Abstract
Context:
Loss-of-function mutations in makorin ring finger 3 (MKRN3), an imprinted gene located on the long arm of chromosome 15, have been recognized recently as a cause of familial central precocious puberty (CPP) in humans. MKRN3 has a potential inhibitory effect on GnRH secretion.
Objectives:
The objective of the study was to investigate potential MKRN3 sequence variations as well as copy number and methylation abnormalities of the 15q11 locus in patients with apparently sporadic CPP.
Setting and Participants:
We studied 215 unrelated children (207 girls and eight boys) from three university medical centers with a diagnosis of CPP. All but two of these patients (213 cases) reported no family history of premature sexual development. First-degree relatives of patients with identified MKRN3 variants were included for genetic analysis.
Main Outcome Measures:
All 215 CPP patients were screened for MKRN3 mutations by automatic sequencing. Multiplex ligation-dependent probe amplification was performed in a partially overlapping cohort of 52 patients.
Results:
We identified five novel heterozygous mutations in MKRN3 in eight unrelated girls with CPP. Four were frame shift mutations predicted to encode truncated proteins and one was a missense mutation, which was suggested to be deleterious by in silico analysis. All patients with MKRN3 mutations had classical features of CPP with a median age of onset at 6 years. Copy number and methylation abnormalities at the 15q11 locus were not detected in the patients tested for these abnormalities. Segregation analysis was possible in five of the eight girls with MKRN3 mutations; in all cases, the mutation was inherited on the paternal allele.
Conclusions:
We have identified novel inherited MKRN3 defects in children with apparently sporadic CPP, supporting a fundamental role of this peptide in the suppression of the reproductive axis.
Gonadotropin-dependent precocious puberty, also known as central precocious puberty (CPP), is clinically defined by the development of secondary sexual characteristics before age 8 years in girls and 9 years in boys. CPP has a striking predominance in girls. It results from premature reactivation of the hypothalamic-pituitary-gonadal axis with pubertal levels of gonadotropins and consequent gonadal stimulation (1, 2). Affected children have premature and progressive sexual development associated with acceleration in linear growth and advancement in bone age. No hypothalamic tumors or lesions are detected in the great majority of CPP patients, suggesting a different underlying mechanism such as genetic, epigenetic, and/or environmental factors (1, 3).
Despite efforts to establish a genetic cause for CPP, only rare genetic variants had been demonstrated until recently (4–8). Gene selection in previous studies was based mainly on a candidate gene approach, including genes involved in physiological processes regulating GnRH secretion in animal studies as well as genes implicated in the etiology of congenital isolated hypogonadotropic hypogonadism. To date, mutations associated with CPP have been identified in only two genes, KISS1 and KISS1R (4, 5). Very recently, using whole exome sequencing as a hypothesis-free approach to identify genetic variants, we were able to identify loss-of-function mutations in a single gene, MKRN3, encoding the makorin ring finger protein 3, in 5 of 15 families with CPP (9). These findings suggest that MKRN3 mutations may be a cause of familial CPP in humans, establishing a clear genetic component to this reproductive condition.
MKRN3 is an intronless gene located on chromosome 15q11.2, in the Prader Willi syndrome critical region. The maternal allele of this gene is silenced, and only the paternal allele is expressed (10), which is consistent with the paternal inheritance pattern in families with CPP due to MKRN3 defects (9). The makorin ring finger 3 (MKRN3) protein structure suggests an association with protein ubiquitination, but the precise mechanism by which its inactivation leads to precocious puberty remains to be elucidated (11).
Genomic imprinting is an epigenetic process that involves DNA methylation to achieve monoallelic gene expression without altering the genetic sequence (12). DNA methylation usually takes place on cytosine nucleotides located within CpG islands in the promoter sequences of genes, resulting in transcriptional silencing. Imprinted genes play an etiological role in several human diseases, such as Prader Willi syndrome, Angelman syndrome, and Beckwith-Wiedemann syndrome (13). To date, it is unknown whether CPP could arise from the loss of MKRN3 expression by the paternal allele due to a de novo deletion, maternal uniparental disomy, or an imprinting defect, mechanisms recognized in the pathogenesis of Prader Willi syndrome (14). In the present study, we investigated potential MKRN3 sequence variations in 215 patients with CPP as well as copy number and methylation abnormalities of the 15q11 locus in a partially overlapping cohort of 52 patients.
