Abstract
Context:
Ketosis-prone diabetes (KPD), defined by presentation with diabetic ketoacidosis (DKA), comprises 4 subgroups based on the presence or absence of islet cell autoantibodies (A− or A+) and β-cell functional reserve (β− or β+). Among A+ KPD, autoantibody epitope reactivity to 65-kDa glutamate decarboxylase (GAD65), defined by monoclonal GAD65Ab(DPD), was associated with greater β-cell functional reserve. In a majority of healthy individuals, GAD65Ab are present in the sera but are masked by anti-idiotypic antibodies; in contrast, overtly GAD65Ab-positive patients with autoimmune type 1 diabetes patients lack these anti-idiotypic antibodies.
Objective:
Our objective was to determine the presence of masked and overt GAD65Ab(DPD) in relation to β-cell function and genetic risk factors in KPD patients.
Design:
We investigated the associations of masked and overt GAD65Ab(DPD) with β-cell functional reserve, and their relationship with human leukocyte antigen (HLA) class II haplotypes linked to autoimmune diabetes susceptibility or resistance, in a large KPD cohort.
Patients:
Adult KPD patients (n = 384) were followed longitudinally in a research clinic.
Main Outcome Measures:
β-Cell function, autoantibody status, GAD65Ab epitopes, and HLA class II haplotypes were evaluated.
Results:
Overall, KPD patients with β-cell functional reserve (β+ subgroups) showed significantly higher frequency of masked GAD65Ab(DPD) than patients without β-cell functional reserve (β− subgroups): 112 of 144 (79%) compared with 59 of 100 (59%), respectively (P = .002). Masked or overt GAD65Ab(DPD) were also more frequent among autoantibody-positive patients with preserved β-cell functional reserve (A+β+ KPD) than those lacking β-cell function (A+β− KPD): 77% compared with 55% (P = .01). The susceptibility HLA haplotypes DQA1*0301/DQB1*0302 and DQA1*0301/DQB1*0201 were associated with absence of overt or masked GAD65Ab(DPD) (odds Ratios 2.3 and 2.2, respectively).
Conclusions:
Masked GAD65Ab(DPD) are strongly associated with preserved β-cell functional reserve among patients with KPD. Absence of GAD65Ab(DPD) reactivity is associated with 2 HLA class II susceptibility haplotypes for autoimmune type 1 diabetes.
Ketosis-prone diabetes (KPD), defined by presentation with diabetic ketoacidosis (DKA), comprises 4 subgroups based on the presence or absence of islet cell autoantibodies (A− or A+) and β-cell functional reserve (β− or β+) (1). This classification is highly predictive of long-term β-cell function and clinical behavior (2, 3). However, the mechanisms underlying severe, permanent β-cell dysfunction in some subgroups of KPD, and marked, sustained improvement in β-cell function in other subgroups, remain unclear.
Previously, we investigated factors related to humoral autoimmunity that distinguish the different natural histories of β-cell function in the autoantibody-positive KPD subgroups (A+β− and A+β+). Specifically, we evaluated the autoimmune response expressed by autoantibodies directed toward specific epitopes of the autoantigen 65-kDa glutamate decarboxylase (GAD65) and discovered significant associations between GAD65Ab specific for the epitope DPD (defined by monoclonal GAD65Ab DPD), presence of β-cell functional reserve, and the A+β+ phenotype (4).
Our previous study evaluated only overt GAD65Ab – however, GAD65Ab may be masked and thus escape detection in standard antibody tests, as demonstrated in our recent report of masked GAD65Ab in most randomly surveyed healthy Caucasians (5). These GAD65Ab are masked by specific anti-idiotypic antibodies (5). Extrapolating from these findings, we hypothesized that masked GAD65Ab(DPD) might be present in patients lacking overt autoantibodies, particularly in A− KPD patients with preserved β-cell functional reserve (A−β+ KPD). To test this hypothesis, we assayed plasma samples of a large, longitudinally followed cohort of KPD patients of all 4 subgroups for evidence of both masked and overt GAD65Ab.
Subjects and Methods
The study protocols were approved by the Institutional Review Board for Human Studies of Baylor College of Medicine. Written informed consent was obtained from all subjects. Adult patients (≥18 years of age) admitted to Ben Taub General Hospital with DKA and consecutively enrolled in our prospective study of KPD (n = 384) were identified at the time of their hospital stay and followed as outpatients in a dedicated research clinic between July 1999 and March 2010. DKA was defined as previously validated and described (2). Patients were classified as β+ or β− as previously validated and described (1). Clinical parameters of the patient cohort are presented in Supplemental Table 1.
