Abstract
Context:
Lower birth weight has been reported in conjunction with high maternal free T4 (FT4) in euthyroid pregnancies, raising concerns for suboptimal outcomes.
Objective:
The objective of the study was to explore the relationships between high maternal FT4 and pregnancy complications in euthyroid women and to further examine the relationships among maternal size, FT4, and birth weight.
Design:
This was an observational multicenter cohort study.
Setting:
The study was conducted at prenatal clinics.
Study Subjects:
A total of 9209 euthyroid women with singleton pregnancies participated in the study.
Interventions:
There were no interventions.
Main Outcome Measures:
Relationships between second-trimester high maternal FT4 and pregnancy/delivery complications and, among FT4, maternal weight and birth weight were measured.
Results:
Women in the highest FT4 quintile are younger and weigh less than women in quintiles 1–4; gestational diabetes and preeclampsia occur less often (P = < .001, P < .001, P < .001, and P = .05, respectively). Lowest median birth weight occurs among women in the highest FT4 quintile (P = < .001), but deliveries less than 37 weeks' gestation are not increased. Labor/delivery complications do not differ by FT4 quintile. Restricting analyses to maternal weight-adjusted small-for-gestational-age deliveries yields similar results, except for preeclampsia. In the highest maternal weight decile, adjusted median birth weight is 266 g higher (8.3%) than in the lowest weight decile; adjusted median FT4 is 0.91 pmol/L lower (6.8%). Among women in the highest FT4 decile, adjusted median birth weight is 46 g lower (1.3%) than in the lowest FT4 decile. All three relationships are statistically significant (P < .001, P < .001, and P = .004, respectively).
Conclusions:
Lower median birth weight among euthyroid women with high FT4 is not associated with adverse pregnancy outcomes. Further investigation is indicated to determine how the variations in thyroid hormone concentration influence birth weight.
An association between high maternal free T4 (FT4) levels and lower mean birth weight has recently been described in two studies (1, 2). Although attenuated after adjustment for relevant covariates [including maternal body mass index (BMI)], this association persists. Based on the connection between high FT4 and low birth weight, one of the reports raised concerns that high FT4 might be a risk factor for adverse pregnancy outcomes (1). However, FT4 concentration is also inversely related to maternal BMI (3–6), and low maternal BMI is well known to be associated with constitutionally lower mean birth weight (7, 8). Therefore, the relationship between FT4 and birth weight is confounded by maternal weight. At any given interval of low birth weight, risk for adverse events needs to take maternal size into account because lower birth weight associated with smaller mothers may not always be explained by intrauterine growth restriction. The present analysis explores relationships between high maternal FT4 levels and selected problems with pregnancy and delivery in the First and Second Trimester Assessment of Aneuploidy Risk (FaSTER) data set. The analysis also further examines the documented relationships between maternal weight and FT4, maternal weight and birth weight, and FT4 and birth weight, focusing on the interrelationships among all three variables.
Materials and Methods
The multicenter First and Second Trimester Assessment of Aneuploidy Risk trial has been previously described (3, 9). Briefly, maternal serum samples were collected from singleton pregnancies at 11.0–13.9 weeks' and 15.0–18.9 weeks' gestation. Maternal weights and heights were recorded at the time of initial sampling. At five recruitment centers, participants were asked to give supplementary consent to allow use of residual sample and pregnancy-related information for additional research. Samples were collected between 1999 and 2002 and stored at −80°C. Levels of TSH, FT4, and antithyroperoxidase were measured between July 2004 and May 2005 using the Immulite 2000 chemiluminescence analyzer (Siemens Medical Solutions Diagnostics). Assay characteristics and normative data involving these analytes have been published (10).
Women whose pregnancies were affected by Down syndrome or other chromosome structural abnormalities were excluded. Among women who consented, inclusion criteria for the present study required the following: 1) TSH and FT4 measurement available and gestational age established by ultrasound [10074 women (100%)], 2) no known thyroid disease [9670 women (96.0%)], 3) TSH between the second and 98th centiles [9330 women (92.6%)], 4) gestational age 28 weeks or longer [9221 women (91.5%)], and 4) BMI available [9209 women (91.4%)].
