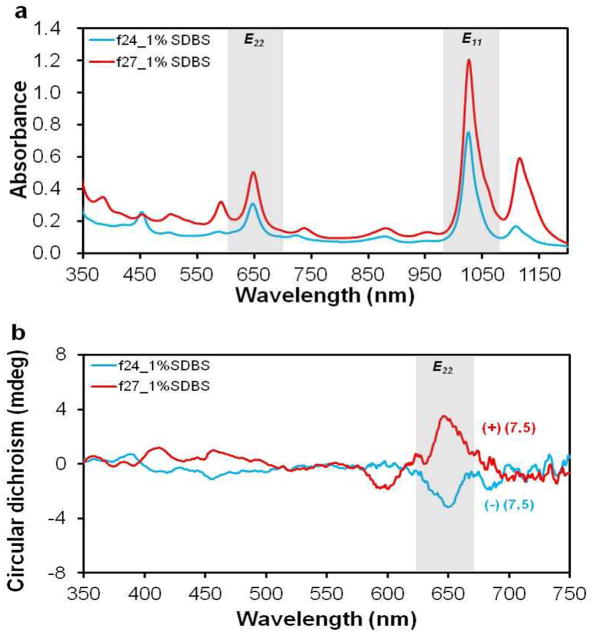
To determine whether enantiomer separation really occurred, we measured CD spectra of fractions f24 and f27, corresponding to the two peaks in the (7,5) bimodal elution profile (Figure 4). UV-vis-NIR spectra of f24 and f27 after exchange into the achiral SDBS are shown in Figure 5a. The two strong peaks, E11 at 1027 nm and E22 at 649 nm correspond to the excitonic transitions of (7,5) species. The CD spectra of the two fractions (Figure 5b) show positive and negative peaks (respectively) at ≈ 649 nm corresponding to the E22 transition of (7,5), indicating that the two fractions are enriched in (7,5)s of opposite handedness. The two enantiomer enriched fractions give ~ 20 mdeg per OD at E11 after taking into account the path length difference in our absorbance and CD measurements. This is comparable with the best enantiomer enrichment results reported in the literature. (ref. Weisman et al, ref. 5) This result clearly shows that CCC is capable of resolving enantiomers of SWCNTs.
Figure 5.
(a) UV-vis-NIR spectra of f24 and f27 in 1% SDBS (path length = 10 mm). (b) CD spectra of the two fractions (path length = 1 mm).
CONCLUSIONS AND OUTLOOK
In this work, we have demonstrated CCC as an effective way to purify single-chirality SWCNTs. While our observations also indicate enantiomer based separation by CCC, the broad elution profiles associated with certain (n,m) species does limit resolution of chirality-based separation. The use of non-chiral polymers for the ATP system and non-chiral dispersant for SWCNTs may eliminate enantiomer separation leading to narrower elution peak width. These will be explored in our future studies. In addition to being an effective separation technique for SWCNTs, CCC may also be used as an analytical tool to map chirality distribution of polydispersed SWCNT populations. A unique advantage of CCC is its near complete recovery of SWCNT materials. Because CCC uses liquid for both stationary and mobile phases, it avoids the irreversible adsorption of SWCNTs on solid stationary phases inevitable in other types of liquid chromatography. We note that due to its high recovery yield, CCC enables purification of not only highly abundant species (e g. (6,5)), but also species of very low abundance (e.g. (9,4)). This feature may allow relative abundance of different species in the starting mixture to be mapped out if extinction coefficients for different (n, m) tubes are known. Existing methods for chirality distribution mapping include fluorescence-based method16 and resonance Raman based method,17 the former works only for fluorescent semiconducting tubes, whereas the latter requires multiple excitation laser lines to bring different species into resonance. The high yield total fractionation of SWCNTs by CCC shown in this work suggests a new way to map chirality distribution by optical absorption, which is by far the simplest and most reliable spectroscopy method. With further improvement in resolution and speed, CCC could become a very useful metrology tool to both SWCNT material manufacturers and application developers.
Acknowledgments
The authors would like to thank Dr. Martha Knight and Moon-Jun Brian Kim at CC Biotech for their kindly providing the photographs of mixer-settler CCC.
Footnotes
The authors declare no competing financial interests.
References
- 1.Tu X, Hight Walker AR, Khripin CY, Zheng M. Journal of the American Chemical Society. 2011;133:12998. doi: 10.1021/ja205407q. [DOI] [PubMed] [Google Scholar]
- 2.Tu X, Manohar S, Jagota A, Zheng M. Nature. 2009;460:250. doi: 10.1038/nature08116. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Nishide D, Tanaka T, Kataura H. Nat Commun. 2011;2:309. doi: 10.1038/ncomms1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Tanaka T, Urabe Y, Kataura H. Nano Letters. 2013 doi: 10.1021/nl400128m. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, Bachilo SM, Weisman RB. Nat Nano. 2010;5:443. doi: 10.1038/nnano.2010.68. [DOI] [PubMed] [Google Scholar]
- 6.Khripin CY, Fagan JA, Zheng M. Journal of the American Chemical Society. 2013;135:6822. doi: 10.1021/ja402762e. [DOI] [PubMed] [Google Scholar]
- 7.Fagan JA, Khripin CY, Batista CS, Simpson JR, Haroz EH, Walker ARH, Zheng M. Advanced Materials. 2014 doi: 10.1002/adma.201304873. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y. Nature. 1987;326:419. doi: 10.1038/326419a0. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Bowman RL. Science. 1970;167:281. doi: 10.1126/science.167.3916.281. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y. Separation & Purification Reviews. 2005;34:131. [Google Scholar]
- 11.Ito Y. Journal of Chromatography A. 2005;1065:145. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Clary R, Powell J, Knight M, Finn TM. Journal of Chromatography A. 2009;1216:4193. doi: 10.1016/j.chroma.2008.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Clary R, Sharpnak F, Metger H, Powell J. Journal of Chromatography A. 2007;1172:151. doi: 10.1016/j.chroma.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 14.Shastry TA, Morris-Cohen AJ, Weiss EA, Hersam MC. Journal of the American Chemical Society. 2013;135:6750. doi: 10.1021/ja312235n. [DOI] [PubMed] [Google Scholar]
- 15.Green A, Duch M, Hersam M. Nano Research. 2009;2:69. [Google Scholar]
- 16.Bachilo SM, Balzano L, Herrera JE, Pompeo F, Resasco DE, Weisman RB. Journal of the American Chemical Society. 2003;125:11186. doi: 10.1021/ja036622c. [DOI] [PubMed] [Google Scholar]
- 17.Jorio A, Santos AP, Ribeiro HB, Fantini C, Souza M, Vieira JPM, Furtado CA, Jiang J, Saito R, Balzano L, Resasco DE, Pimenta MA. Physical Review B. 2005;72:075207. [Google Scholar]