Autoantibodies
Autoantibodies to GAD65, the tyrosine-phosphatase-like protein insulinoma antigen 2 (IA-2), and zinc transporter 8 (ZnT8) were determined in radioligand binding assays (6) as standardized in the International Combined Autoantibody Workshop (7). The intra-assay coefficient of variation was 7.6% for GAD65Ab, 7.4% for IA2-Ab, 7.9% for ZnT8Ab-Arg, 8.1% for ZnT8Ab-Trp, and 7.2% for ZnT8Ab-Glu. In the International Combined Autoantibody Workshop (7), our assay showed 70% sensitivity and 98% specificity for GAD65Ab, and 66% sensitivity and 98% specificity for IA-2Ab. ZnT8Ab were not assessed in that workshop.
Antibody-positive and -negative samples were included in every assay to correct for interassay variation, and used to calculate an antibody index for GAD65Ab and IA-2Ab as described (6). For ZnT8Ab, a panreactive positive control serum from a patient with autoimmune type 1 diabetes (T1D) was included as a standard and used to express Ig binding levels as a relative unit. Samples were considered ZnT8Ab-positive if binding to either ZnT8-Arg, ZnT8-Trp, or ZnT8-Glu was detected. Cutoffs were set at 15 U/mL for autoantibodies to ZnT8-Arg, 26 U/mL for ZnT8-Trp, 30 U/mL for ZnT8-Glu, and an index of 0.05 for GAD65Ab and IA-2Ab based on the 98th percentile observed in 50 healthy human control sera.
Overt GAD65Ab(DPD)
Epitope mapping of overt GAD65Ab was performed as previously described (4). The cutoff for specific competition was determined as more than 15% as previously described (4).
Anti-Id to GAD65Ab
The complexes of GAD65Ab and anti-idiotypic antibodies in plasma samples were dissociated as described earlier (5, 8). Masked GAD65Ab levels were calculated as the observed increase in GAD65Ab levels after absorption compared with the GAD65Ab level before absorption as previously reported (9). Antibody levels were expressed as a relative index as above. Cutoff levels for DPD positivity were set at an index of 0.05. An in-house control sample from a healthy donor was included on every plate as a positive control. The median index after absorption to DPD-crosslinked to protein A Sepharose for this sample was 0.27 (range, 0.24–0.31) with a coefficient of variation of 6.83%.
Human leukocyte antigen class II genotyping
Genotyping for human leukocyte antigen (HLA) class II alleles associated with susceptibility or resistance to autoimmune T1D was performed as previously described (10). HLA class II analysis was performed for DRB1, DQA1, and DQB1 alleles using either an in-house oligotyping assay or One Lambda SSP kits (LABType SSO RSSO2B1 and RSSO2Q) to capture all major alleles. When using One Lambda SSP kits for DQA1/DQB1 typing, DQB1 alleles were processed through allelic amplification followed by oligotyping and/or by nucleotide sequencing to obtain further allelic clarity. Any ambiguities in the first round of typing were clarified via nucleotide sequencing for the second exons of the respective loci.
Statistical analysis
All figures were drawn and statistical analyses performed using Prism version 4 (GraphPad Software). Categorical variables were summarized as frequencies and χ2 tests were used to test group differences. P < .05 was considered significant.
Results
We analyzed plasma samples of all KPD patients (n = 384) for GAD65Ab, IA-2Ab, and ZnT8Ab. These results, together with longitudinal assessment of β-cell functional reserve, were used to classify the patients into the 4 KPD subgroups as previously described (1). The frequencies of patients in the 4 KPD subgroups were 18% (n = 71) A+β−, (autoantibody-positive and lacking β-cell function), 22% (n = 86) A−β−, 9% (n = 34) A+β+, and 50% (n = 193) A−β+. Autoantibody distributions in the A+β− and A+β+ groups did not differ significantly. We cannot exclude that some of the A− patients may have lost previous autoantibody positivity. We did not find a significant difference in disease duration between autoantibody-positive and autoantibody-negative patients (data not shown). Furthermore, we have shown previously (1) that A−β− KPD patients have a significantly lower frequency of HLA class II T1D susceptibility alleles than A+β− KPD patients, limiting the likelihood that the former were misclassified.
Demographic, glycemic, and β-cell functional characteristics of the KPD subgroups are presented (Supplemental Table 1). Compared with β+ patients, β− patients (irrespective of autoantibody status) were younger at the time of diagnosis and had lower BMI, a higher frequency of recurrent DKA episodes, and worse glycemic control. None of the β− patients regained β-cell function. Six percent (12 of 193) of A−β+ patients lost β-cell functional reserve over 0.5 to 9 years (median 5 years); 19% (37 of 193) of A−β+ patients did not complete 12-month follow-up C-peptide measurements. Thirty-two percent (11 of 34) of the A+β+ patients lost β-cell function over 0.7 to 8 years (median 4 years); 4 A+β+ patients did not complete the 12-month follow-up C-peptide measurements.