Postdelivery follow-up was performed by the research coordinator at each site or by telephone interview. A single perinatologist and a pediatric geneticist reviewed maternal and pediatric medical records for the following patient subsets: abnormal first- and/or second-trimester screening, adverse obstetric or pediatric outcomes, and 10% of normal subjects randomly selected from the trial database. A purpose-designed computerized tracking system with up to 10 contacts per subject was used to ensure complete outcome collection. Pregnancy and pediatric outcomes were obtained in more than 98% of cases. Gestational diabetes was classified as two abnormal values on a 3-hour glucose challenge test.
Statistical analyses
Data analyses were performed using SAS version 9.2 (SAS Institute). Birth weight was adjusted for gestational age at delivery. To accomplish this, birth weight was first converted to a multiple of the median (MoM) by dividing by the overall median birth weight (3430 g). Then a linear regression of birth weight and gestational age at delivery was performed, resulting in the following equation: predicted birth weight = −3658.9 + 179.099 (gestational age at delivery), where gestational age could vary from 28 to 42 weeks' gestation. The predicted birth weights were then converted to MoMs. Each individual birth weight was then adjusted by dividing by the predicted birth weight MoM multiplied by the overall median birth weight. Potential confounders of birth weight and FT4 were examined using stepwise regression. Adjustments for birth weight and FT4 described below were also performed using MoM in a similar fashion.
Differences in continuous variables within FT4 quintiles were tested using the Student's t test. Categorical variables were tested using the χ2 test. Potential confounders for small-for-gestational-age (SGA) newborns were examined using a stepwise regression. The linear regression was used to test for trends. Statistical significance was two tailed at the P = .05 level.
Results
Pregnancy and delivery characteristics
Table 1 lists selected pregnancy and delivery characteristics for the study population as a whole and after stratification by quintile of FT4. Quintile intervals are used due to the relatively low percentages of pregnancy/delivery-related adverse events. When compared with women with FT4 levels in quintiles 1–4, women in quintile 5 are younger, weigh less, and are diagnosed with gestational diabetes and preeclampsia less often; the median TSH is lower, and a lower percentage have elevated levels of thyroid antibodies. The lowest median birth weight occurs among women in the highest FT4 quintile. When tested for linear trend among quintiles, the above characteristics of the study population remain significant except for thyroid antibodies, but the extent of significance is somewhat diminished (data not shown). Labor/delivery complications (including deliveries less than 37 wk gestation) are not increased in FT4 quintile 5, as compared with quintiles 1–4, and tests for linear trend are not significant.
Table 1.
Pregnancy and Delivery Characteristics of 9209 Euthyroid Women, Stratified by FT4 Concentration at 15.0–18.9 Weeks' Gestation
| Characteristic | All | FT4 Quintilea |
P Valueb | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| n | 9209 | 1679 | 1980 | 1842 | 1741 | 1967 | |
| FT4, pmol/L | 13.03 (1.96)c | 10.58 (0.94) | 12.00 (0.34) | 13.03 (0.26) | 13.93 (0.29) | 15.35 (1.39) | |
| TSH, mIU/L | 1.23 (0.27) | 1.27 (0.28) | 1.28 (0.26) | 1.25 (0.27) | 1.22 (0.26) | 1.15 (0.26) | <.001 |
| Antibody positive, % | 11.2 | 16.0 | 10.6 | 11.0 | 9.7 | 9.5 | .005 |
| Age, y | 29 (5.5) | 31 (5.4) | 30 (5.5) | 29 (5.4) | 27 (5.4) | 26 (5.1) | <.001 |
| Weight, kg | 65.0 (14.6) | 68.2 (16.4) | 65.9 (15.1) | 64.5 (14.5) | 64.1 (13.4) | 62.3 (12.3) | <.001 |
| BMI, kg/m2 | 23.5 (5.0) | 25.0 (5.8) | 23.9 (5.2) | 23.3 (4.9) | 23.1 (4.6) | 22.6 (4.6) | <.001 |
| Gestational diabetes, % | 2.9 | 5.0 | 2.9 | 2.4 | 3.1 | 1.3 | <.001 |
| Gestational age, wk | 39.4 (1.5) | 39.6 (1.7) | 39.4 (1.5) | 39.4 (1.4) | 39.4 (1.4) | 39.4 (1.5) | .36 |
| GA < 37 wk, % | 5.8 | 7.4 | 5.5 | 5.4 | 4.9 | 5.9 | .83 |
| Birth weight, g | 3402 (494) | 3437 (531) | 3402 (496) | 3402 (468) | 3374 (472) | 3345 (496) | <.001 |
| Preeclampsia, % | 0.9 | 1.4 | 1.1 | 1.1 | 0.6 | 0.6 | .05 |
| Preterm-PROM, % | 1.1 | 1.5 | 1.0 | 1.0 | 1.2 | 1.0 | .47 |
| Premature labor, % | 5.9 | 6.6 | 6.1 | 5.4 | 5.5 | 6.0 | .81 |
| Placental abruption, % | 0.9 | 1.0 | 0.7 | 0.8 | 1.0 | 1.0 | .56 |
| Placenta previa, % | 0.4 | 0.4 | 0.3 | 0.5 | 0.3 | 0.5 | .46 |
| Intrauterine fetal death, % | 0.02 | 0.06 | 0.0 | 0.0 | 0.05 | 0.0 | .46 |
Abbreviations: GA, gestational age; PROM, premature rupture of membranes.
