Abstract
Context:
Postprandial hypoglycemia, a late complication of gastric bypass (GB) surgery, is associated with an exaggerated insulin response to meal ingestion.
Objective:
The purpose of this study was to characterize insulin secretion and other glucoregulatory hormone responses to meal ingestion after GB based on hypoglycemia and clinical symptoms.
Methods:
We conducted a cross-sectional analysis of insulin secretion rate and islet and gastrointestinal hormone responses to liquid mixed meal ingestion in 65 subjects with GB and 11 body mass index-matched controls without surgery. The GB subjects were stratified by clinical history for analysis of their responses to the test meal.
Results:
The glucose and insulin responses to meal ingestion were shifted upward and to the left after GB, with the largest early insulin response and the lowest nadir glucose levels in patients with a history of hypoglycemia, particularly those with neuroglycopenic symptoms. Hypoglycemic GB subjects had lower postprandial insulin clearance rates and higher insulin secretion rates during the glucose decline after the test meal. Meal-induced glucagon was enhanced in all GB subjects but did not differ between subjects who did and did not develop hypoglycemia. Plasma gastric inhibitory polypeptide and glucagon-like peptide-1 concentrations did not differ between asymptomatic and neuroglycopenic GB subjects.
Conclusion:
Among GB subjects with a clinical history of hypoglycemia, hyperinsulinemia is the result of inappropriate insulin secretion and reduced insulin clearance. In subjects with symptoms of postprandial hypoglycemia, insulin secretion is higher in the latter stages of meal glucose clearance, and despite elevated meal-induced glucagon, there is no further response to hypoglycemia. These abnormalities in islet function are most pronounced in subjects who report neuroglycopenic symptoms.
Roux-en-Y gastric bypass (GB) surgery leads to postprandial hyperinsulinemia independent of weight loss. Previous investigation indicates that insulin and glucagon-like peptide 1 (GLP-1) responses to meal ingestion are shifted to the left and upward as early as 1 week after surgery (1), consistent with a process rapidly engaged by the gastrointestinal (GI) alterations of GB.
Recently, it has been reported that a subgroup of individuals with GB develop postprandial hypoglycemia several years after surgery (2). This often debilitating complication is associated with larger insulin and GLP-1 responses to meal ingestion compared to asymptomatic individuals after GB (2). A common hypothesis proposed to explain this condition is that exaggerated secretion or action of GLP-1 enhances the β-cell response to meal ingestion, resulting in hypoglycemia. Persons with GB do have higher plasma levels of GLP-1 and significantly greater GLP-1 action to promote insulin release than individuals without surgery (3). However, based on studies using a GLP-1 receptor antagonist during meal ingestion and clamped hyperglycemia, the postprandial actions of GLP-1 do not differ between those with and without hypoglycemia (3).
At present, the mechanisms underlying postprandial hypoglycemia in GB subjects remain unknown. Moreover, there is no consensus on the diagnosis or definition of this condition, which in the past was often labeled as late dumping syndrome. A previous study of 32 subjects with GB who had oral glucose tolerance tests reported that 80% had reactive hypoglycemia defined as serum glucose <60 mg/dL or a drop of 100 mg/dL of serum glucose, but without mention of associated symptoms (4). In another study, ingestion of a liquid mixed meal resulted in blood glucose <60 mg/dL in three of nine asymptomatic GB subjects (2). Thus, it is not clear whether postprandial hypoglycemia in the absence of symptoms or postmeal hypoglycemic symptoms with relatively normal blood glucose levels signifies glucose dysregulation among people with GB.
To determine the relationships among symptomatology and physiological responses, we measured blood glucose and islet and GI hormones after ingestion of a test meal in a cohort of patients with GB and a group of comparable nonoperated control subjects; this study includes the largest number of GB subjects with hypoglycemia yet reported. The GB subjects were characterized based on clinical history of hypoglycemic symptoms and the type of symptoms (autonomic or neuroglycopenic), as well as their glucose response to the meal tolerance test (MTT). We predicted that the subgroup of subjects with a positive clinical history would have a distinct pattern of insulin secretion compared to asymptomatic GB subjects, and that this would be most apparent in the most symptomatic, ie, those with neuroglycopenia.
Subjects and Methods
Subjects
Sixty-five subjects with GB at least 2 years before the study were recruited. Thirty-six of the GB subjects reported symptoms consistent with postprandial hypoglycemia (Sym), and 29 reported no symptoms suggesting hypoglycemia (Asym). Subjects were studied in order of their presentation to the clinic or response to advertisement. Exclusion criteria included GI obstruction, active diabetes, renal dysfunction, and liver disorders. In addition to the Sym and Asym groups, 11 healthy subjects (CON) with body mass index (BMI) comparable to the surgical group, normal glucose tolerance, and no GI surgery or postprandial symptoms were included as a comparator group to demonstrate the impact of GB on postprandial glycemia. The study was approved by the Institutional Review Board of the University of Cincinnati, and all participants provided written informed consent before the studies.
Subjects with GB were screened for postprandial symptoms. The Sym group was defined by the presence of recurrent episodes of autonomic symptoms (palpitation, shaking, sweating, dizziness, fatigue, nausea, weakness, coldness and clamminess, anxiety, and paresthesia) or neuroglycopenic symptoms (cognitive dysfunction, loss of consciousness, and/or seizure) occurring within 1–5 hours of eating (5, 6), which resolved immediately with carbohydrate ingestion. The onset of hypoglycemic symptoms varied from several months to years after surgery, long after nadir body weight was achieved within the first year from surgery. Among the Sym individuals, 12 suffered from autonomic symptoms only (SymA), and 24 had neuroglycopenic symptoms, with or without autonomic symptoms (SymN). Also, 21 of the Sym group as well as seven of the Asym group experienced symptoms consistent with the dumping syndrome (postprandial nausea, diarrhea, weakness, sleepiness, palpitation, dizziness, headaches, feeling warmth, and abdominal fullness) (7). Dumping symptoms were distinguished from autonomic responses to hypoglycemia as starting almost immediately after surgery (<3 months), and not being relieved by carbohydrate ingestion. Eleven subjects from the Sym group and five from the Asym group had a preoperative history of type 2 diabetes controlled with diet or insulin sensitizers with no known diabetes-related complication that resolved completely after surgery. No subjects were taking medication known to affect glucose metabolism.
Experimental protocols
Subjects were instructed to maintain their usual carbohydrate ingestion and not to engage in intense physical activity for 3 days before the study. They were admitted to the General Clinical Research Center at Cincinnati Children's Hospital after an overnight fast. Blood was sampled through an iv catheter in an antecubital vein, and the arm was continuously warmed to arterialize venous blood.
