Abstract
Context:
Promoter mutations chr5:1,295,228C>T and chr5:1,295,250C>T (termed C228T and C250T, respectively) in the gene for telomerase reverse transcriptase (TERT) have been reported in various cancers and need to be further investigated in thyroid cancer.
Objective:
The aim of the study was to explore TERT promoter mutations in various thyroid tumors and examine their relationship with BRAF V600E mutation, iodine intake, and clinicopathological behaviors of thyroid cancer.
Design:
TERT promoter and BRAF mutations were identified by sequencing genomic DNA of primary thyroid tumors from normal- and high-iodine regions in China, and clinicopathological correlation was analyzed.
Results:
The C228T mutation was found in 9.6% (39 of 408) of papillary thyroid cancer (PTC), C250T was found in 1.7% (7 of 408) of PTC, and they were collectively found in 11.3% (46 of 408) of PTC. C228T was found in 31.8% (7 of 22) and C250T in 4.6% (1 of 22) of follicular thyroid cancer (FTC), and they were collectively found in 36.4% (8 of 22) of FTC. No TERT mutation was found in 44 benign thyroid tumors. The two mutations occurred in 3.8% (6 of 158) of BRAF mutation-negative PTC vs 16.0% (40 of 250) of BRAF mutation-positive PTC (P = 5.87 × 10−4), demonstrating their association. Unlike BRAF mutation, TERT promoter mutations were not associated with high iodine intake, but they were associated with older patient age, larger tumor size, extrathyroidal invasion, and advanced stages III/IV of PTC. Coexisting TERT and BRAF mutations were even more commonly and more significantly associated with clinicopathological aggressiveness.
Conclusions:
In this large cohort, we found TERT promoter mutations to be common, particularly in FTC and BRAF mutation-positive PTC, and associated with aggressive clinicopathological characteristics.
Telomerase reverse transcriptase (TERT) is the catalytic subunit of telomerase, a ribonucleoprotein complex that plays a key role in cellular immortality by maintaining telomere length at the end of chromosomes (1, 2). TERT has long been known to be overexpressed in many human cancers, suggesting an important role of this protein in human tumorigenesis (3). This role is directly supported by the demonstration that in transgenic mouse models, induced expression of TERT led to increased development of tumors (4, 5). Two interesting somatic mutations, chr5:1,295,228C>T and chr5:1,295,250C>T (termed here as C228T and C250T, respectively), in the promoter of the TERT gene have been identified in melanoma, which represent nucleotide changes of −124 C>T and −146 C>T from the ATG translation start site of the TERT gene, respectively (6, 7). These mutations confer TERT increased transcriptional activities by creating binding sites for ETS transcription factors in the TERT promoter, providing a mechanism for the overexpression of TERT observed in human cancers. The two TERT promoter mutations, particularly the C228T mutation, have also been demonstrated in other cancers, including bladder cancer and glioblastoma, as well as many other human cancers (8, 9), suggesting a wide role of TERT promoter mutations in human tumorigenesis.
Follicular cell-derived thyroid cancer is the most common endocrine malignancy, with a rapidly rising incidence in recent years (10, 11). This cancer can be classified into several histological types, including papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC) (12). PTC and FTC are differentiated thyroid cancers, and ATC is a deadly undifferentiated thyroid cancer. PTC is the most common type of thyroid cancer, accounting for 85–90% of all thyroid malignancies. Thyroid cancer, like other human cancers, is a genetically driven malignancy. The most common genetic alteration in PTC is the BRAF V600E mutation, which, through constitutively activating the MAPK pathway, plays an important role in the tumorigenesis of PTC (13, 14). Recently, we for the first time reported common occurrence of the C228T and C250T TERT promoter mutations, particularly the C228T mutation, in thyroid cancers in an American cohort of patients (15). Interestingly, in this study we found the C228T mutation to be associated with the BRAF V600E mutation and to be particularly highly prevalent in aggressive types of thyroid cancer, such as poorly differentiated thyroid cancer and ATC. In the present study, we explored TERT promoter mutations and their characteristics in a Chinese cohort of thyroid cancer patients to further examine the role of TERT promoter mutations in human thyroid tumorigenesis.