Cutoffs for FT4 quintiles are 11.481, 12.642, 13.545, and 14.577 pmol/L.
Student's t test is for quintile 5 vs quintiles 1–4; χ2 test for categorical dependent variables.
Results are reported as median (SD); for TSH, results are expressed as median (log SD).
If the association between high FT4 and lower birth weight were also to include adverse events of pregnancy and delivery, these health end points might be more obvious in women with SGA outcomes. Birth weights of 920 of the 9209 offspring are less than the 10th centile for gestational age (≤2915 g) and are classified as SGA. Women in the SGA subgroup are younger, on average, than the remaining population (P = .024), weigh less (P ≤ .001), and a higher percentage deliver at gestation of 37 weeks or less (P ≤ .001). When the SGA subgroup is stratified by FT4 quintile (Table 2), there is no birth weight gradient, and gestational diabetes and preeclampsia do not differ significantly (FT4 quintile 5 vs quintiles 1–4). Labor/delivery complications once again are not significantly different in FT4 quintile 5, as compared with quintiles 1–4.
Table 2.
Pregnancy and Delivery Characteristics of 920 Euthyroid Women With SGA Babies, Stratified by FT4 Concentration at 15.0–18.9 Weeks' Gestation
| Characteristic | All | FT4 Quintilea |
P Valueb | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| n | 920 | 146 | 182 | 154 | 201 | 237 | |
| FT4, pmol/L | 13.42 (1.95)c | 10.64 (0.98) | 12.13 (0.27) | 13.03 (0.27) | 13.93 (0.29) | 15.35 (1.07) | |
| TSH, mIU/L | 1.23 (0.27) | 1.30 (0.26) | 1.20 (0.28) | 1.31 (0.26) | 1.33 (0.27) | 1.15 (0.28) | .04 |
| Antibody positive, % | 10.3 | 14.4 | 8.2 | 10.4 | 12.9 | 7.2 | .06 |
| Age, y | 28 (5.8) | 31 (5.5) | 29 (5.8) | 29 (5.9) | 27 (5.8) | 26 (5.3) | <.001 |
| Maternal weight, kg | 62.5 (14.0) | 66.8 (14.9) | 63.6 (14.6) | 62.7 (14.6) | 61.4 (13.6) | 60.0 (12.0) | <.001 |
| BMI, kg/m2 | 23.1 (5.1) | 25.0 (5.9) | 23.5 (5.3) | 22.8 (5.2) | 22.7 (4.6) | 22.2 (4.3) | <.001 |
| Gestational diabetes, % | 2.5 | 3.4 | 1.6 | 3.9 | 2.5 | 1.7 | .35 |
| Gestational age, wk | 39.3 (2.4) | 39.4 (2.7) | 39.1 (2.5) | 39.6 (2.2) | 39.3 (2.1) | 39.1 (2.3) | .18 |
| GA < 37 wk, % | 15.8 | 17.8 | 15.4 | 16.2 | 11.9 | 17.7 | .34 |
| Birth weight, g | 2743 (437) | 2750 (479) | 2695 (436) | 2778 (410) | 2750 (397) | 2722 (458) | .06 |
| Preeclampsia, % | 2.8 | 2.1 | 4.9 | 3.9 | 1.5 | 2.1 | .44 |
| Preterm-PROM, % | 2.8 | 2.7 | 3.8 | 1.3 | 2.0 | 3.8 | .30 |
| Premature labor, % | 8.0 | 7.5 | 6.6 | 5.8 | 9.0 | 10.1 | .17 |
| Placental abruption, % | 1.4 | 0.7 | 1.1 | 1.3 | 2.0 | 1.7 | .68 |
| Placenta previa, % | 1.3 | 0.0 | 0.5 | 1.9 | 1.0 | 2.5 | .05 |
| Intrauterine fetal death, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | .99 |
Abbreviations: PROM, premature rupture of membranes; GA, gestational age. SGA was defined as below the 10th centile for gestational age (bwt_adj = −3658.9 + 179.099 41 [ga_birth (weeks)].