After fasting blood samples were taken, subjects consumed a 237-mL liquid mixed meal (350 kcal; 57% carbohydrate, 15% protein, and 28% fat; Ensure Plus, Abbott Laboratories) within 10 minutes. Blood samples were taken from 0–180 minutes, and plasma was separated within 60 minutes for storage at −80°C. Blood glucose concentrations were determined using an automated glucose analyzer. Hypoglycemia was defined as symptoms consistent with hypoglycemia associated with blood glucose <2.78 mmol/L and relief of symptoms immediately after carbohydrate ingestion (Whipple's triad) (7). For subjects developing hypoglycemia, the study was terminated (65 GB subjects completed their study at 75 min, 64 went to 90 min, and 59 completed the 120 min of protocol).
Assays
Blood samples were collected as described previously (8). Insulin was measured by a previously described RIA (9). C-Peptide and glucagon were measured using RIA (Millipore), and total GLP-1 (Meso Scale Diagnostics, LLC) and total gastric inhibitory polypeptide (GIP) (Millipore) were measured using ELISA techniques according to the manufacturers' instructions.
Calculations and analysis
The three samples before meal ingestion (time 0) were averaged as fasting levels of glucose and hormones. Insulin secretion rates (ISRs) were derived from plasma C-peptide concentrations using deconvolution with population estimates of C-peptide (10). Glucose, insulin, and ISR values from 0–180 minutes; GLP-1 and glucagon levels from 0–120 minutes; and GIP levels from 0–150 minutes were used to compute area under the curve (AUC) using the trapezoidal rule. Insulin clearance was calculated by dividing the AUCISR(0,180 min) by the AUCInsulin(0,180 min) (11, 12). AUC for all parameters were also calculated for 0–60 minutes to evaluate the early response to meal ingestion given the known differences in the pattern of glucose, islet, and GI hormone response between surgical and nonsurgical subjects. MTT-derived insulin sensitivity (OGIS120 min) (13, 14) as well as β-cell function (rate sensitivity and glucose sensitivity) were determined as described previously (15, 16).
Statistical analysis
Data are presented as mean ± SEM. The primary analysis was a comparison of insulin secretion among the Sym, Asym, and control subjects. Secondary analyses included measures of glycemic change, insulin clearance, and glucagon and incretin secretion. Subset analyses were performed comparing: 1) SymA, SymN, and Asym GB subjects to identify the phenotypic characteristics among GB subjects with different clinical profiles, including the most exaggerated postprandial glucose abnormalities; and 2) SymN and Asym subjects with and without hypoglycemia during the MTT to determine the endocrine profile associated with hypoglycemia independent of symptoms. Parameters were compared among specific groups using ANOVA; for the second subset analysis, two-way ANOVA was used with previous symptoms (SymN/Asym) and hypoglycemia during the meal test (present/absent) as the two factors. Nonparametric variations of ANOVA models were used when a significant departure of the data from parametric assumptions was noted. Each of these comparisons was performed at an unadjusted α = 0.05, two-tailed. The Tukey's honest significant difference was used for post hoc analysis for the first two comparisons. Baseline nominal characteristics among surgical groups were compared using the χ2 test. Spearman correlation was used to seek relationships between nadir glucose levels and subject characteristics or hormonal profile within surgical group. Statistical analyses were performed using SPSS version 20 (SPSS Inc).
Results
Comparisons of GB patients with and without symptoms of hypoglycemia and nonsurgical controls
Subject characteristics (Table 1)
Table 1.
Clinical Characteristics and Glucose and Insulin Responses to Test Meal Ingestion in GB Subjects With (Sym) and Without (Asym) Previous Postprandial Hypoglycemic Symptoms and Nonsurgical Controls (CON)
| Sym | Asym | CON | Statistical Tests (P Values) | |
|---|---|---|---|---|
| n | 36 | 29 | 11 | |
| Preoperative BMI, kg/m2 | 51 ± 1 (37–74) | 52 ± 2 (38–95) | .434 | |
| Initial weight loss, kga | 64 ± 3 (34–127) | 55 ± 5 (21–114) | .124 | |
| Total weight loss, kgb | 52 ± 3 (22–96) | 54 ± 4 (11–130) | .678 | |
| Weight regain, kgc | 12 ± 3 (−43 to 48) | 1 ± 5 (−48 to 68) | .050 | |
| Time after surgery, y | 4.9 ± 0.5 (2–17) | 5.0 ± 0.5 (2–11) | .940 | |
| History of T2DM (presence/ absence) | 11/25 | 5/24 | .172 | |
| History of dumping (presence/absence) | 21/13 | 7/22 | .003 | |
| Gender (F/M) | 34/2 | 23/6 | 9/2 | .121 |
| Age, y | 45 ± 2 (23–63)* | 51 ± 2 (25–65)** | 36 ± 3 (23–52) | .001 |
| Current BMI, kg/m2 | 31 ± 1 (18–48) | 33 ± 1 (26–42) | 34 ± 2 (25–42) | .410 |
| HbA1C, % | 5.2 ± 0.1 (4.2–6.0) | 5.4 ± 0.1 (4.7–6.3) | 5.1 ± 0.1 (4.6–5.7) | .098 |
| Fasting glucose, mmol/L | 4.4 ± 0.1 | 4.7 ± 0.1 | 4.6 ± 0.1 | .085 |
| Peak glucose, mmol/L | 9.8 ± 0.3** | 9.8 ± 0.3** | 6.6 ± 0.2 | .000 |
| Nadir glucose, mmol/L | 2.8 ± 0.12**‡ | 3.8 ± 0.2** | 4.4 ± 0.1 | .000 |
| Time to reach peak glucose, min | 30 ± 1** | 30 ± 1** | 67 ± 9 | .000 |
| Time to reach nadir glucose, min | 103 ± 4** | 120 ± 8** | 166 ± 6 | .000 |
| Fasting insulin, pmol/L | 47 ± 4** | 51 ± 4** | 107 ± 24 | .000 |
| Peak insulin, pmol/L | 2010 ± 186** | 1262 ± 131** | 708 ± 116 | .000 |
| Fasting ISR, pmol/L/m2 | 50 ± 4* | 60 ± 4* | 84 ± 16 | .002 |
| Peak ISR, pmol/L/m2 | 1014 ± 77** | 803 ± 66* | 530 ± 95 | .003 |
| Glucose sensitivity, pmol · min−1 · m−2 · mm−1 | 243 ± .22 | 255 ± .22 | 285 ± .69 | .704 |
| Rate sensitivity, pmol · m−2 · mm−1 | 1426 ± 200** | 1271 ± 188** | 3547 ± 849 | .001 |
| AUCInsulin:Glucose (0,180 min) | 107 ± 11*‡ | 60 ± 5 | 68 ± 10 | .001 |
| OGIS120 min | 376 ± 18 | 375 ± 16 | 367 ± 16 | .960 |
| Insulin clearance | 1.1 ± 0.1*† | 1.5 ± 0.1 | 1.6 ± 0.2 | .005 |
Abbreviations: T2DM, type 2 diabetes mellitus; F, female; M, male. Data are presented as mean ± SEM (range) unless otherwise specified. P values for ANOVA or χ2 comparison among the two surgical groups (rows 2–8) and the three groups are provided in the far right column. Significant P values are bold.