Patients and Methods
In this study, we analyzed 215 consecutive unrelated children (207 girls and eight boys) with idiopathic CPP from three different university medical centers: Sao Paulo University (Brazil), Sao Paulo campus (96 cases), and Ribeirao Preto campus (87 cases); Campinas University (four cases); and Medical Faculty Skopje, Macedonia (28 cases). When available, family members of patients with MKRN3 mutations were invited to participate in the study. Fourteen relatives of five probands agreed to participate in the genetic analyses. No family history of premature sexual development was reported in 213 cases, whereas two female patients had first-degree relatives who were found to have precocious puberty.
CPP was diagnosed by clinical signs of pubertal development before age 8 years in girls and 9 years in boys, pubertal basal and/or GnRH-stimulated LH levels, advanced bone age (Greulich and Pyle method), and normal central nervous system magnetic resonance imaging (15–17). Each medical center had its own protocol that was approved by local ethics committees. Written informed consent was obtained from all participants or their parents/guardians. Clinical and hormonal data from all patients are summarized in http://press.endocrine.org/doi/suppl/10.1210/JC.2013-.3126/suppl_file/JC-13-3126.pdf Supplemental Appendix Table 1.
The single exon of MKRN3 was sequenced in all 215 patients. For gene dosage and methylation analysis, 40 patients from this cohort were randomly selected, together with 12 children with familial CPP from the previous study in whom no MKRN3 mutations were described (9). KISS1 and KISS1R genes were previously sequenced in 206 of 215 patients (96%) with CPP in this cohort, and the LIN28B gene was sequenced in 178 of 215 patients (83%). Only two mutations (one in KISS1 and one in KISS1R) were identified in the patients with sporadic and familial CPP in the previous studies (4–6).
Hormone assays
Serum LH, FSH, T, and estradiol concentrations were measured by immunofluorometric assay (IFMA) and immunochemiluminometric assay (ICMA). The interassay coefficient of variation was 5% or less for all assays. For the acute GnRH stimulation test, serum LH and FSH were measured at −15, 0, 15, 30, 45, and 60 minutes after iv administration of 100 μg GnRH. Basal LH greater than 0.6 U/L (IFMA) or greater than 0.15 U/L (ICMA) were considered as pubertal levels for both sexes, and a GnRH-stimulated LH peak greater than 6.9 U/L for girls and greater than 9.6 U/L for boys (IFMA) or greater than 5.0 U/L for both sexes (ICMA) were considered as a pubertal response (15, 16). Basal estradiol levels higher than 21 pg/mL and basal T levels higher than 19 ng/dL were considered as pubertal.
Genetic analysis
PCR amplification and sequencing of MKRN3
Genomic DNA was isolated from peripheral blood leukocytes from all patients using standard procedures. The entire coding region of MKRN3 (GenBank accession number NC_000015.9) was amplified by PCR followed by automatic sequencing of the products using the Sanger method (see Supplemental Table 2 for more details). Two databases (1000 Genomes and NHLBI EVS) were used to exclude all common variants (minor allele frequency >1%) (18, 19). Computational algorithms (PolyPhen, SIFT, Panther, and MutationTaster) were used to predict the pathogenicity of the missense variants (20).
Methylation-specific multiplex ligation-dependent probe amplification (MLPA)
Methylation-specific multiplex ligation-dependent probe amplification (SALSA methylation-specific MLPA Kit ME028; MRC Holland) was used to detect potential copy number changes as well as to analyze CpG island methylation of chromosome 15q11 in a semiquantitative manner. The Angelman/Prader Willi syndrome locus at 15q11–13 contains at least eight imprinted genes, including the MKRN3 gene, which are regulated by a bipartite imprinting center associated with the small nuclear ribonucleoprotein polypeptide N (SNRPN) gene (21). The MLPA commercial assay includes 32 probes for nucleotide sequences in or near the Prader Willi syndrome critical region of chromosome 15q11, including a specific MKRN3 probe (172 bp in exon 1). These probes were used to test for deletions and duplications of one or more sequences of the 15q11 region in the DNA samples. Five of these probes are specific for imprinted sequences, four for SNRPN and one for the necdin (NDN) gene, and contain one recognition site each for the methylation-sensitive restriction enzyme HhaI. Digestion with HhaI after hybridization of the DNA samples with these probes, followed by PCR amplification, was used to test for the presence of aberrant methylation patterns in the 15q11 locus, caused by either uniparental disomy or by imprinting defects, as previously described (13) (see Supplemental Appendix for additional details).