Materials and Methods
Tumor samples and DNA isolation
The study included 44 benign thyroid tumors, 22 classical FTC, and 408 classical PTC. To try to be representative of the general Chinese population, we obtained paraffin-embedded surgical primary PTC specimens from five regions in China, spanning from the south to the north and including Shanghai, Shenyang, Qingdao, Heza, and Binzhou. Clinicopathological data were obtained from the medical records of the patients. As reported previously (16), these regions had different iodine content levels in natural drinking water, ranging from the normal levels of 10–21 μg/L in Shanghai, Shenyang, and Qingdao to a high level of 104–287 μg/L in Heza and Binzhou. Urinary iodine levels in individuals living in these regions were previously documented to be correspondingly normal or high (16). The study was approved by related institutional review boards or ethical committees. Patient consent was obtained where required.
Tissues dissected from paraffin-embedded specimens were treated for 8 hours at room temperature with xylene to remove paraffin. This was followed by digestion with 1% sodium dodecyl sulfate and 0.5 mg/mL proteinase K at 48°C for 48 hours. Mid-interval additions of a spiking aliquot of concentrated sodium dodecyl sulfate-proteinase K were added to the samples to facilitate the digestion. DNA was isolated from the digested tissues by standard phenol-chloroform extraction and ethanol precipitation procedures.
Identification of mutations by genomic DNA sequencing
Identification of the BRAF V600E mutation on tumor genomic DNA was accomplished by amplifying exon 15 of the BRAF gene using the primers and PCR conditions that we established previously (16). TERT promoter C228T and C250T mutations were identified on genomic tumor DNA as we recently described (15). Briefly, a 235-bp region of the TERT promoter containing the hotspots of C228T and C250T mutations was PCR-amplified using primers 5′-AGTGGATTCGCGGGCACAGA-3′ (sense) and 5′-CAGCGCTGCCTGAAACTC-3′ (antisense) and 40–50 ng of genomic DNA. The efficiency of this PCR was enhanced by the use of the GC-RICH PCR System (Roche Applied Science) according to the manufacturer's instructions. After gel electrophoresis to confirm the quality of the PCR products, sequencing PCR was performed using a Big Dye terminator version 3.1 cycle sequencing ready reaction kit (Applied Biosystems), and DNA sequence was analyzed on an ABI PRISM 3730 automated genetic analyzer (Applied Biosystems). When a mutation was identified, an independent PCR amplification/sequencing, both in forward and reverse directions, was performed to confirm the result.
Statistical analysis
Categorical data were summarized using frequencies and percentiles. Comparison of two groups of categorical variables was performed using the Pearson χ2 test or Fisher's exact test if the number was < 5. Comparison of two groups of continuous variables was performed using Wilcoxon-Mann-Whitney test. All reported P values were two-sided. P ≤ .05 was considered to be statistically significant. Analysis was performed using SPSS software version 11.5 (SPSS Inc).