Cutoffs for FT4 quintiles are 11.481, less than 12.642, less than 13.545, and less than 14.577 pmol/L.
Student's t test is for quintile 5 vs quintiles 1–4; χ2 test for categorical dependent variables.
Results are reported as median (SD); for TSH, results are expressed as median (log SD).
Interrelationships between maternal weight, FT4, and birth weight
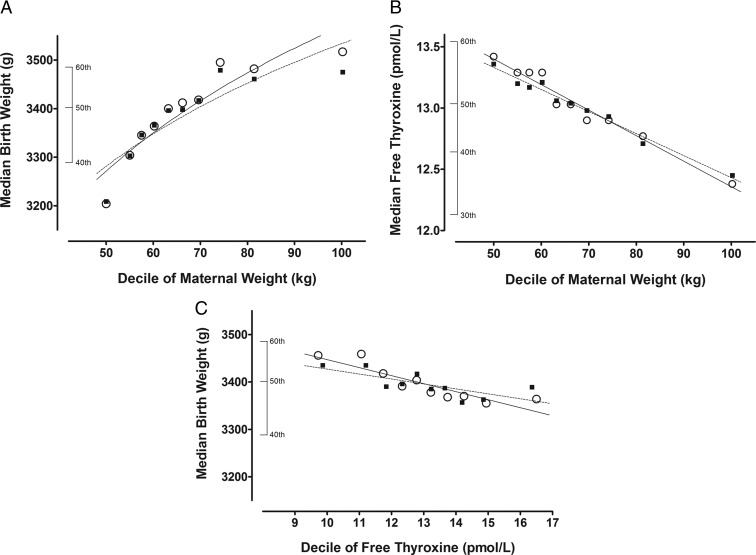
Figure 1 summarizes unadjusted (open circles) and adjusted (filled squares) relationships for maternal weight vs birth weight (Figure 1A), maternal weight vs FT4 (Figure 1B), and FT4 vs birth weight (Figure 1C). FT4 measurements are from samples obtained at 15.0–18.9 weeks' gestation. Maternal weights are from the time of entry into the study at 11.0–13.9 weeks' gestation. In Figure 1, A and C, all birth weights are corrected for gestational age at birth. Birth weights are then also adjusted for additional factors (race, smoking, gestational diabetes, education, and others; see figure legends). Among women with weight in the highest decile, the observed median birth weight is 313 g higher than in the lowest maternal weight decile (9.8% increase, P < .001, Figure 1A); median FT4 is 1.04 pmol/L lower than in the lowest maternal weight decile (7.7% decrease, P < .001, Figure 1B). After adjustment, birth weight is 266 g higher (8.3% increase, P < .001, Figure 1A) and FT4 is 0.91 pmol/L lower (6.8% decrease, P < .001, Figure 1B). Among women with FT4 in the highest decile, observed median birth weight is 192 g less than in the lowest FT4 decile (2.7% decrease, P < .001, Figure 1C) but is only 46 g less (1.3% decrease, P = .004, Figure 1C) after adjustment.
Figure 1.