P < .05; and ‡ P < .01, compared to Asym.
P < .05; and ** P < .01, compared to CON using post hoc analysis.
Initial weight loss at 6–12 months after surgery;
total weight loss since surgery;
weight regain since the nadir body weight after surgery.
The two groups of GB subjects had comparable age, BMI, total weight loss, and time since surgery. The control subjects, nine females and two males, were younger than the GB surgical subjects but had similar gender distribution, BMI, and glycated hemoglobin (HbA1C) level.
Plasma glucose, islet function, and hormone profile (Figure 1 and Table 1)
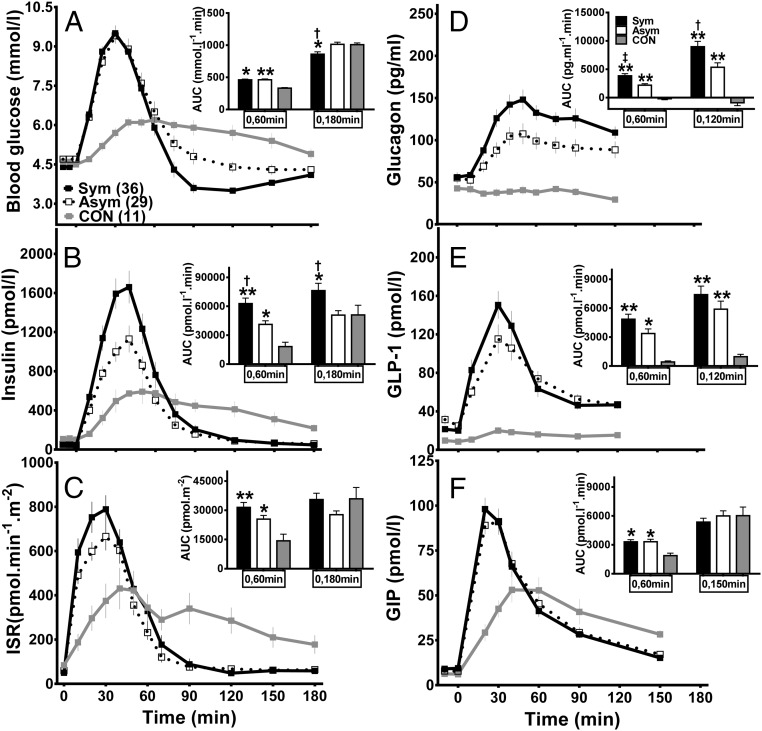
Figure 1.
Plasma glucose (A), islet hormone (B–D), and incretin (E and F) responses to meal ingestion in GB subjects, with (Sym, solid line and closed square, black bar) and without (Asym, dashed line and open square, white bar) previous hypoglycemia, and controls (CON, gray solid line and closed square, gray bar). †, (P < .05) compared to Asym; *, P < .05, and **, P < .01 compared to CON using post hoc analysis.
Fasting blood glucose levels were comparable in the Sym, Asym, and CON groups, and the overall glucose response to meal ingestion (AUCGlucose(0,180 min)) was not different between the Asym and CON subjects. However, the glucose response was shifted upward and to the left because the peak glucose values were significantly higher in surgical patients and the time to reach the peak glycemia was shorter. Surgical subjects reached their lowest glucose values more rapidly and had a lower nadir glucose and larger decrement from fasting glucose compared to the controls. The history of postprandial hypoglycemia (ie, the Sym group) was associated with lower nadir glucose compared to the Asym subjects, despite similar peak glucose and AUCGlucose(0,60 min) values.
Fasting insulin and ISR levels were significantly higher in the control subjects compared to the two GB groups. There was no difference in the total insulin response to meal ingestion (AUCISR(0,180 min)) between the Asym and CON groups. However, the β-cell response of the GB subjects to meal ingestion paralleled their glucose curves with the early response (AUCInsulin(0,60 min) and AUCISR(0,60 min)) significantly larger than the controls. However, the Sym subjects had a significantly greater insulin excursion after the test meal (AUCInsulin(0,60 min) and AUCInsulin(0,180 min)) compared to the Asym despite similar postprandial glycemia. Model-derived parameters of β-cell glucose sensitivity did not differ among the three cohorts, but the GB groups had reduced β-cell rate sensitivity to changes in plasma glucose compared to the controls.
Values of OGIS were similar among the surgical and nonsurgical controls. The Sym individuals differed from the Asym and CON subjects in having significantly greater postprandial insulin:glucose ratios and reduced rates of insulin clearance.
Fasting glucagon levels were similar among the three groups. However, there was a 2- to 3-fold rise in postprandial glucagon in the surgical groups with the higher levels in the Sym compared to Asym individuals, whereas glucagon was unchanged or slightly suppressed from basal levels after the test meal in the controls. Similar to the results with glucagon, plasma GLP-1 was much higher in the GB than the CON subjects, with a trend toward larger levels in the Sym group compared to the Asym group that was not significant. GIP levels rose more quickly after eating in the surgical subjects, but the total GIP response was similar in the GB and control groups.
Comparisons of GB subjects with a history of neuroglycopenic or autonomic hypoglycemia symptoms and asymptomatic GB subjects (Table 2)
Table 2.