Results
Common TERT promoter mutations in thyroid cancer in a Chinese cohort
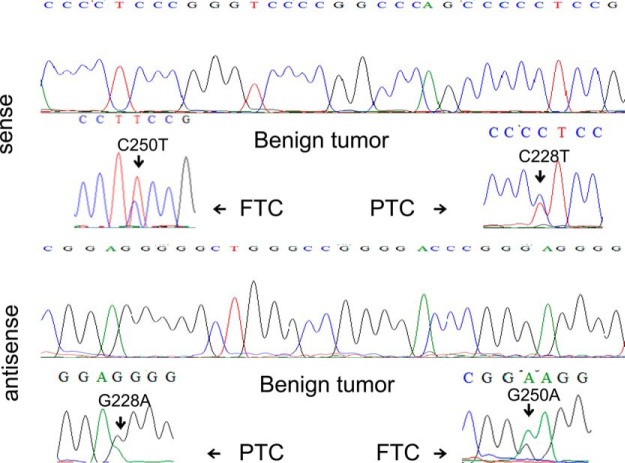
Figure 1 illustrates representative electropherograms of the two TERT promoter mutations, C228T and C250T, in PTC and FTC, respectively, detected by sense (upper panel) and antisense (lower panel) primers. Shown at the top of each panel is also the wild-type allele of the TERT promoter from a benign thyroid tumor. The C228T mutation was far more common than C250T in both PTC and FTC. As summarized in Table 1, we found C228T in 9.6% (39 of 408) of PTC and C250T in 1.7% (7 of 408) of PTC. The two mutations were collectively found in 11.3% (46 of 408) of PTC. In FTC, C228T was found in 31.8% (7 of 22) and C250T in 4.6% (1 of 22) of samples, and they were collectively found in 36.4% (8 of 22) of FTC. The two mutations were mutually exclusive in both FTC and PTC, and they were more prevalent in the former than the latter in this Chinese cohort (36.4 vs 11.3%; P = .00054). No TERT promoter mutation of any type was found in 44 benign thyroid tumors. All the TERT promoter mutations found in this Chinese cohort of thyroid cancers were heterozygous. The germline A>C (T>G on the opposite strand) mutation at −57 bp from the ATG translation start site of the TERT gene previously reported in familial melanoma (6) was not found in thyroid tumors in the present study.
Figure 1.
Representative electropherograms of the two TERT promoter mutations. Shown are C228T and C250T in a PTC tumor and a FTC tumor, respectively, which were detected both by sense (upper panel) and antisense (lower panel) primers. Shown at the top of each panel is also the wild-type allele of the TERT promoter in a benign thyroid tumor.
Table 1.
TERT Promoter Mutations in Thyroid Tumors in a Chinese Cohort
| Samples | Mutation C228T | Mutation C250T | Collective Mutations |
|---|---|---|---|
| Benign tumor | 0/44 (0) | 0/44 (0) | 0/44 (0) |
| PTC | 39/408 (9.6) | 7/408 (1.7) | 46/408 (11.3) |
| FTC | 7/22 (31.8) | 1/22 (4.6) | 8/22 (36.4) |
Data are expressed as number of mutations/number of tumors (percentage).
Association of TERT promoter mutations with BRAF V600E mutation in PTC
As summarized in Table 2, TERT mutation C228T was found in 3.2% (5 of 158) of BRAF V600E mutation-negative PTC vs 13.6% (34 of 250) of BRAF mutation-positive PTC, revealing a significantly higher prevalence of C228T in the BRAF mutation-positive PTC (P = 2.95 × 10−3). There was a higher trend of TERT C250T mutation in the BRAF mutation-positive PTC, but this was not statistically significant, probably due to the small number of TERT C250T mutation events (Table 2). The two TERT promoter mutations were collectively found in 3.8% (6 of 158) of BRAF V600E mutation-negative PTC vs 16.0% (40 of 250) BRAF mutation-positive PTC, again showing a significantly higher prevalence of TERT promoter mutations in the BRAF mutation-positive PTC (P = 5.87 × 10−4). Thus, these data demonstrate a significant association of TERT promoter mutations with the BRAF V600E mutation in this Chinese cohort of PTC.
Table 2.
Association of TERT Promoter Mutations with BRAF V600E Mutation in PTC
|
TERT C228T |
TERT C250T |
Collective TERT mutations |
|||
|---|---|---|---|---|---|
| BRAF− | BRAF+ | BRAF− | BRAF+ | BRAF− | BRAF+ |
| 5/158 (3.2) | 34/250 (13.6) | 1/158 (0.6) | 6/250 (2.4) | 6/158 (3.8) | 40/250 (16) |
| P = 2.95 × 10−3 | P = .26 | P = 5.87 × 10−4 | |||
Data are expressed as number of mutations/number of tumors (percentage).