Interrelationships between maternal free thyroxine (FT4), maternal weight, and birth weight. Open circles and solid lines indicate observed values; closed squares and dashed lines indicate adjusted values. Covariates used for adjusting relationships are listed individually for panels A–C. A, Median birth weight in relation to maternal weight. Adjustment factors include the following equation: predicted birth weight = 3466.65 − 15.558 38(FT4) + 80.677(race) − 190.549 36(smoking) + 143.927 54(gestational diabetes) + 10.045 78(education) + 114.743 24(parity). B, Median FT4 concentration in relation to maternal weight. Adjustment factors include the following equation: predicted FT4 = 1.598 97 − 0.02371(log TSH) − 0.422 98(maternal log age) + 0.019 65(race) − 0.029 48(smoking) − 0.0391(gestational diabetes) + 0.013 02(education). C, Median birth weight in relation to FT4 concentration. Adjustment factors include the following equation: predicted birth weight = 3262.6 + 76.239 42(race) − 185.518 77(smoking) + 155.15369(gestational diabetes) + 12.958 78(education) + 119.238 94(parity) and predicted FT4 = 1.974 93 − 0.238 98(log weight, kilograms) − 0.025 25(log TSH) − 0.381 88(maternal log age) + 0.022 91(race) − 0.028 31(smoking) − 0.026 67(gestational diabetes) + 0.007 11(education).
Pregnancy and delivery characteristics of SGA pregnancies after adjustment for maternal weight and other relevant covariates
To exclude SGA pregnancies that might be due to constitutional factors (eg, smaller babies from smaller mothers), we redefined the SGA subgroup, taking into account maternal characteristics known to influence birth weight (eg, maternal weight, parity, smoking). This adjustment removed 394 of the 920 pregnancies (42.8%) initially classified as SGA and added 394 that did not initially qualify for inclusion. Table 3 shows selected pregnancy and delivery characteristics for these 920 pregnancies, stratified once again by FT4 quintile. The format is the same as in Tables 1 and 2. In this redefined SGA subgroup, the median gestational age at delivery is earlier than in the remaining women (P < .001), and a considerably greater percent of SGA deliveries are at a gestation of 37 weeks or less (P < .001). As in Table 1, women in the highest FT4 quintile are younger in comparison with women in FT4 quintiles 1–4 and weigh less (P < .001 for both). The rate of gestational diabetes is lower (P = .007), but preeclampsia does not occur significantly less often. Rates of other complications of labor and delivery in the highest FT4 quintile do not differ significantly from quintiles 1–4.
Table 3.
Pregnancy and Delivery Characteristics of 920 Euthyroid Women With SGA Babies, Stratified by FT4 Concentration at 15.0–18.9 Weeks' Gestation, After Accounting for Maternal Weight and Other Relevant Variables
| Characteristic | All | FT4 Quintilea |
P Valueb | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| n | 920 | 170 | 200 | 166 | 169 | 215 | |
| FT4, pmol/L | 13.03 (1.92)c | 10.58 (0.92) | 12.13 (0.33) | 13.03 (0.27) | 13.93 (0.28) | 15.35 (1.02) | |
| TSH, mIU/L | 1.22 (0.26) | 1.24 (0.29) | 1.26 (0.26) | 1.25 (0.26) | 1.23 (0.26) | 1.16 (0.25) | .13 |
| Antibody positive, % | 11.5 | 16.5 | 11.0 | 11.4 | 9.5 | 9.8 | .36 |
| Age, y | 29 (5.7) | 32 (5.3) | 30 (5.6) | 28 (5.7) | 28 (6.0) | 27 (5.4) | <.001 |
| Maternal weight, kg | 65.9 (16.4) | 70.5 (17.0) | 66.8 (17.2) | 65.9 (17.9) | 63.6 (16.0) | 62.3 (12.2) | <.001 |
| BMI, kg/m2 | 24.1 (5.9) | 26.0 (6.6) | 24.4 (6.0) | 24.2 (6.1) | 23.7 (5.6) | 22.8 (4.4) | <.001 |
| Gestational diabetes, % | 3.4 | 6.5 | 2.5 | 3.6 | 4.7 | 0.5 | .007 |
| Gestational age, wk | 37.4 (2.3) | 37.0 (2.5) | 37.6 (2.4) | 37.3 (2.0) | 37.7 (2.2) | 37.4 (2.4) | .72 |
| GA < 37 wk, % | 40.7 | 48.2 | 41.5 | 43.4 | 31.4 | 39.1 | .59 |
| Birth weight, g | 2608 (394) | 2621 (421) | 2580 (392) | 2637 (348) | 2608 (372) | 2580 (420) | .03 |
| Preeclampsia, % | 3.6 | 4.1 | 5.5 | 4.2 | 1.2 | 2.8 | .47 |
| Preterm-PROM, % | 8.6 | 12.4 | 9.5 | 6.6 | 7.1 | 7.4 | .49 |
| Premature labor, % | 21.5 | 23.5 | 23.5 | 19.9 | 19.5 | 20.9 | .81 |
| Placental abruption, % | 3.9 | 4.7 | 4.5 | 4.2 | 4.7 | 1.9 | .08 |
| Placenta previa, % | 1.7 | 0.6 | 1.5 | 2.4 | 1.2 | 2.8 | .18 |
| Intrauterine fetal death, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | .99 |
Abbreviations: GA, gestational age; PROM, premature rupture of membranes; Adjustments use the MoM methodology described in Materials and Methods: predicted birth weight = 1495.17 + 981.71 (log weight) + 47.93 (race) − 182.77 (smoking) + 40.23 (parity) + 70.92 (college). SGA is defined as below the 10th centile for a given gestational age and maternal weight.