Plasma samples obtained 1 to 6 months after the index DKA episode were analyzed for the presence of masked GAD65Ab(DPD). Availability of sufficient plasma volume determined which of the samples in each group were analyzed (Supplemental Figure 1).
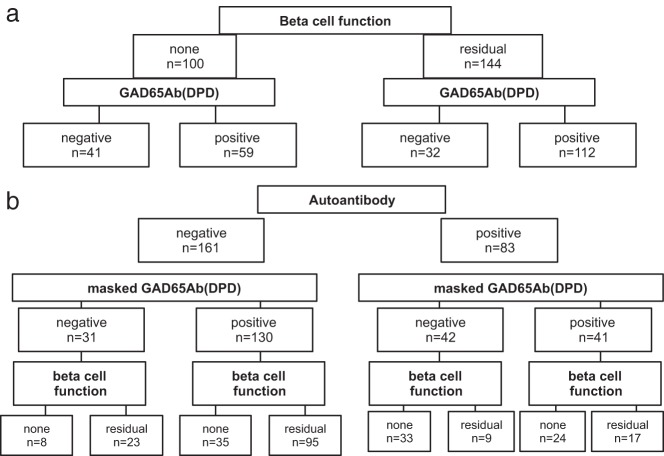
Initially, we compared the frequencies of masked GAD65Ab among patients with or without β-cell functional reserve, regardless of their autoantibody status (Figure 1A). Patients belonging to the 2 β+ KPD subgroups had a significantly higher frequency of masked GAD65Ab(DPD) (112 of 144, 78%) compared with patients belonging to the 2 β− KPD subgroups (59 of 100, 59%) (P = .002). The relative risk for lack of β-cell functional reserve in patients without masked GAD65Ab(DPD) was 1.8 (95% confidence interval [95% CI], 1.2–2.7, P = .0013) compared with patients with masked GAD65Ab(DPD). Peak glucagon-stimulated C-peptide levels correlated significantly with masked GAD65Ab(DPD) levels (P = .003) (data not shown).
Figure 1.
Flowcharts of frequencies of autoantibodies, masked GAD65Ab(DPD), and β-cell functional reserve. A, KPD patients were categorized into subgroups of patients with and without preserved β-cell functional reserve (n = 144 and 100, respectively). Frequencies of masked GAD65Ab(DPD) are compared in both groups. Patients in the β-cell function-negative subgroup had significantly lower frequency of masked GAD65Ab(DPD) (59%) compared with the frequency in patients with preserved β-cell functional reserve (78%) (P = .002). B, KPD patients were categorized into subgroups of patients with and without autoantibodies (n = 83 and 161, respectively). Frequencies of masked GAD65Ab(DPD) are compared in both groups. Patients in the autoantibody-negative subgroup had significantly higher frequency of masked GAD65Ab(DPD) (80%) compared with the frequency in autoantibody-positive patients (49%) (P < .0001). Among autoantibody-positive patients with masked GAD65Ab(DPD), the frequency of patients with preserved β-cell functional reserve was significantly higher compared with that in patients without masked GAD65Ab(DPD) (41% and 21%, respectively; P = .04).
We next analyzed the results in relation to the autoantibody status. Among A+ KPD patients, preserved β-cell functional reserve was significantly associated with the presence of masked GAD65Ab(DPD). The odds ratio (OR) for preserved β-cell functional reserve among A+ KPD patients with masked GAD65Ab(DPD) was 2.6 (95% CI, 0.9–2.9; P = .04) (Figure 1B).
Analysis of the frequencies of overt or masked GAD65Ab(DPD) among A+ KPD patients with or without β-cell functional reserve showed that the A+β+ KPD subgroup had a significantly higher frequency of masked and/or overt GAD65Ab(DPD) (22 of 26, 77%) than the A+β− KPD subgroup (32 of 58, 55%) (P = .01). Furthermore, 30% (8 of 26) of A+β+ KPD patients had both masked and overt GAD65Ab(DPD) present concurrently, whereas only 7% (4 of 58) of the A+β− patients had both masked and overt GAD65Ab(DPD) (P = .007). The A+β− KPD subgroup had a significantly lower frequency of masked GAD65Ab(DPD) than all other subgroups.