Lack of association of TERT promoter mutations with iodine intake in PTC
Among the 408 cases of PTC, 206 cases were from normal-iodine regions, and 202 cases were from high-iodine regions as described in Materials and Methods. Because BRAF V600E mutation was previously shown to be associated with high iodine intake (16), we were curious about the relationship of TERT promoter mutations with iodine intake. We explored this issue by taking advantage of our PTC tumors available from both normal-iodine and high-iodine regions. As summarized in Table 3, the two TERT promoter mutations were collectively found in 10.7% (22 of 206) of PTC from normal-iodine regions vs 11.9% (24 of 202) of PTC from high-iodine regions (P = .73), revealing no significant association of TERT promoter mutations with high iodine intake in patients. In contrast, the BRAF V600E mutation was found in 49.5% (102 of 206) of PTC from normal-iodine regions vs 73.3% (148 of 202) of PTC from high-iodine regions (P = 8.46 × 10−7). This shows a significant association of BRAF V600E mutation with high iodine intake in patients, consistent with our previous findings (16).
Table 3.
Relationship of Mutations in PTC with Iodine Intake
| Mutation Type | Normal Iodine Intake | High Iodine Intake | P Value |
|---|---|---|---|
| TERT promoter mutations (both types) | 22/206 (10.7) | 24/202 (11.9) | .73 |
| BRAF V600E | 102/206 (49.5) | 148/202 (73.3) | 8.46 × 10−7 |
Data are expressed as number of mutations/number of tumors (percentage).
Association of TERT promoter mutations with aggressive clinicopathological characteristics of PTC
We next examined the relationship of TERT promoter mutations with the classical clinicopathological characteristics of PTC. As shown in Table 4, patient age at the diagnosis of PTC was significantly older in the TERT promoter mutation-positive group than the mutation-negative group, being 53.40 ± 16.14 years in the former vs 43.66 ± 12.91 years in the latter (P = 1.08 × 10−5). There was no significant difference in the occurrence rate of TERT promoter mutations between male and female sexes, although there was a trend of higher prevalence of TERT promoter mutations in male patients. Tumor size was significantly bigger in the TERT promoter mutation-positive group than the mutation-negative group, being 3.14 ± 1.62 cm in the former vs 2.48 ± 1.58 cm in the latter (P = .029). Extrathyroidal extension of PTC was also more common in the TERT promoter mutation-positive group than the mutation-negative group, being 28.1% (9 of 32) in the former vs 8.2% (17 of 207) in the latter (P = .00076). We did not find a significant difference in neck lymph node metastasis rate between the TERT promoter mutation-positive and mutation-negative groups in this cohort of patients. Stage III/IV disease was significantly more common in the TERT promoter mutation-positive group than the mutation-negative group (P = .05). Thus, overall, TERT promoter mutations were associated with aggressive clinicopathological characteristics of PTC.
Table 4.
Relationship of TERT Promoter Mutations With Clinicopathological Characteristics of PTC
| Clinicopathological Characteristics | TERT Mutations | TERT Wild-Type | P Value |
|---|---|---|---|
| Age at diagnosis in y, mean ± SD (n) | 53.40 ± 16.14 (42) | 43.66 ± 12.91 (325) | 1.08 × 10−5 |
| Gender | .259 | ||
| Male | 12/42 (28.6) | 68/325 (20.9) | |
| Female | 30/42 (71.4) | 257/325 (79.1) | |
| Tumor size in cm, mean ± SD (n) | 3.14 ± 1.62 (31) | 2.48 ± 1.58 (291) | .029 |
| Extrathyroidal extension | 9/32 (28.1) | 17/207 (8.2) | .00076 |
| Lymph node metastasis | 7/26 (26.92) | 68/213 (31.9) | .604 |
| Cancer stages | .05 | ||
| Stages I/II | 15/26 (57.69) | 161/213 (75.59) | |
| Stages III/IV | 11/26 (42.31) | 52/213 (24.41) |
Data are expressed as number of mutations/number of tumors (percentage), unless otherwise indicated.