Cutoffs for FT4 quintiles are 11.481, less than 12.642, less than 13.545, and less than 14.577 pmol/L.
Student's t test is for quintile 5 vs quintiles 1–4; χ2 test for categorical dependent variables.
Results are reported as median (SD); for TSH, results are expressed as median (log SD).
Discussion
The present analysis confirms earlier observations that median birth weight is lower among euthyroid pregnant women with high FT4 (1, 2). The strength of this association is reduced after adjustment for several covariates (including maternal weight) but remains significant. In addition to birth weight, several maternal demographic and pregnancy-related characteristics segregate with FT4 stratification. When FT4 concentrations are in the highest quintile, women are younger, weigh less, and are diagnosed with gestational diabetes and preeclampsia less frequently. A linear test of trend confirms an FT4-dependent gradient of risk for both gestational diabetes and preeclampsia. TSH levels and frequency of thyroid antibodies both are lower. Despite a relationship between high maternal serum FT4 and low birth weight, gestational age at delivery and complications relating to delivery are not different from the cohort as a whole, nor does there appear to be a FT4-dependent gradient of risk. When SGA pregnancies are stratified and analyzed in the same format, there is once again no indication that high FT4 among euthyroid women is associated with higher risk for adverse events, even though median birth weight is lower.
There is no obvious explanation for the connection between FT4 concentration and either birth weight or maternal weight among euthyroid women. However, the fact that both birth weight and maternal weight are lower, on average, with high FT4 raises the possibility that shifting thyroid hormone concentrations might play a causal role in influencing fetal growth and maternal metabolism, even within the normal TSH range. If, in fact, thyroid hormones are causal, multivariate adjustments for FT4 and birth weight that include maternal weight might underestimate the impact of shifts in thyroid hormone concentrations. The lower frequency of gestational diabetes among euthyroid women with high FT4 levels may also be explained by observed changes in thyroid hormones. Establishing a causal connection, however, would require that a reciprocal relationship exist between concentrations of FT4 and free triiodothyronine (FT3). FT3, the active thyroid hormone, is known to stimulate endogenous glucose production (11). Assuming that a high normal FT4 might act as a surrogate marker for reduced endogenous maternal glucose production, this would also offer a possible explanation for the lower birth weight.
Unfortunately, the present study did not include measurements of FT3. Two large, population-based studies in women during early pregnancy, however, support not only our observed relationship between lower BMI and higher FT4 but also report lower FT3 in that context (4, 5). In two other studies, FT4 and the FT3 to FT4 ratio are examined in euthyroid pregnant women in relation to BMI and glucose metabolism at the time of glucose screening (24–28 wk gestation) (12, 13). The FT3 to FT4 ratio is used as an index of conversion of T4 to T3 by peripheral deiodinase activity (14). In both of these studies, higher FT4 and lower FT3 to FT4 ratio are associated not only with lower BMI but also with lower hemoglobin A1c, lower insulin resistance, and lower triglycerides. The FT3 to FT4 ratio is more strongly associated with these metabolic end points than FT4 alone. Collectively, these published data involving euthyroid pregnancies are consistent with an indirect link between high FT4, lower peripheral deiodinase activity, and lower endogenous glucose production.