Clinical Characteristics and Glucose and Insulin Responses to Meal Ingestion in GB Subjects With Previous Neuroglycopenic (SymN) or Autonomic (SymA) Symptoms or no Symptoms of Hypoglycemia (Asym)
| SymN | SymA | Asym | Statistical Tests (P Values) | |
|---|---|---|---|---|
| n | 24 | 12 | 29 | |
| History of T2DM (presence/absence) | 9/15 | 2/10 | 5/24 | .097 |
| History of dumping (presence/absence) | 14/9 | 7/4 | 7/22 | .007 |
| Gender (F/M) | 23/1 | 11/1 | 23/6 | .007 |
| Age, y | 43 ± 2 (23–63)* | 49 ± 3 (29–63) | 51 ± 2 (25–65) | .025 |
| Current BMI, kg/m2 | 32 ± 1 (18–44) | 31 ± 3 (22–48) | 33 ± 1 (26–42) | .570 |
| Preoperative BMI, kg/m2 | 50 ± 2 (37–74) | 52 ± 3 (41–66) | 52 ± 2 (38–94) | .523 |
| Initial weight loss, kga | 66 ± 4 (34–127) | 60 ± 5 (36–85) | 55 ± 5 (21–114) | .240 |
| Total weight loss, kgb | 50 ± 4 (22–96) | 56 ± 5 (29–89) | 54 ± 4 (11–130) | .600 |
| Weight regain, kgc | 16 ± 3 (−13 to 48)* | 4 ± 6 (−43 to 37) | 1 ± 6 (−48 to 68) | .050 |
| Time after surgery, y | 4.8 ± 0.6 (2–17) | 5.2 ± 0.8 (2–10) | 5.0 ± 0.5 (2–11) | .900 |
| HbA1C, % | 5.2 ± 0.1 (4.3–6.0) | 5.0 ± 0.1 (4.2–5.6) | 5.4 ± 0.1 (4.7–6.3) | .094 |
| Fasting glucose, mmol/L | 4.4 ± 0.1 | 4.5 ± 0.1 | 4.7 ± 0.1 | .110 |
| Peak glucose, mmol/L | 9.8 ± 0.3 | 9.9 ± 0.4 | 9.8 ± 0.3 | .980 |
| Nadir glucose, mmol/L | 2.6 ± 0.2* | 3.3 ± 0.1 | 3.8 ± 0.2 | .000 |
| Fasting insulin, pmol/L | 53 ± 6† | 35 ± 3* | 51 ± 4 | .024 |
| Peak insulin, pmol/L | 2257 ± 227* | 1516 ± 287 | 1262 ± 131 | .011 |
| Fasting ISR, pmol/L/m2 | 47 ± 5 | 47 ± 5 | 61 ± 4 | .063 |
| Peak ISR, pmol/L/m2 | 1057 ± 1001 | 927 ± 113 | 803 ± 66 | .096 |
| Glucose sensitivity, pmol · min−1 · m−2 · mm−1 | 247 ± 28 | 236 ± 33 | 255±.22 | .296 |
| Rate sensitivity, pmol · m−2 · mm−1 | 1504 ± 284 | 1293 ± 243 | 1271 ± 188 | .774 |
| AUCInsulin:Glucose (0,180 min) | 126 ± 15**‡ | 69 ± 11 | 60 ± 5 | .000 |
| OGIS120 min | 359 ± 26 | 400 ± 21 | 375 ± 16 | .530 |
| Insulin clearance | 1.0 ± 0.1** | 1.3 ± 0.1 | 1.5 ± 0.1 | .006 |
Abbreviations: F, female; M, male. Data are presented as mean ± SEM (range) unless otherwise specified. P values for ANOVA or χ2 comparison among the three groups are provided in the far right column. Significant P values are bold.
P < .05; and ‡ P < .01, compared to Asym.
P < .05; and ** P < .01, compared to CON using post hoc analysis.
Initial weight loss at 6–12 months after surgery;
total weight loss since surgery;
weight regain since the nadir body weight after surgery.
Subject characteristics
Among the 36 Sym subjects, 24 had predominantly neuroglycopenic symptoms (SymN), and 12 had only autonomic symptoms (SymA). The SymN, SymA, and Asym groups had comparable BMI and time from surgery and similar preoperative weight and weight loss. The SymN individuals were slightly younger and had a greater weight regain from their lowest postoperative level compared to the SymA and Asym subjects. All three groups (SymN, SymA, and Asym) had a predominance of female to male subjects and similar presurgery rates of diabetes. The number of subjects who suffered from postoperative dumping symptoms, which started shortly after surgery, was higher in the SymN and SymA groups compared to the Asym group.
Plasma glucose, islet function, and hormone profile (Figure 2 and Table 2)
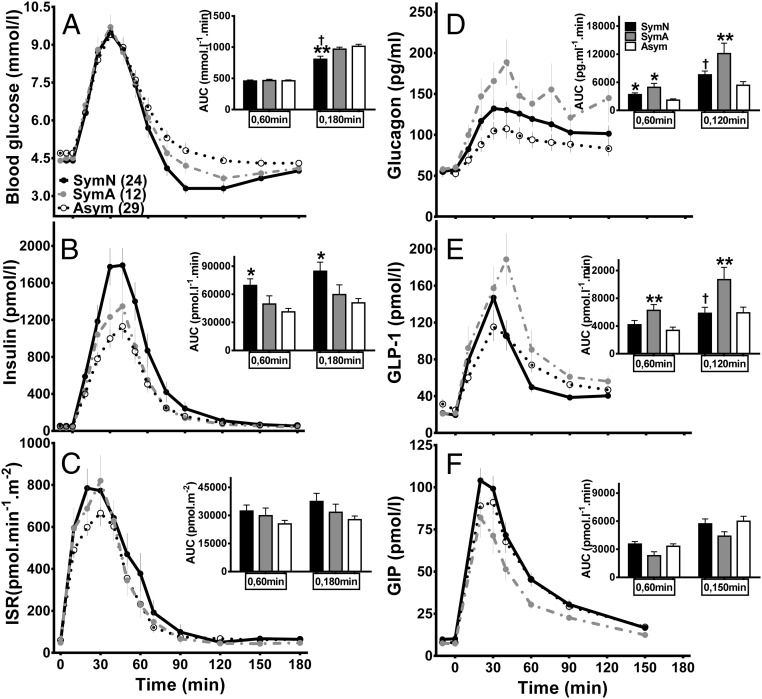
Figure 2.
Plasma glucose (A), islet hormone (B–D), and incretin (E and F) responses to meal ingestion in postsurgical subjects categorized by previous clinical symptoms. Neuroglycopenic symptoms (SymN, solid line and closed circle, black bar); autonomic symptoms (SymA, gray dashed line and closed circle, gray bar); and asymptomatic (Asym, dashed line and open circle, white bar). †, P < .05, compared to SymA; *, P < .05, and **, P < .01, compared to Asym using post hoc analysis.