We also analyzed the individual effects of BRAF V600E alone, TERT promoter mutation alone, and coexistence of BRAF V600E and TERT promoter mutations on clinicopathological outcomes of PTC (Table 5). Interestingly, whereas the effects of BRAF alone became weaker and the effects of TERT promoter mutations alone were lost, coexistence of BRAF and TERT mutations was more commonly and more significantly associated with the aggressive clinicopathological characteristics of PTC. Specifically, tumor size was 3.32 ± 1.67 cm vs 2.15 ± 1.62 cm (P = .001) in the patients with coexisting BRAF V600E and TERT promoter mutations vs the patients with neither mutation, demonstrating larger PTC tumors associated with the coexistence of the two types of mutations. Extrathyroidal invasion was 39.13% (9 of 23) vs 7.25% (5 of 69) (P = .0002) in patients with coexisting BRAF V600E and TERT promoter mutations vs the patients with neither mutation. Stage III/IV disease was 47.83% (11 of 23) vs 24.64% (17 of 69) (P = .036) in patients with coexisting BRAF V600E and TERT promoter mutations vs the patients with neither mutation. The age at the diagnosis of thyroid cancer was older in patients with coexisting BRAF V600E and TERT promoter mutations than the patients with neither mutation, being 54.89 ± 16.17 years vs 43.73 ± 13.23 years (P < .0001) in the former vs the latter. These results demonstrated a unique role of the coexisting BRAF V600E and TERT promoter mutations in the tumorigenesis and aggressiveness of PTC.
Table 5.
Impact of BRAF V600E or TERT Promoter Mutations or Their Coexistence on Clinicopathological Outcomes of PTC
| Clinicopathological Outcomes | No Mutation | BRAF Mutation Only | P Value | TERT Mutation Only | P Value | BRAF + TERT Mutation | P Value |
|---|---|---|---|---|---|---|---|
| Age at diagnosis in y, mean ± SD (n) | 43.73 ± 13.23 (141) | 43.61 ± 12.69 (184) | .936 | 44.5 ± 13.97 (6) | .889 | 54.89 ± 16.17 (36) | <.0001 |
| Gender, no. of males/total (%) | 28/141 (19.86) | 40/184 (21.74) | .679 | 0/6 (0) | .225 | 12/36 (33.33) | .084 |
| Tumor size in cm, mean ± SD (n) | 2.15 ± 1.62 (129) | 2.74 ± 1.50 (162) | .0014 | 2.2 ± 1.01 (5) | .946 | 3.32 ± 1.67 (26) | .0011 |
| Extrathyroidal invasion | 5/69 (7.25) | 18/137 (13.14) | .205 | 0/3 (0) | 1 | 9/23 (39.13) | .00023 |
| Lymph node metastasis | 20/69 (28.99) | 49/137 (35.77) | .33 | 0/3 (0) | .555 | 7/23 (30.43) | .895 |
| Disease stage | |||||||
| I + II | 52/69 (75.36) | 102/137 (74.45) | 3/3 (100) | 12/23 (52.17) | |||
| III + IV | 17/69 (24.64) | 35/137 (25.55) | .887 | 0/3 (0) | 1 | 11/23 (47.83) | .036 |
Data are expressed as number of mutations/number of tumors (percentage), unless otherwise indicated. P values are from the comparison of the indicated genetic group in the column immediately left to the P value column with the “No mutation” group.
Discussion
Our previous exciting documentation of TERT promoter mutations in thyroid cancer in an American cohort of patients (15) prompted us to extend the studies to this large Chinese cohort of thyroid cancer patients. The present study, together with several other recent studies on TERT promoter mutations in thyroid cancer from different ethnic populations (17–19), now firmly establishes the wide occurrence of this novel genetic alteration in human thyroid cancers and provides definitive evidence to support its important role in thyroid tumorigenesis. The prevalence of TERT promoter mutations in PTC in this Chinese cohort is very similar to that in our initial report on TERT promoter mutations in a large American cohort (15), which was around 11%. It is also similar to that in a large Portuguese cohort recently reported by Soares' group (18). Interestingly, in the Chinese cohort in the present study, the prevalence of TERT promoter mutations in FTC was 36%, which was significantly higher than that in PTC. It is also higher than that in FTC reported in the American cohort (15) and in the Portuguese cohort (18). It thus seems that TERT promoter mutations may play a more extensive role in the tumorigenesis of FTC in Chinese patients, but this needs to be confirmed in a larger cohort of patients. Unlike BRAF V600E mutation, which has been shown to be associated with high iodine intake, a presumptive high risk factor for thyroid cancer (20, 21), the present study showed no association of TERT promoter mutations with high iodine intake, suggesting that iodine may not be a risk factor for the occurrence of TERT promoter mutations.