Statistical analyses
Statistical and computational analyses were performed using the software SigmaStat3.5. To compare clinical and hormonal data between patients with and without MKRN3 mutations, the nonparametric Mann-Whitney U test was used. Statistical significance was set at P < .05.
Results
DNA sequencing
Automatic sequencing of MKRN3 in 215 patients with CPP revealed five novel heterozygous mutations in eight unrelated girls, including four indel variants (ie, insertions or deletions) and one missense variant (Table 1 and Supplemental Appendix Figure 1). These five variants were absent in two databases (1000 Genomes and NHLBS EVS). All indel variants (p.Pro161Argfs*10, p.Pro161Argfs*16, p.Gln226Thrfs*6, p.Glu256Glyfs*36) were located in the N-terminal region of the MKRN3 protein. The missense mutation (p.Phe417Ile), located within a zinc finger, was predicted to be deleterious by four different in silico prediction programs (Mutation Taster, PolyPhen-2, SIFT, and Panther). The remaining patients did not have any detectable rare coding variants (minor allele frequency < 1%) in MKRN3.
Table 1.
Clinical and Hormonal Features of Eight Girls With CPP Associated With MKRN3 Mutations
| Patient Number | Initial Clinical Manifestation (Age, y) | Initial Diagnosis |
LH, IU/L |
FSH, IU/L Basal | E2, pg/mL | Mutation cDNA and Protein | Additional Family Members With MKRN3 Mutations# | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Breast Tanner Stage | Pubic Hair Tanner Stage | BMI (Z) | BA, y | Basal | After GnRH | ||||||
| 1 | Thelarche and pubarche (6.4) | 7.8 | 3 | 4 | 0.4 | 11 | 1.0 | 12 | 5.9 | 15 | c.482delC | Father and sister (20 y) |
| p.Pro161Argfs*10 | ||||||||||||
| 2 | Thelarche (5.4) | 6.1 | 3 | 1 | 0.2 | 8.8 | 1.3 | 5 | 36 | c.482_483insC | Father and sister (4 y) | |
| p.Pro161Argfs*16 | ||||||||||||
| 3 | Thelarche (6.0) | 7.9 | 4 | 3 | 2.0 | 11 | 3.4 | 10 | 80 | c.482_483insC | NA | |
| p.Pro161Argfs*16 | ||||||||||||
| 4 | Thelarche (3.0) and pubarche (6.0) | 6.7 | 3 | 1 | 1.5 | 8.8 | 1.8 | 62.5 | 4.9 | 60 | c.482delC | Father |
| p.Pro161Argfs*10 | ||||||||||||
| 5 | Thelarche (4.0) | 6.8 | 4 | 3 | 1.3 | 12 | 6.1 | 4.7 | 62 | c.675_676insA | NA | |
| p.Gln226Thrfs*6 | ||||||||||||
| 6 | Thelarche and pubarche (6.0) | 6.6 | 3 | 2 | 1.4 | 7.8 | 1.6 | 4.6 | 20 | c.766_767delA | NA | |
| p.Glu256Glyfs*36 | ||||||||||||
| 7 | Thelarche (6.0) | 7.1 | 3 | 1 | 0.2 | 8.8 | 0.3 | 7.5 | 4.4 | 44 | c.1249T>A | Father |
| p.Phe417Ile | ||||||||||||
| 8 | Thelarche (6.0) and pubarche (6.3) | 6.9 | 3 | 2 | 0.5 | 9.3 | 2.7 | 16.6 | 6.5 | 47 | c.482_483insC | Father |
| p.Pro161Argfs*16 | ||||||||||||
Abbreviations: BA, bone age; BMI, body mass index; E2, estradiol; NA, not available. Normal values are as follows: E2, prepubertal levels less than 21 pg/mL; LH, prepubertal basal levels less than 0.6 (IU/L), peak less than 6.9 (IU/L) in girls. Breast and pubic hair Tanner stage and bone age were assessed at the time of diagnosis.