Fasting and peak glucose levels, as well as the early glucose response to meal ingestion, were similar among the groups. The SymN individuals had the lowest nadir glucose levels as well as the highest insulin response to meal ingestion (AUCInsulin(0,60 min), AUCInsulin(0,180 min), and AUCInsulin:glucose (0,180 min)) compared to the other two groups.
However, there were no differences in ISR or OGIS, and neither β-cell glucose sensitivity nor rate sensitivity differed among the SymN, SymA, and Asym subjects. The increased plasma insulin in the SymN group was likely a function of reduced insulin clearance compared to the SymA and Asym subjects. The SymA subjects had higher glucagon and GLP-1 responses than the other groups, but GIP responses to the test meal were similar among the three groups.
Comparison of SymN and Asym subjects with and without hypoglycemia during the meal test
Subject characteristics and postprandial symptom profile
Of the 29 GB subjects with no prior history of postprandial hypoglycemia, three developed asymptomatic hypoglycemia (blood glucose <2.78 mmol/L) during MTT. In contrast, among the 24 subjects with a history of postprandial neuroglycopenic symptoms, 12 had glucose nadirs <2.78 mmol/L after meal consumption. There were no obvious differences in the clinical characteristics of the euglycemic and hypoglycemic subjects in the Asym and SymN groups, nor was there any difference between the rates of diabetes before surgery or prior history of dumping symptoms.
Plasma glucose, islet function, and hormone profile (Figure 3)
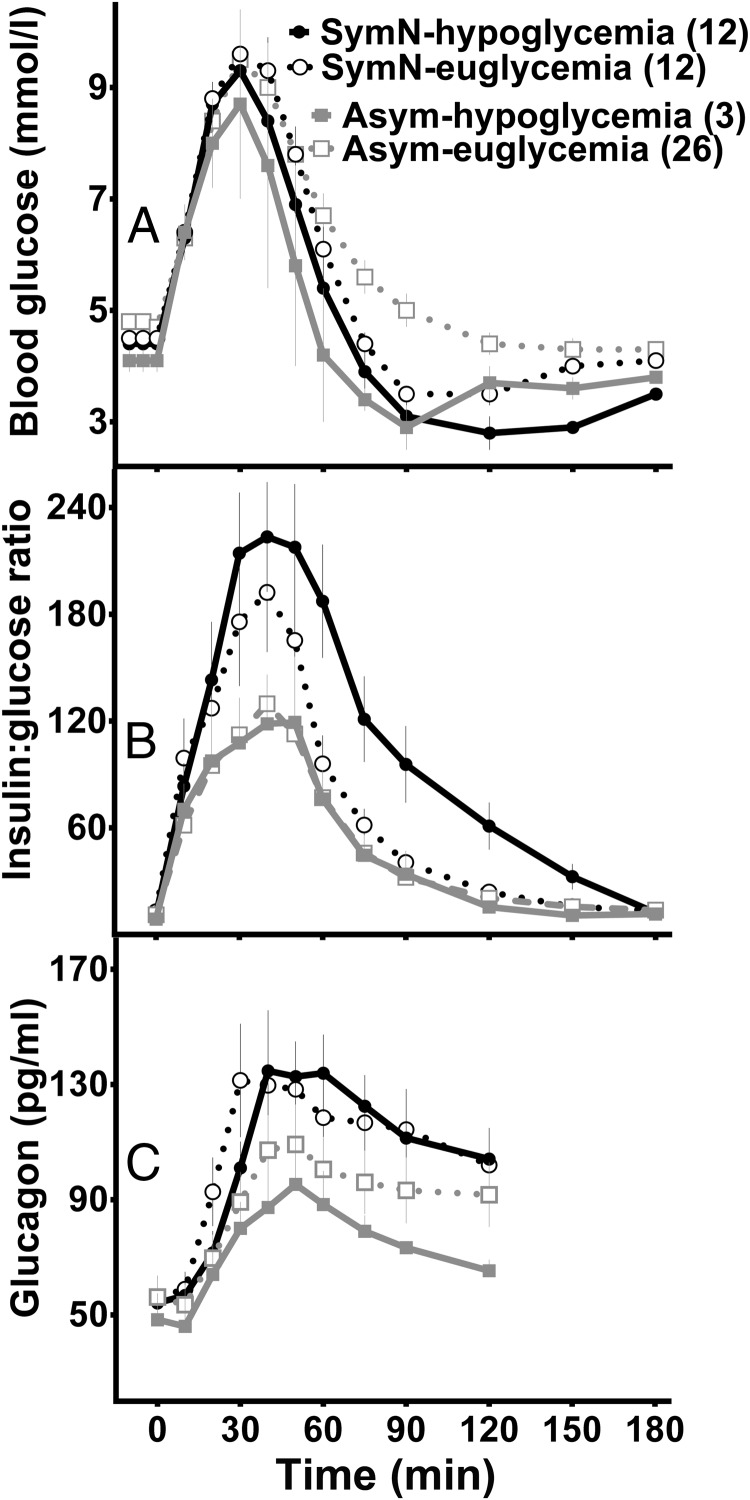
Figure 3.
Plasma glucose (A), insulin:glucose ratios (B), and glucagon response (C) in postsurgical subjects stratified by previous clinical symptoms and glycemic response during the MTT. Neuroglycopenic symptoms and hypoglycemia during MTT (SymN-hypoglycemia, solid line and closed circle); neuroglycopenic symptoms and blood glucose ≥2.78 mmol/L during MTT (SymN-euglycemia, dashed line and open circle); asymptomatic and hypoglycemia during MTT (Asym-hypoglycemia, gray solid line and closed square); asymptomatic and blood glucose ≥2.78 mmol/L during MTT (Asym-euglycemia, gray dashed line and open square).
There were no differences in fasting or peak glucose concentrations among the SymN and Asym subjects grouped by their glycemic response to the meal test except that subjects with blood glucose <2.78 mmol/L had a shorter time to reach their glycemic nadir than those who did not become hypoglycemic. Peak insulin levels as well as total insulin responses were greater in the SymN subjects than the Asym group and did not segregate with the glucose response to meal ingestion. The SymN subjects had lower insulin clearance than Asym subjects, but again there were no differences within groups that correlated with the test meal glucose response. During the MTT, the insulin:glucose ratio was higher in the SymN subjects compared to the Asym group, but it was even larger in those SymN subjects with hypoglycemia during MTT compared with the SymN subjects without hypoglycemia. Although the glucagon response to meal ingestion was larger in the SymN individuals compared to the Asym subjects, the increase in glucagon appeared by the first 30 minutes after eating and remained stable with no further elevation in response to hypoglycemia in either group. Finally, there were no differences in the incretin responses between subjects who became hypoglycemic during the meal and those who did not. Overall, there was concordance between the postprandial hormone concentrations and the subject's assigned clinical group, but not with the glycemic response to the MTT.