In our initial report on TERT promoter mutations in a American cohort of 257 PTC patients (15), we observed a significant association of TERT promoter mutations with the BRAF V600E mutation. A similar finding was also reported in the study of Soares' group (18) on a Portuguese cohort of 169 PTC patients. In contrast, Fagin's group (17) reported a significantly inverse relationship between the TERT promoter mutations and the BRAF mutation in PTC; we cannot explain this discrepancy, but the small size of the study could be an explanation. Our present study on a Chinese cohort of 408 PTC patients represents the largest single study to address the issue of the relationship of TERT promoter mutations with the BRAF mutation. The demonstration of a significant association of TERT promoter mutations with BRAF V600E mutation in this large study on Chinese patients confirms our initial finding of this interesting phenomenon in the American patients (15); these large studies from different ethnic backgrounds, taken together, strongly support the existence of an associative relationship of the two types of genetic events in PTC. We have previously speculated and proposed that this association of TERT promoter mutations with BRAF V600E mutation potentially has important biological relevance and may confer to thyroid cancer a unique survival advantage (15). The C228T and C250T promoter mutations create binding sites for ETS transcription factors (6, 7), and ETS factors are targets of the MAPK signaling pathway (22–24). Thus, coexisting TERT promoter mutations and BRAF V600E mutation forms a unique mechanism in which BRAF V600E-activated MAPK pathway promotes the up-regulation of the TERT gene through generating and enhancing the interaction of ETS factors with the TERT promoter. In fact, increased TERT expression was observed in PTC tumors harboring both the TERT promoter and BRAF V600E mutations (18). This may be an important mechanism in promoting thyroid tumorigenesis and aggressiveness of thyroid cancer. Indeed, we have recently demonstrated in an American cohort of PTC that coexistence of TERT promoter mutations and BRAF V600E mutation was most commonly and significantly associated with clinicopathological aggressiveness of PTC (25), similar to the findings that coexisting BRAF V600E and TERT promoter mutations were more commonly associated with aggressive clinicopathological characteristics of PTC in the present study. These results are consistent with an interesting observation in our previous study that several PTC tumors that harbored both TERT promoter and BRAF V600E mutations also contained anaplastic features (15), raising the possibility that coexistence of TERT promoter and BRAF mutations may be a genetic mechanism driving conversion of PTC to ATC. The molecular mechanisms in the process of this aggressive progression of thyroid cancer are likely complex and multifaceted, and one speculative mechanism could involve pathological changes in the microenvironments of thyroid cancer that drive thyroid cancer progression (26, 27). It is important to note that our present study demonstrated a significant association of TERT promoter mutations with several conventional high-risk factors for poor prognosis of PTC, including older patient age, large tumor size, extrathyroidal extension, and advanced disease stages III/IV. The lack of significant association of TERT promoter mutations with lymph node metastasis in the present study likely reflects the possibility that patients studied here mostly did not have, or only had limited, surgical neck dissection at their thyroidectomy. In our initial study on TERT promoter mutations in thyroid cancer, a striking finding was the association of such mutations with aggressive types of thyroid cancers, such as tall-cell PTC variant, poorly differentiated thyroid cancer, and ATC (15). Based on this finding, we proposed that TERT promoter mutations play an important role in thyroid cancer aggressiveness. This was further supported by subsequent reports that also reported association of TERT promoter mutations with aggressive types or aggressive features of thyroid cancer (17–19). Thus, our present study, together with other recent studies, strongly suggests that TERT promoter mutations play an important role in the aggressiveness of thyroid cancer and thus represent potential novel prognostic molecular markers that may be useful in assisting risk stratification of thyroid cancer patients. This role of TERT promoter mutations, however, seems to require and cooperate with additional genetic alterations, such as BRAF V600E mutation, which aberrantly activate other tumor-promoting signaling pathways in promoting the tumorigenesis and development of progression and aggressiveness of PTC. This concept is supported by the fact that, when separated from BRAF V600E mutation, TERT promoter mutations alone showed a limited effect on clinicopathological outcomes of PTC. This needs to be further investigated, given the small number of cases positive only for TERT promoter mutations.