Dietary influences on deiodinase activity have been explored in an overfeeding study in mice in which a high-fat diet induced higher serum T3 levels, lower T4 levels, and an increased T3 to T4 ratio. Deiodinase-1 activity and deiodinase-1 mRNA levels in the liver both increased. This study provides evidence that an obesity-induced increase in deiodinase activity may explain the lower FT4 levels (15). Similar findings were reported in an overfeeding study in men carried out by Danforth et al in 1979 (16). The authors concluded that overfeeding likely accelerated the peripheral conversion of FT4 to FT3, again adding plausibility to our speculation that high FT4 in pregnant women with low BMI might be a marker for low FT3 and low deiodinase activity.
Under the conditions of our study, the thyroid gland is functioning normally. When put together with published evidence involving euthyroid pregnancies from other sources, the high FT4 associated with lower maternal BMI is expected to be accompanied by low FT3, and this pattern appears to be explained by lower peripheral deiodinase activity. FT4 measurement thus reflects a combination of thyroid gland output and peripheral deiodinase activity in the euthyroid state. Our data indicate that high FT4 (and lower deiodinase activity) is not associated with higher rates of pregnancy/delivery complications. In the event that an advantage might be envisioned for adjusting peripheral deiodinase activity, the only available option would be through dietary manipulation. Data from the present study therefore offer limited implications for modifying clinical practice.
Strengths of the present study include second-trimester measurements of TSH, FT4, and thyroid antibodies in a large cohort of pregnant women. Gestational dates are reliable and complete, and verified outcome information is available in nearly all cases. Absence of FT3 measurements is a major shortcoming, given the possible connection between variations in thyroid hormone function in euthyroid women that might explain lower birth weight among women with high FT4 concentrations. Also, assessment of adverse events is necessarily limited to pregnancy and delivery; longer-term physical and neuropsychological consequences were not included in the study protocol. The present observations suggest that further investigation into a possible connection between maternal thyroid hormone activity and birth weight among euthyroid women might be of interest.
Acknowledgments
This work was supported by Grant RO1 HD 38652 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- FT3
- free triiodothyronine
- FT4
- free T4
- MoM
- multiple of the median
- SGA
- small for gestational age.
References
- 1. Medici M, Timmermans S, Visser W, et al. Maternal thyroid hormone parameters during early pregnancy and birth weight: the Generation R Study. J Clin Endocrinol Metab. 2013;98:59–66 [DOI] [PubMed] [Google Scholar]
- 2. Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011;96:E934–E938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haddow JE, Craig WY, Palomaki GE, et al. Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid. 2013;23:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mannisto T, Surcel HM, Ruokonen A, et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21:291–298 [DOI] [PubMed] [Google Scholar]
- 5. Ashoor G, Kametas NA, Akolekar R, Guisado J, Nicolaides KH. Maternal thyroid function at 11–13 weeks of gestation. Fetal Diagn Ther. 2010;27:156–163 [DOI] [PubMed] [Google Scholar]
- 6. Pop VJ, Biondi B, Wijnen HA, Kuppens SM, Lvader H. Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin Endocrinol (Oxf). 2013;79:577–583 [DOI] [PubMed] [Google Scholar]
- 7. Ay L, Kruithof CJ, Bakker R, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG. 2009;116:953–963 [DOI] [PubMed] [Google Scholar]
- 8. Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339:283–287 [DOI] [PubMed] [Google Scholar]
- 9. Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambert-Messerlian G, McClain M, Haddow JE, et al. First- and second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First- and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am J Obstet Gynecol. 2008;199:62 e61–e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pisarev MA. Interrelationships between the pancreas and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2010;17:437–439 [DOI] [PubMed] [Google Scholar]
- 12. Bassols J, Prats-Puig A, Soriano-Rodriguez P, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. 2011;96:3717–3723 [DOI] [PubMed] [Google Scholar]
- 13. Knight B, Shields B, Hattersley A, Vaidya B. Thyroid hormone levels in normal pregnancy are associated with altered metabolic parameters. Eur Thyroid J. 2012;1(suppl 1):89 [Google Scholar]
- 14. Agnihothri RV, Courville AB, Linderman JD, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pelletier P, Gauthier K, Sideleva O, Samarut J, Silva JE. Mice lacking the thyroid hormone receptor-α gene spend more energy in thermogenesis, burn more fat, and are less sensitive to high-fat diet-induced obesity. Endocrinology. 2008;149:6471–6486 [DOI] [PubMed] [Google Scholar]
- 16. Danforth E, Jr, Horton ES, O'Connell M, et al. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J. Clin Invest. 1979;64:1336–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]