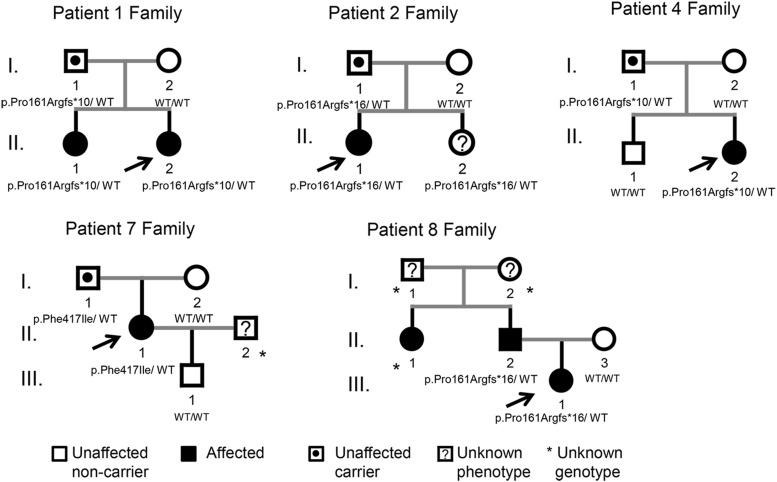
The familial segregation analysis is illustrated in Figure 1.
Clinical features of CPP patients with MKRN3 defects
Patients with CPP due to MKRN3 mutations had typical clinical and hormonal features of premature activation of the reproductive axis, including early pubertal signs, such as breast and pubic hair development, accelerated linear growth, advanced bone age, and elevated basal and/or GnRH-stimulated LH levels (Table 1 and Supplemental Data). Patient 6 had mild nonspecific syndromic features, such as high-arched palate, dental abnormalities, clinodactyly, and hyperlordosis.
The age of pubertal onset in these girls ranged from 3.0 to 6.4 years (mean 5.3 y and median 6.0 y). Simultaneous development of breasts and pubic hair, as the initial clinical manifestations, were observed in four of eight patients, whereas the remaining four patients initially presented only with thelarche. Bone age was advanced in all cases. Median basal and GnRH-stimulated LH levels were 1.7 U/L (ranging from 0.3 to 6.1 U/L) and 14.3 U/L (ranging from 7.5 to 62.5 U/L), respectively. All affected girls were treated with GnRH analogs for a mean period of 3.4 years. Satisfactory clinical and hormonal control was obtained in six of eight patients, who had menarche at an appropriate age, and they reached a near predicted final height. The two other patients were noncompliant with treatment (Supplemental Data). Except for the significantly (P = .016) higher levels of basal FSH in patients with MKRN3 mutations (median 4.9, ranging from 4.4 to 10) compared with those without MKRN3 mutations (median 3.6, ranging from 1.0 to 9.8), no other clinical or hormonal differences were identified between these groups (Supplemental Appendix Table 2).
Familial segregation analysis
Genetic analysis was carried out in 14 first-degree relatives of five of eight children with MKRN3 mutations (Figure 1 and Table 1). Among these five families, genomic DNA from all parents (10 individuals), three siblings (families 1, 2, and 4), and one son (family 7) was available for MKRN3 analysis. Family members of patients 3, 5, and 6 were not accessible for genetic studies. Except for the older sister of patient 1 and the father and paternal aunt of patient 8, who had histories of premature sexual development, indicating familial CPP, all other relatives studied reported normal onset of pubertal development. Segregation analysis demonstrated that the mutant allele was paternally inherited in all five families because the fathers were carriers of the same mutation as their affected daughters, whereas the mothers were wild type for MKRN3 (Figure 1). Patient 2 has a younger sister (aged 4 y) who carries the same mutation (p.Pro161Argfs*16) but has not yet shown signs of puberty.
Figure 1.
Pedigrees of the patients with MKRN3 mutations. Squares indicate male family members, circles female members, black symbols clinically affected family members, symbols with black circles unaffected carriers, and question mark unknown phenotype. *, Unknown genotype. Arrows the proband in each family. The MKRN3 genotypes are shown for family members whose DNA was available for genetic studies. WT, wild-type. Families 1 and 8 are typical familial CPP cases. Patient II.2 from family 2 (4 y old) has not yet shown signs of puberty.
Gene dosage and methylation analysis of the 15q11-13 locus
No abnormalities in gene copy number or methylation pattern were detected by methylation-specific MLPA in the chromosome 15q11 locus in any of the 52 patients studied (Supplemental Appendix Figure 2 and data not shown).