In summary, we present here a study with the largest cohort of PTC patients to investigate TERT promoter mutations and demonstrate common occurrence of this novel genetic alteration. The study also demonstrates a significant association of TERT promoter mutations with BRAF V600E mutation and several aggressive clinicopathological characteristics of PTC. This study, together with other recent studies, unequivocally establishes an important role of TERT promoter mutations in the tumorigenesis of human thyroid cancers.
Acknowledgments
This study was supported by National Institutes of Health Grant R01 CA113507 (to M.X.).
Disclosure Summary: M.X. received royalties as a coholder of a licensed United States patent related to the discovery and clinical characterization of BRAF V600E mutation in thyroid cancer. The other authors have nothing to declare.
Footnotes
- ATC
- anaplastic thyroid cancer
- FTC
- follicular thyroid cancer
- PTC
- papillary thyroid cancer
- TERT
- telomerase reverse transcriptase.
References
- 1. Smekalova EM, Shubernetskaya OS, Zvereva MI, Gromenko EV, Rubtsova MP, Dontsova OA. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc). 2012;77:1120–1128 [DOI] [PubMed] [Google Scholar]
- 2. Mocellin S, Pooley KA, Nitti D. Telomerase and the search for the end of cancer. Trends Mol Med. 2013;19:125–133 [DOI] [PubMed] [Google Scholar]
- 3. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622 [DOI] [PubMed] [Google Scholar]
- 4. González-Suárez E, Samper E, Ramírez A, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. González-Suárez E, Flores JM, Blasco MA. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol. 2002;22:7291–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961 [DOI] [PubMed] [Google Scholar]
- 7. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Wu G, Shan Y, Hartmann C, von Deimling A, Xing M. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle. 2013;12:1637–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90 [DOI] [PubMed] [Google Scholar]
- 11. Howlader N, Noone AM, Krapcho M, et al. 2013 SEER Cancer Statistics Review, 1975–2010. National Cancer Institute. http://seer.cancer.gov/csr/1975_2010/. Published June 12, 2013 Accessed January 10, 2014
- 12. DeLellis RA, Lloyd RV, Heitz PU. Pathology and Genetics: Tumours of Endocrine Organs. WHO Classification of Tumors. Lyon, France: IARC Press; 2004 [Google Scholar]
- 13. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262 [DOI] [PubMed] [Google Scholar]
- 14. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762 [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612–1617 [DOI] [PubMed] [Google Scholar]
- 17. Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 19. Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas [published online October 21, 2013]. Oncogene. doi:10.1038/onc.2013.446 [DOI] [PubMed] [Google Scholar]
- 20. Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86 [DOI] [PubMed] [Google Scholar]
- 21. Knobel M, Medeiros-Neto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:701–712 [DOI] [PubMed] [Google Scholar]
- 22. Janknecht R, Ernst WH, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216 [PubMed] [Google Scholar]
- 23. Strahl T, Gille H, Shaw PE. Selective response of ternary complex factor Sap1a to different mitogen-activated protein kinase subgroups. Proc Natl Acad Sci USA. 1996;93:11563–11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407 [DOI] [PubMed] [Google Scholar]
- 25. Xing M, Liu X, Liu R, Pai SI, Zeiger M, Bishop J. TERT promoter mutation corporates with BRAF mutation to promote thyroid cancer recurrence. In: Proceedings from the American Thyroid Association; October 16–20, 2013; San Juan, Puerto Rico [Google Scholar]
- 26. Nucera C, Porrello A, Antonello ZA, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci USA. 2010;107:10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011;71:2417–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]