β-Cell responses to increasing and decreasing glucose during the meal test
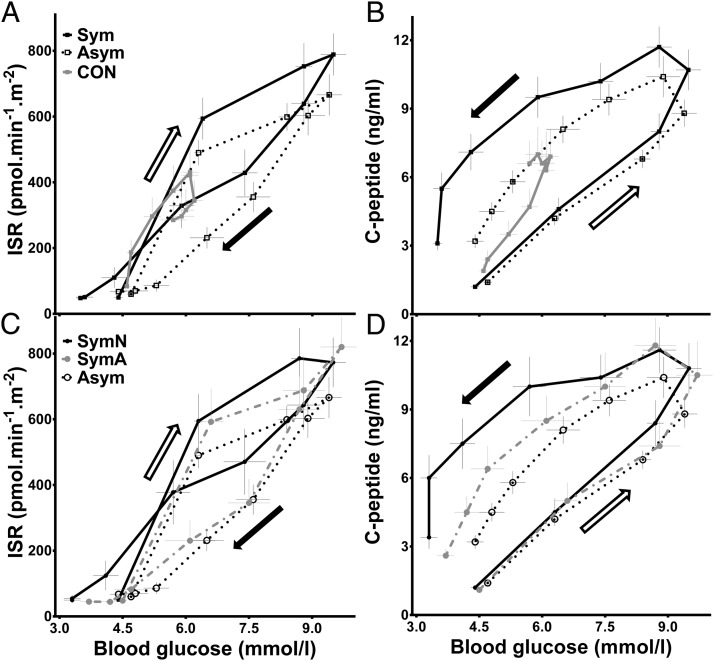
To assess β-cell responses as a function of blood glucose across the MTT, plasma ISR and C-peptide levels were plotted against pre- and postprandial glycemia for the first 120 minutes of MTT (Figure 4). This relationship includes stimulation of the β-cell as postprandial glucose increases in the first 30 minutes in surgical patients and in the first 70 minutes in nonoperated controls, and reduction of β-cell output as meal stimuli wane and glucose is corrected in the latter part of the MTT. The rise of ISR was similar among the Sym, Asym, and CON groups immediately after meal ingestion (Figure 4A). Both the Asym and CON subjects had lower ISR relative to blood glucose levels in the latter part of the meal test; ie, for any level of glucose, the ISR was lower as blood glucose declined. However, the Sym individuals had a smaller difference between ISRs with the rising and falling of blood glucose, and secretion was higher as glucose approached basal levels. The profile of C-peptide vs glucose had a different pattern than ISR, due to its long half-life and accumulation during the meal test, but the largest C-peptide:glucose ratio in the latter part of the meal period was observed in the Sym subjects (Figure 4B). When the SymN, SymA, and Asym groups were compared (Figure 4, C and D), the results were similar, with the SymN subjects having persistent elevations of ISR and C-peptide in the latter phases of the meal, most notable when blood glucose dipped below the fasting level.
Figure 4.
Relationship between β-cell secretion and blood glucose values during MTT (0–120 min). ISR (A) and C-peptide (B) vs glucose levels in GB subjects with (Sym, solid line and closed square) and without (Asym, dashed line and open square) a history of hypoglycemia, and controls (CON, gray solid line and closed square). ISR (C) and C-peptide (D) vs glucose levels in GB patients with a history of neuroglycopenic (SymN, solid line and closed circle) or autonomic (SymA, gray dashed line and closed circle) hypoglycemic symptoms and asymptomatic surgical subjects (Asym, dashed line and open circle). Each data point represents ISR/glucose or C-peptide/glucose values at 0, 10, 20, 30, 40, 50, 60, 75, 90, and 120 minutes. White arrow shows the initial phase of the MTT, and black arrow the latter phase of the MTT (declining glucose).
Associations of nadir glucose with clinical and β-cell parameters
When all the surgical subjects were analyzed together, there were positive associations of nadir glucose during the MTT with age (r = 0.35; P = .005) and BMI (r = 0.29; P = .02) such that younger, less obese subjects had lower postprandial glucose.
The glucose nadirs were inversely correlated with the size of the β-cell response to meal ingestion, especially the early response (AUCISR(0, 60), r = −0.33; P = .008). Glucose nadirs were also correlated with insulin clearance (r = 0.45; P = .001), such that those with lower insulin clearance had the lowest glucose nadir levels. There was no association of nadir glucose levels with peak glucose values, time to reach peak glycemia, OGIS, or parameters of β-cell function.
Discussion
This study was performed to identify physiological characteristics that cause some GB patients to have postprandial hypoglycemia. We recruited relatively large cohorts of GB patients with and without postprandial symptoms of hypoglycemia sequentially and analyzed them based on clinical history; although the groups were not tightly matched for age, the subjects spanned middle age and had considerable overlap. Among the GB groups, those with symptoms of postprandial hypoglycemia had relative hyperinsulinemia, and this was most pronounced among the subjects with neuroglycopenic symptoms. In the SymN subjects, postprandial hyperinsulinemia was associated with reduced insulin clearance and enhanced insulin secretion in the latter portion of the meal test. Moreover, whereas plasma glucagon was elevated in the GB patients, there was no further compensatory α-cell response to hypoglycemia. Differences in the glycemic response to a mixed nutrient meal in surgical subjects were not dependent on relative changes in body weight, amount of weight loss, or insulin sensitivity. These findings demonstrate the significant changes in islet hormone secretion induced by GB and suggest that these processes are accentuated by reduced insulin clearance among individuals who develop the postprandial hypoglycemia syndrome.
In this study, we used a mixed nutrient meal (1, 2, 17, 18) rather than a glucose solution for testing in order to mimic the composition of daily meal consumption and to limit the postprandial glucose excursion while maintaining robust islet and incretin stimulation (19). For the initial analysis, postsurgical subjects were categorized based on clinical history because this is how individuals present to caregivers, and it reflects the chronicity and recurrence of hypoglycemia.