Discussion
The estimated incidence of CPP in American girls is 1:5000–1:10 000 (3). Among Danish girls, the prevalence of precocious puberty was 1:500, based on national registries over a 9-year period (22). Precocious puberty can result in short stature and psychological and behavioral disorders in untreated patients (1). Accumulating evidence suggests an association between early timing of puberty and adverse health outcomes in later life (23). Early age at menarche has been associated with increased risk of obesity, hypertension, type 2 diabetes, ischemic heart disease and stroke, cancer, and cardiovascular mortality (24–27).
In the present study, we identified five novel MKRN3 defects, including four frame shift mutations and one missense variant, in eight unrelated patients with idiopathic CPP (Table 1 and Supplemental Appendix Figure 1). Notably, frame shift mutations are generally loss-of-function mutations. However, they could potentially affect regulatory regions, promoting overexpression or having other consequences. Therefore, definitive confirmation that these indels and the missense variant cause loss of function awaits the availability of a MKRN3 functional assay. Nevertheless, the absence of the missense variant in databases such as 1000 Genomes and NHLBI EVS, and the in silico predictions of a deleterious effect on protein function suggest that this variant is related to the patient's phenotype. The identification of these novel variants in MKRN3 strengthens previous evidence that mutations in this gene are linked to CPP (9).
Two indel mutations of MKRN3, p.Pro161Argfs*10 and p.Pro161Argfs*16 described in the current study, recurred in apparently unrelated girls with CPP, patients 1 and 4 and patients 2, 3, and 8, respectively, suggesting a mutational hot spot codon (Table 1). Interestingly, these mutations affect a poly-C region of MKRN3, a potential hypermutable site.
Combining the previous (familial CPP) and current patients with MKRN3 defects, the mean age of puberty onset was 6.0 years in both sexes (9). The youngest case (patient 4) was a 3-year-old girl with Tanner stage 3 breast development and advanced bone age. This mean age of pubertal onset contrasts with those reported in the two patients with activating mutations involving the kisspeptin system, who started pubertal development in the first year of life (4, 5). No difference was observed in the median age of pubertal onset between patients with and without MKRN3 mutations: 6.0 years (range 3.0–6.4 y) vs 6.0 years (range 0.1–9.0 y), respectively (P = .44) (Supplemental Appendix Table 2). Given the median age of pubertal onset of affected children with MKRN3 mutations, we speculate that the prepubertal inhibitory tonus on GnRH secretion apparently took place normally but was lost prematurely. This clinical observation suggests that MKRN3 is not crucial for GnRH suppression after the minipuberty of early infancy but that its down-regulation plays a relevant role for the reemergence of GnRH pulses in the pubertal phase. Interestingly, down-regulation of Mkrn3 was recently demonstrated in the arcuate nucleus of mice prior to the onset of puberty (9).
The basal and GnRH-stimulated LH levels as well as the LH to FSH peak ratio are the major hormonal parameters for the diagnosis of activation of reproductive axis in children with central precocious puberty. CPP patients with MKRN3 mutations had significantly higher basal FSH levels than patients without mutations (Supplemental Appendix Table 2). Although statistically significant, this result must be interpreted with caution because it is based on a small number of MKRN3 mutation carriers. There is no definitive explanation for this difference at this time. However, it is possible that MKRN3 could play distinct roles in the release of the two pituitary gonadotropins, LH and FSH.
All patients with MKRN3 mutations had typical clinical features of pubertal development and hormonal profiles compatible with hypothalamic-pituitary-gonadal axis activation. In contrast to the near equal distribution of boys and girls with familial CPP due to MKRN3 loss-of-function mutations, MKRN3 mutations were identified only in girls in the current study (9). This finding may be explained by the smaller proportion of boys in the present cohort (one male and 26 females) compared with the previous study (one male and five females) (9). However, the exact reason for this sexual dimorphism is not well understood (28–30).