Sixty percent (216 of 353) of the KPD patients analyzed for masked GAD65Ab also had HLA-DQ haplotypes determined (10) (Supplemental Figure 1). We analyzed relationships between GAD65Ab(DPD) and HLA-DQ haplotypes associated with risk or protection against autoimmune T1D (Table 1) (11–13). DQA1*0301/DQB1*0302, a strong risk marker for autoimmune T1D, was associated with the absence of either overt or masked GAD65Ab(DPD). Whereas 51% (33 of 65) of GAD65Ab(DPD)-negative plasma samples carried at least 1 DQA1*0301/DQB1*0302 haplotype, only 30% (48 of 152) of overt or masked GAD65Ab(DPD)-positive samples carried this risk marker (OR 2.2 [95% CI 1.2–4.0. P = .006]). Susceptibility haplotype DQA1*0301/DQB1*0201 showed a similar distribution, as 30% (19 of 65) of GAD65Ab(DPD)-negative samples carried this haplotype, compared with 15% (23 of 152) of GAD65Ab(DPD)-positive samples (OR 2.3 [95% CI 1.1–4.6], P = .01).
Table 1.
Association of HLA Class II DQ Haplotypes and Presence of GAD65Ab(DPD)
| Association With Autoimmune T1D | DPD-negative (n = 65) | DPD-positive (n = 152) | P Value | |
|---|---|---|---|---|
| DQA1*0301/DQB1*0302 | Susceptibility | 51% (33/65) | 30% (48/152) | .006 |
| DQA1*0301/DQB1*0201 | Susceptibility | 30% (19/65) | 15% (23/152) | .014 |
| DQA1*0501/DQB1*0201 | Susceptibility | 27% (17/62) | 29% (45/152) | NS |
| DQA1*0401/DQB1*0402 | Susceptibility | 10% (7/65) | 16% (25/152) | NS |
| DQA1*0101/DQB1*0501 | Susceptibility | 14% (9/62) | 18% (28/152) | NS |
| DQA1*0102/DQB1*0602 | Resistance | 8% (5/62) | 10% (16/152) | NS |
Abbreviation: NS, not significant.
Discussion
The present study indicates that masked GAD65Ab(DPD) are strongly associated with preserved β-cell functional reserve among patients with KPD, irrespective of the presence of circulating islet autoantibodies (78% β+ vs 59% β−). This corroborates our previous finding that overt GAD65Ab(DPD) are associated with better preserved β-cell function and with the milder clinical phenotype of A+β+ KPD (4). KPD patients who lack masked GAD65Ab(DPD) have a 1.8 times higher risk of lacking β-cell functional reserve.
Lack of masked and overt GAD65Ab(DPD) was associated with 2 susceptibility HLA class II haplotypes (DQA1*0301/DQB1*0302 and DQA1*0301/DQB1*0201). The association between high-risk HLA haplotypes and specific autoantibodies is well documented in patients with T1D (14–17). However, such associations have not been established in patients with KPD. Based on our previous and current observations, we hypothesize that the presence of high-risk HLA haplotypes in KPD does confer increased risk for β-cell autoimmunity, but this may be manifested in cellular rather than humoral autoimmunity, as outlined below. Most autoantibody-negative (A−) KPD patients have masked GAD65Ab(DPD), regardless of residual β-cell function. This indicates presence of an intact immune network, hence the cause of β-cell dysfunction in these patients is likely to be nonautoimmune. Conversely, about 20% of A− KPD patients lack masked GAD65Ab(DPD); β-cell dysfunction in this subset (particularly A−β− KPD) may be manifested in cellular autoimmunity. In support of this notion we have recently shown that T cell reactivity to islet antigens occurs in a significant proportion of patients with A−β− and A−β+ KPD (18). Moreover, decline in masked GAD65Ab levels in autoantibody-negative type 2 diabetes patients preceded islet T-cell reactivity (19).
In conclusion, the present data provide novel insights into the pathophysiology of β-cell dysfunction in patients with different phenotypic forms of KPD. Our data suggest that the humoral autoimmune response network associated with the GAD65Ab(DPD) epitope is linked to a pathway of β-cell dysfunction that is relatively mild. The presence of masked GAD65Ab, favorable GAD65Ab epitope specificity (4), and inheritance of protective HLA class II alleles (10) comprise mechanisms underlying the unique phenotype of A+β+ KPD.
Acknowledgments
The study was sponsored by the National Institutes of Health (DK26190 and DK017047) and the Juvenile Diabetes Research Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- DKA
- diabetic ketoacidosis
- GAD65
- 65-kDa glutamate decarboxylase
- IA-2
- insulinoma antigen 2
- HLA
- human leukocyte antigen
- KPD
- ketosis-prone diabetes
- OR
- odds ratio
- T1D
- type 1 diabetes
- ZnT8
- zinc transporter 8.