Recognizing the possible overlap between postprandial autonomic symptoms of hypoglycemia and dumping syndrome, we differentiated the two by the time of onset as well as the response to carbohydrate ingestion. Moreover, we refined our initial stratification to select for the most affected individuals by differentiating autonomic and neuroglycopenic symptoms, using specific criteria, and finally by a hypoglycemic response to the MTT (blood glucose <2.78 mmol/L). Previous studies indicate that neuroglycopenic symptoms have a stronger correlation with the degree of hypoglycemia than other complaints (20, 21), and based on the glycemic response to a mixed nutrient meal, this was a useful distinction because 50% of SymN (12 of 24) subjects in our cohort had hypoglycemia during MTT, as opposed to 8–10% of the SymA (1 of 12) and Asym (3 of 29) individuals. A history of dumping in the past was not associated with a higher risk of developing hypoglycemia during MTT in the GB subjects because eight of 28 individuals with a prior history of dumping symptoms and eight of 37 subjects with no dumping developed blood glucose <2.78 mmol/L during the MTT (Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) (P = .5). However, the results of a single MTT do not distinguish the underlying physiology of symptomatic and asymptomatic subjects very well; plasma insulin levels were similar in the SymN group with and without hypoglycemia, as were the responses in the Asym subjects who did and did not drop their glucose below 2.78 mmol/L after the test meal. Overall, these results support neuroglycopenic rather than autonomic symptoms to identify subjects with the underlying physiology likely to cause recurrent hypoglycemia.
Since the early reports of hypoglycemia occurring after GB (22, 23), there has been a growing awareness of this syndrome. Although the pathophysiology is still unclear, several hypotheses have been advanced to explain the phenomenon including: rapid glucose absorption from the gut due to loss of the reservoir function of the stomach, enhanced insulin secretion as a result of increased GLP-1 secretion or action, and greater insulin action due the increased insulin sensitivity associated with massive weight loss. Each of these explanations has some experimental support (2, 24–27), but in fact all of these factors are common to GB with and without clinical hypoglycemia. Here we did not find differences between the Sym and Asym subjects in the rate of rise or peak of blood glucose during the MTT, suggesting that glucose absorption was comparable in these groups. Nor were there substantial differences in plasma incretins or insulin sensitivity. We did observe consistent hyperinsulinemia in the Sym subjects, particularly those with neuroglycopenic symptoms.
The postprandial hyperinsulinemia in the Sym subjects could be accounted for by two differences with the Asym subjects—decreased insulin clearance, and enhancement of insulin secretion in the waning portion of the MTT. These factors were accentuated in the SymN subjects who manifest the most pronounced symptoms of recurrent hypoglycemia. The Sym subjects had approximately 30% reduced clearance of insulin compared with the Asym and control subjects. The computation of insulin clearance used here includes both hepatic extraction and systemic (mostly renal) elimination of circulating peptide, a distinction that cannot be made from our dataset. However, it seems unlikely that systemic insulin clearance would differ significantly in a population of subjects of similar body size with normal renal function. Hepatic insulin extraction is a saturable process (28) that varies with insulin secretion, both before and after meals (12, 29, 30), or as a function of insulin sensitivity (31). However, the differences in insulin clearance between the Sym and Asym subjects cannot be easily explained by differences in ISR or insulin sensitivity, and so seems to contribute to, rather than result from, hyperinsulinemia. One possibility is that there are individuals prone to postprandial hypoglycemia after GB because of inherently reduced insulin clearance that is unmasked by surgery. An alternative is that factors associated with surgery, such as variable denervation, lead to different rates of insulin clearance among individuals. Regardless, the lower rates of insulin clearance are a key difference between the Sym and Asym groups in our cohort, and this process will require more directed study to fully understand glucose regulation after GB.
In broad terms, there were not dramatic differences in insulin secretion between the Sym and Asym subjects. Both groups had lower fasting ISR compared to the controls, consistent with lower basal insulin concentrations and greater fasting insulin sensitivity. The GB subjects had left-shifted ISR responses to the MTT, with a disproportionate percentage of their total insulin secretion in the first postprandial hour. The β-cell sensitivity to glucose did not differ in GB and control subjects, and the β-cell sensitivity to rate of change in plasma glucose during the early phase of meal absorption was actually lower in the surgical subjects. However, when the GB subjects were stratified based on symptoms, there were no differences in these parameters of β-cell function. The Sym subjects had relatively greater β-cell output per unit of plasma glucose reduction compared to the Asym group, and this characteristic was relatively greater in the SymN compared to both the SymA and Asym individuals. However, β-cell function among the GB subjects varied along a continuum rather than dichotomously based on the tendency to have hypoglycemia. Overall, the findings from this study indicate that in GB subjects with neuroglycopenic symptoms, alterations in β-cell function are most apparent as delayed cessation of insulin release in response to declining blood glucose. This phenomenon has been previously described in obese subjects with impaired glucose tolerance (32).
In addition to the changes in β-cell responses among the Sym, Asym, and control groups, we noted important differences in α-cell function. A number of studies have previously reported that the glucagon response to meal ingestion is elevated after GB (1, 3, 33–36). In our cohort, the glucagon response to meal ingestion was larger in the Sym patients compared to the Asym subjects, particularly in the early part of the MTT before glucose levels reached their nadir. Further stratification of surgical patients showed that SymA subjects had the highest overall glucagon response compared to the Asym or SymN subjects, possibly reflecting increased sympathetic nervous system activation. Importantly, there was no further augmentation of glucagon secretion during the fall in blood glucose among the GB subjects, and those with hypoglycemia during MTT had similar glucagon responses to those who did not. The failure of counter-regulatory glucagon secretion to postprandial hypoglycemia in GB subjects has not been noted previously, but abnormal α-cell function could in fact contribute to the hypoglycemic syndrome. It will be important to directly assess the dynamics of glucagon release in response to controlled changes in blood glucose and hypoglycemia in cohorts, similar to those described here.
Although there were not significant differences in incretin release among the Sym and Asym groups, among the GB subjects the SymA group had the largest GLP-1 response to meal ingestion. Glucagon and GLP-1 both are processed from proglucagon in a tissue-specific fashion because the former is produced in the islet α-cells and the latter from the intestinal L-cells. A large body of evidence has shown that GB exaggerates secretion of L-cell products (1, 33–35, 37–39). Given the strong correlation between glucagon and GLP-1 response to the test meal in our cohort, it is possible that both peptides are produced from L-cells and the tissue specificity is modified after GB or that our assays are not able to differentiate between a gut-derived glucagon-like molecule and pancreatic glucagon. However, recent studies suggest that hyperglucagonemia after GB may have some physiological consequences (27).