Familial segregation analysis was conducted in five of eight patients with MKRN3 abnormalities and paternal inheritance was shown in all five, indicating that the familial nature of CPP is likely underrecognized. Notably, most children with CPP present for medical consultation accompanied by their mothers and the paternal family history is often unknown. Another limitation to the detection of familial cases is in determining the precise onset of puberty in boys and in their fathers because testicular enlargement is not as obvious as breast development and menarche in girls (31). Another point to consider is the age of the proband's siblings at the first medical visit. Because the median age of pubertal onset in patients with MKRN3 mutations is 6 years, it is important to follow up the younger siblings of patients with MKRN3 mutations carefully, such as the youngest, currently asymptomatic sister of patient 2, who harbors a p.Pro161Argfs*16 mutation of MKRN3 to detect signs of precocious puberty and to implement treatment as early as possible.
Interestingly, genes located on the long arm of chromosome 15 have been involved in human pubertal development based on clinical and genetic studies (32–34). It is well known that patients with Prader Willi syndrome typically have incomplete sexual development, but, paradoxically, some begin to exhibit signs of puberty much earlier than expected (35). No major signs of Prader Willi syndrome were detected in any patient with CPP in the present study. The incidence of CPP in Prader Willi syndrome is not entirely clear, but it is evident that normal onset and progression of puberty is frequently disrupted. To date, few cases (14 patients) of children with Prader Willi syndrome and precocious puberty have been reported (35). Here we hypothesized that deletions and imprinting abnormalities of chromosome 15 could be implicated in the pathogenesis of CPP in those patients without identified MKRN3 mutations. Therefore, gene dosage and the DNA methylation pattern of the chromosome 15q locus were analyzed in 52 patients without MKRN3 mutations. However, no interstitial deletion or methylation abnormalities were identified in these analyses. Due to the limitations of the technique performed (only five probes were available to detect aberrant methylation patterns) and because the mechanisms by which imprinting and expression of paternal genes within the Prader Willi syndrome domain, such as MKRN3 and necdin, are regulated by the Prader Willi syndrome-imprinting center are unclear (21), we cannot rule out defects located outside the target sequences of the methylation-specific MLPA probes.
To date, MKRN3 defects represent the most frequent known genetic cause of familial CPP. We have demonstrated novel MKRN3 mutations in children with and without a family history of CPP, supporting a role of MKRN3 in the control of puberty initiation. Because of the imprinting pattern of MKRN3, the CPP phenotype can be inherited from an apparently asymptomatic father, highlighting the importance of obtaining a detailed family history, especially from the paternal side, in children with apparently idiopathic CPP. MKRN3 gene analysis may provide an additional tool for the diagnosis of familial CPP, allowing early diagnosis and adequate treatment of children with CPP.
Acknowledgments
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 2013/06391-1 (to D.B.M.); National Institutes of Health (NIH) Grant 1F05HD072773-01 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Grant 3806-11-1 (to A.P.A.); NIH Grant 1K23HD073351 (to A.D.); Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and Grant R21HD066495 (to U.B.K.); Brazilian Grant 302825/2011-8 (to A.C.L.), Grant 300982/2009-7 (to I.J.P.A.), and Grant 305743/2011-2 (to B.B.M.) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico; and Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 2005/04726-0 (to A.C.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CPP
- central precocious puberty
- ICMA
- immunochemiluminometric assay
- IFMA
- immunofluorometric assay
- MKRN3
- makorin ring finger 3
- MLPA
- multiplex ligation-dependent probe amplification.