References
- 1. Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and β-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab. 2003;88:5090–5098 [DOI] [PubMed] [Google Scholar]
- 2. Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes mellitus (KPDM). Diabetes Care. 2006;29:2575–2579 [DOI] [PubMed] [Google Scholar]
- 3. Nalini R, Ozer K, Maldonado M, et al. Presence or absence of a known diabetic ketoacidosis precipitant defines distinct syndromes of “A−β+” ketosis-prone diabetes based on long-term β-cell function, human leukocyte antigen class II alleles, and sex predilection. Metabolism. 2010;59:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hampe CS, Nalini R, Maldonado MR, et al. Association of amino-terminal-specific anti-glutamate decarboxylase (GAD65) autoantibodies with β-cell functional reserve and a milder clinical phenotype in patients with GAD65 antibodies and ketosis prone diabetes mellitus. J Clin Endocrinol Metab. 2007;92:462–467 [DOI] [PubMed] [Google Scholar]
- 5. Oak S, Gilliam LK, Landin-Olsson M, et al. The lack of anti-idiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci U S A. 2008;105:5471–5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grubin CE, Daniels T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–350 [DOI] [PubMed] [Google Scholar]
- 7. Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2) autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes. 1998;47:1857–1866 [DOI] [PubMed] [Google Scholar]
- 8. Routsias JG, Dotsika E, Touloupi E, et al. Idiotype-anti-idiotype circuit in non-autoimmune mice after immunization with the epitope and complementary epitope 289–308aa of La/SSB: implications for the maintenance and perpetuation of the anti-La/SSB response. J Autoimmun. 2003;21:17–26 [DOI] [PubMed] [Google Scholar]
- 9. Oak S, Radtke J, Landin-Olsson M, Torn C, Lernmark A, Hampe CS. Comparison of three assays for the detection of GAD65Ab-specific anti-idiotypic antibodies. J Immunol Methods. 2009;351:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nalini R, Gaur LK, Maldonado M, et al. HLA class II alleles specify phenotypes of ketosis-prone diabetes. Diabetes Care. 2008;31:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenbarth GS, Srikanta S, Fleischnick E, et al. Progressive autoimmune β-cell insufficiency: occurrence in the absence of high-risk HLA alleles DR3, DR4. Diabetes Care. 1985;8:477–480 [DOI] [PubMed] [Google Scholar]
- 12. Monos DS, Spielman RS, Gogolin KJ, et al. HLA-DQw3.2 allele of the DR4 haplotype is associated with insulin-dependent diabetes; correlation between DQβ restriction fragments and DQβ chain variation. Immunogenetics. 1987;26:299–303 [DOI] [PubMed] [Google Scholar]
- 13. Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M. Aspartic acid at position 57 of the HLA-DQ ß-chain protects against type 1 diabetes: a family study. Proc Natl Acad Sci. 1988;85:8111–8115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knip M, Kukko M, Kulmala P, et al. Humoral β-cell autoimmunity in relation to HLA-defined disease susceptibility in preclinical and clinical type 1 diabetes. Am J Med Genet. 2002;115:48–54 [DOI] [PubMed] [Google Scholar]
- 15. Kordonouri O, Hartmann R, Charpentier N, Knip M, Danne T, Ilonen J. Genetic risk markers related to diabetes-associated autoantibodies in young patients with type 1 diabetes in berlin, Germany. Exp Clin Endocrinol Diabetes. 2010;118:245–249 [DOI] [PubMed] [Google Scholar]
- 16. Hagopian WA, Sanjeevi CB, Kockum I, et al. Glutamate decarboxylase-, insulin- and islet cell-antibodies and HLA typing to detect diabetes in a general population-based study of Swedish children. J Clin Invest. 1995;95:1505–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham J, Hagopian WA, Kockum I, et al. ; Diabetes Incidence in Sweden Study Group, Swedish Childhood Diabetes Study Group. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355 [DOI] [PubMed] [Google Scholar]
- 18. Brooks-Worrell BM, Iyer D, Coraza I, et al. Islet-specific T-cell responses and proinflammatory monocytes define subtypes of autoantibody-negative ketosis-prone diabetes (KPD). Diabetes Care. 2013;36:4098–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortqvist E, Brooks-Worrell B, Lynch K, et al. Changes in GAD65Ab-specific antiidiotypic antibody levels correlate with changes in C-peptide levels and progression to islet cell autoimmunity. J Clin Endocrinol Metab. 2010;95:E310–E318 [DOI] [PMC free article] [PubMed] [Google Scholar]