There are important limitations to our study that merit consideration. Although our group of GB subjects with symptoms of hypoglycemia is larger than previously published cohorts, the number is still relatively small, especially given the variability we find among GB subjects. However, because our subjects were recruited on simple clinical criteria, ie, the presence or absence of hypoglycemic symptoms, they are generally reflective of GB patients encountered in practice. The CON group was small relative to the others, and this may have reduced the likelihood of detecting differences in specific parameters of β-cell function such as glucose sensitivity between subjects with and without surgery. However, this group was included to emphasize the substantial impact of GB on postprandial physiology, and based on the patterns of glucose and hormones presented here demonstrate this clearly. Finally, the primary intervention here, a MTT, is a relatively imprecise tool for studying specific aspects of islet hormone regulation. Nonetheless, it was important to use this method in this study to bring out the defining outcome under investigation—postprandial hypoglycemia.
In summary, we report here that subjects with GB who report a clinical history suggestive of postprandial neuroglycopenic hypoglycemia are more likely to have low blood glucose after meal ingestion. These subjects have relative hyperinsulinemia that is due to excessive ISR in the latter portion of the meal as postprandial hyperglycemia wanes, and by reduced insulin clearance. Our findings also suggest an impaired α-cell response to low glucose in GB subjects with the postprandial hypoglycemia syndrome. These findings support a multifactorial model of glucose dysregulation among some GB subjects. Moreover, the discordance between a history of hypoglycemia and the glycemic responses during MTT suggests that GB subjects span a continuum of likelihood for postprandial hypoglycemia. Further investigation into the changes of islet function and insulin clearance induced by GB is important to develop therapeutic approaches to this rare but frequently debilitating syndrome.
Acknowledgments
We thank Brianne Reedy and Leslie Baum from the Department of Medicine of the University of Cincinnati and the nursing staff from the Clinical Research Center of Cincinnati Children's Hospital for their expert technical assistance. We owe a great debt to our research participants.
This work was supported by National Institutes of Health (NIH) Grants DK083554 (to M.S.) and DK57900 (to D.A.D.), and in part by National Center for Advancing Translational Sciences, National Institute of Health grant 8 UL1 TR000077, as well as the Medical Research Service of the Department of the Veterans Affairs.
Disclosure Summary: The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
- Asym
- asymptomatic surgical subject
- AUC
- area under the curve
- BMI
- body mass index
- GB
- gastric bypass
- GI
- gastrointestinal
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide 1
- HbA1C
- glycated hemoglobin
- ISR
- insulin secretion rate
- MTT
- meal tolerance test
- OGIS
- MTT-derived insulin sensitivity
- Sym
- symptomatic surgical subject
- SymA
- surgical subject with autonomic symptoms
- SymN
- surgical subject with neuroglycopenic symptoms.
References
- 1. Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–E131 [DOI] [PubMed] [Google Scholar]
- 2. Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685 [DOI] [PubMed] [Google Scholar]
- 3. Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roslin M, Damani T, Oren J, Andrews R, Yatco E, Shah P. Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia. Surg Endosc. 2011;25:1926–1932 [DOI] [PubMed] [Google Scholar]
- 5. Service FJ. Hypoglycemias. West J Med. 1991;154:442–454 [PMC free article] [PubMed] [Google Scholar]
- 6. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709–728 [DOI] [PubMed] [Google Scholar]
- 7. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479–486 [PubMed] [Google Scholar]
- 8. Salehi M, Aulinger B, D'Alessio DA. Effect of glycemia on plasma incretins and the incretin effect during oral glucose tolerance test. Diabetes. 2012;61:2728–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salehi M, Aulinger B, Prigeon RL, D'Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 11. Shuster LT, Go VL, Rizza RA, O'Brien PC, Service FJ. Incretin effect due to increased secretion and decreased clearance of insulin in normal humans. Diabetes. 1988;37:200–203 [DOI] [PubMed] [Google Scholar]
- 12. Tillil H, Shapiro ET, Miller MA, et al. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol. 1988;254:E349–E357 [DOI] [PubMed] [Google Scholar]
- 13. Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and β cell function. Diabetologia. 2006;49:2136–2143 [DOI] [PubMed] [Google Scholar]
- 14. Gastaldelli A, Nauck MA, Balena R. Eight weeks of treatment with long-acting GLP-1 analog taspoglutide improves postprandial insulin secretion and sensitivity in metformin-treated patients with type 2 diabetes. Metabolism. 2013;62:1330–1339 [DOI] [PubMed] [Google Scholar]
- 15. Gastaldelli A, Brodows RG, D'Alessio D. The effect of chronic twice daily exenatide treatment on beta-cell function in new onset type 2 diabetes. Clin Endocrinol (Oxf). 2014;80:545–553 [DOI] [PubMed] [Google Scholar]
- 16. Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283:E1159–E1166 [DOI] [PubMed] [Google Scholar]
- 17. Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891 [DOI] [PubMed] [Google Scholar]
- 18. Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and β-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–1096 [DOI] [PubMed] [Google Scholar]
- 20. Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67–E74 [DOI] [PubMed] [Google Scholar]
- 21. Hepburn DA, Deary IJ, Frier BM, Patrick AW, Quinn JD, Fisher BM. Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM. Factor-analysis approach. Diabetes Care. 1991;14:949–957 [DOI] [PubMed] [Google Scholar]
- 22. Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240 [DOI] [PubMed] [Google Scholar]
- 23. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254 [DOI] [PubMed] [Google Scholar]
- 24. Z'graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18:981–988 [DOI] [PubMed] [Google Scholar]
- 25. Shah M, Law JH, Micheletto F, et al. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nannipieri M, Mari A, Anselmino M, et al. The role of β-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E1372–E1379 [DOI] [PubMed] [Google Scholar]
- 27. Camastra S, Muscelli E, Gastaldelli A, et al. Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes. 2013;62:3709–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prigeon RL, Røder ME, Porte D, Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest. 1996;97:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab. 2009;297:E941–E948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Byrne MM, Sturis J, Polonsky KS. Insulin secretion and clearance during low-dose graded glucose infusion. Am J Physiol. 1995;268:E21–E27 [DOI] [PubMed] [Google Scholar]
- 31. Jones CN, Pei D, Staris P, Polonsky KS, Chen YD, Reaven GM. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J Clin Endocrinol Metab. 1997;82:1834–1838 [DOI] [PubMed] [Google Scholar]
- 32. Ehrmann DA, Breda E, Cavaghan MK, et al. Insulin secretory responses to rising and falling glucose concentrations are delayed in subjects with impaired glucose tolerance. Diabetologia. 2002;45:509–517 [DOI] [PubMed] [Google Scholar]
- 33. Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring). 2008;16:298–305 [DOI] [PubMed] [Google Scholar]
- 36. Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235 [DOI] [PubMed] [Google Scholar]
- 37. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bose M, Machineni S, Oliván B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring). 2010;18:1085–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215 [DOI] [PubMed] [Google Scholar]