References
- 1. Carel JC, Léger J. Precocious Puberty. N Engl J Med. 2008;358(22):2366–2377 [DOI] [PubMed] [Google Scholar]
- 2. Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57:2–14 [DOI] [PubMed] [Google Scholar]
- 3. Partsch CJ, Heger S, Sippell WG. Management and outcome of central precocious puberty. Clin Endocrinol (Oxf). 2002;56:129–148 [DOI] [PubMed] [Google Scholar]
- 4. Teles MG, Bianco SD, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silveira-Neto AP, Leal LF, Emerman AB, et al. Absence of functional LIN28B mutations in a large cohort of patients with idiopathic central precocious puberty. Horm Res Paediatr. 2012;78:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao Y, Chen T, Zhou Y, Li K, Xiao J. An association study between the genetic polymorphisms within GnRHI, LHβ, FSHβ genes and central precocious puberty in Chinese girls. Neurosci Lett. 2010;486:188–192 [DOI] [PubMed] [Google Scholar]
- 8. Luan X, Yu H, Wei X, et al. GPR54 polymorphisms in Chinese girls with central precocious puberty. Neuroendocrinology. 2007;86:77–83 [DOI] [PubMed] [Google Scholar]
- 9. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jong MT, Gray TA, Ji Y, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8:783–793 [DOI] [PubMed] [Google Scholar]
- 11. Böhne A, Darras A, D'Cotta H, Baroiller JF, Galiana-Arnoux D, Volff JN. The vertebrate makorin ubiquitin ligase gene family has been shaped by large-scale duplication and retroposition from an ancestral gonad-specific, maternal-effect gene. BMC Genomics. 2010;11:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swain JL, Stewart TA, Leder P. Parental legacy determines methylation and expression of an autosomal transgene: a molecular mechanism for parental imprinting. Cell. 1987;50(5):719–727 [DOI] [PubMed] [Google Scholar]
- 13. Bittel DC, Kibiryeva N, Butler MG. Methylation-specific multiplex ligation-dependent probe amplification analysis of subjects with chromosome 15 abnormalities. Genet Test. 2007;11(4):467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26 [DOI] [PubMed] [Google Scholar]
- 15. Brito VN, Batista MC, Borges MF, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. 1999;84:3539–3544 [DOI] [PubMed] [Google Scholar]
- 16. Neely EK, Hintz RL, Wilson DM, et al. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1995;127(1):40–46 [DOI] [PubMed] [Google Scholar]
- 17. Greulich WW, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed Stanford, CA: Stanford University Press; 1971 [Google Scholar]
- 18. San Lucas FA, Wang G, Scheet P, Peng B. Integrated annotation and analysis of genetic variants from next-generation sequencing studies with variant tools. Bioinformatics. 2012;28:421–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Heart, Lung, and Blood Institute. Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP). 2013. http://evs.gs.washington.edu/EVS/
- 20. Frousios K, Iliopoulos CS, Schlitt T, Simpson MA. Predicting the functional consequences of non-synonymous DNA sequence variants—evaluation of bioinformatics tools and development of a consensus strategy. Genomics. 2013;102(4):223–228 [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez-Jato S, Shan J, Khadake J, et al. Regulatory elements associated with paternally expressed genes in the imprinted murine Angelman/Prader-Willi syndrome domain. PLoS One. 2013;8(2):e52390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teilmann G, Pedersen CB, Jensen TK, Skakkeback NE, Juul A. Prevalence and incidence of precocious pubertal development in Denmark: an epidemiologic study based on national registries. Pediatrics. 2005;116:1323–1328 [DOI] [PubMed] [Google Scholar]
- 23. Widén E, Silventoinen K, Sovio U, et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care. 2012;35:850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51:781–786 [DOI] [PubMed] [Google Scholar]
- 25. Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960 [DOI] [PubMed] [Google Scholar]
- 26. He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from two large prospective cohorts. Am J Epidemiol. 2009;171(3):334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elks CE, Perry J, Sulem P, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bianco SDC. A potential mechanism for the sexual dimorphism in the onset of puberty and incidence of idiopathic central precocious puberty in children: sex-specific kisspeptin as an integrator of puberty signals. Front Endocrinol. 2012;3:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokininB neurons. Eur J Neurosci. 2010;31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 30. Klein KO. Precocious puberty: who has it? Who should be treated? J Clin Endocrinol Metab. 1999;84(2):411–414 [DOI] [PubMed] [Google Scholar]
- 31. Pigneur B, Trivin C, Brauner R. Idiopathic central precocious puberty in 28 boys. Med Sci Monit. 2008;14(1):CR10–CR14 [PubMed] [Google Scholar]
- 32. Beneduzzi D, Iyer A, Trarbach EB, et al. Mutational analysis of the necdin gene in patients with congenital isolated hypogonadotropic hypogonadism. Eur J Endocrinol. 2011;165(1):145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller NL, Wevrick R, Mellon PL. Necdin, a Prader-Willi syndrome candidate gene, regulates gonadotropin-releasing hormone neurons during development. Hum Mol Genet. 2009;18(2):248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanber D, Giltay J, Wieczorek D, et al. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader–Willi syndrome. Eur J Hum Genet. 2009;17(5):582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee HS, Hwang JS. Central precocious puberty in a girl with Prader-Willi syndrome. J Pediatr Endocr Metab. 2013;30:1–4 [DOI] [PubMed] [Google Scholar]