Abstract
Context:
Although adequate luteal hormone production is essential for establishing pregnancy, luteal phase deficiency (LPD) is poorly characterized among eumenorrheic women.
Objective:
We assessed the prevalence and overlap of two established LPD diagnostic criteria: short luteal phase duration less than10 days (clinical LPD) and suboptimal luteal progesterone of 5 ng/mL or less (biochemical LPD) and their relationship with reproductive hormone concentrations.
Design, Setting, and Participants:
We conducted a prospective study in western New York (2005–2007) following 259 women, aged 18–44 years, for up to two menstrual cycles.
Results:
Among ovulatory cycles with recorded cycle lengths (n = 463), there were 41 cycles (8.9%) with clinical LPD, 39 cycles (8.4%) with biochemical LPD, and 20 cycles (4.3%) meeting both criteria. Recurrent clinical and biochemical LPD was observed in eight (3.4%) and five (2.1%) women, respectively. Clinical and biochemical LPD were each associated with lower follicular estradiol (both P ≤ .001) and luteal estradiol (P = .03 and P = .02, respectively) after adjusting for age, race, and percentage body fat. Clinical, but not biochemical, LPD was associated with lower LH and FSH across all phases of the cycle (P ≤ .001).
Conclusions:
Clinical and biochemical LPD were evident among regularly menstruating women. Estradiol was lower in LPD cycles under either criterion, but LH and FSH were lower only in association with shortened luteal phase (ie, clinical LPD), indicating that clinical and biochemical LPD may reflect different underlying mechanisms. Identifying ovulation in combination with a well-timed luteal progesterone measurement may serve as a cost-effective and specific tool for LPD assessment by clinicians and researchers.
Menstrual cycle disorders include a spectrum of conditions, from luteal phase deficiency (LPD) to oligoovulation to chronic anovulation (1). Researchers hypothesize that LPD is caused by impaired corpus luteum function, resulting in the lack of a fully mature secretory endometrium. Alternatively, LPD may reflect a deficiency in the uterine endometrial response to normal hormonal changes during the luteal phase (2). Clinically, LPD has been associated with abnormal estradiol (E2) and progesterone production, shortening of the menstrual cycle by shortening of the luteal phase, irregular menstrual bleeding (3, 4), and pregnancy-related disorders such as infertility and early pregnancy loss (5, 6).
Diagnosis of LPD has historically been based on the histological evaluation of endometrial maturation, serum progesterone concentrations, and luteal phase duration assessed via basal body temperature (BBT) (3). However, the diagnostic accuracy of endometrial histological dating to assess LPD has been questioned due to the normal inter- and intraindividual variation in endometrial maturation (7), with an estimated positive predictive value of less than 10% (8). Identification of suboptimal progesterone production is challenging and potentially unreliable as well. Progesterone concentrations peak 8–10 days after ovulation (4). Consequently, the appropriate timing of serum blood draws depends on identification of ovulation. Clinicians and researchers prefer timing ovulation based on urine LH for predicting the day of ovulation and defining the luteal length because it has been shown to have better accuracy than using BBT (3, 6, 9).
The relevance of LPD as a distinct clinical entity has recently been debated due in part to lack of a reliable test to diagnose (3, 4). With BBT and endometrial histological dating no longer considered reliable LPD diagnostic tools, further research using gold standard methods to time ovulation to determine luteal phase duration and repeated measurements of progesterone to assess normal vs pathological luteal phase sufficiency are needed. Although sporadic LPD may have limited clinical relevance, 28% of recurrent pregnancy losses are thought to be due to LPD (10), highlighting the importance of proper management of LPD when consistently present. Managing recurrent pregnancy loss with targeted interventions has the potential to reduce adverse maternal and neonatal outcomes and health care spending (11, 12).
The primary objective of this study was to evaluate whether LPD occurs in healthy, regularly menstruating women with no known gynecological disorders. To carry out our objective, we evaluated the prevalence and overlap of short luteal phase duration and suboptimal luteal progesterone concentration (the most common clinical and biochemical definitions of LPD) in a prospective cohort of 259 premenopausal women enrolled in the BioCycle Study. Our secondary objective was to explore whether clinical and/or biochemical LPD are associated with cycle and phase-specific reproductive hormone profiles, including E2, LH, and FSH.
Materials and Methods
Study population
The BioCycle Study was a prospective cohort of 259 women followed up for one (n = 9) or two (n = 250) menstrual cycles (13). Participants were recruited from healthy premenopausal volunteers aged 18–44 years from the western New York state. Women were included if they had a self-reported cycle length between 21 and 35 days during the past 6 months and had not used hormonal contraceptives during the past 3 months or been pregnant within the past 6 months. To be enrolled, women could not have ever sought infertility treatment or had any prior diagnosis of endometriosis, polycystic ovary disease, uterine fibroids (past 12 months), untreated gynecological infection (past 6 months), or gynecological surgery (past year). Additionally, women with a history of chronic disease, an unwillingness to abstain from medications (including hormonal contraceptives), or a desire to start trying to conceive were excluded. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the institutional review board designated by the National Institutes of Health under a reliance agreement. All participants provided written informed consent.
Hormone and ovulation assessment
Participants provided serum samples at up to eight clinic visits per cycle, with midcycle visits determined using the assistance of a fertility monitor (FM) (Clearblue Easy fertility monitor; Inverness Medical) (9). Clinic visits were aimed to correspond to biologically relevant windows including menstruation; mid- and late-follicular phase; LH/FSH surge; ovulation; and early-, mid-, and late-luteal phase. FMs measured estrone-3-glucuronide and LH in the first morning urine, starting on day 6 after the first day of menses and continuing for 10–20 days, depending on the timing of the LH surge. FM data were downloaded to obtain daily estrone-3-glucuronide and LH values for each participant. Participants were highly adherent to the study protocol, with 96% and 93% of women bringing in their FM to have data downloaded for cycles 1 and 2, respectively, and 94% of all women completing at least seven clinic visits per cycle.
E2, progesterone, LH, and FSH were measured in serum collected at each clinic visit. Fasting morning blood samples were processed according to standardized protocols (14). Samples were stored frozen at −80°C and sent as complete participant cycle batches to Kaleida Health Center for Laboratory Medicine (Buffalo, New York) for an analysis of hormone concentrations. Total E2, progesterone, LH, and FSH were measured via IMMULITE 2000 solid-phase competitive chemiluminescent enzymatic immunoassay (Siemens Healthcare). Coefficients of variation reported by the laboratory were less than 10% for E2, less than 5% for LH and FSH, and less than 14% for progesterone.
Progesterone generally remains below 1 ng/mL (1 ng/mL = 3.18 nmol/L) in the follicular phase, rises to above 1 ng/mL during the periovulatory period, and rises steadily thereafter until its decline over the days preceding menses (14). Although progesterone greater than 3 ng/mL is indicative of ovulation, concentrations below this have been found to occur in ovulating women with apparently normal endometrial maturation (2, 14). Consequently, we defined ovulatory cycles as any cycle with progesterone greater than 1 ng/mL, which had a urine or serum LH surge prior to the midluteal visit.
Menses assessment
Participants recorded menstrual bleeding (yes/no) via a daily diary. Menses length was defined as a bleeding episode that included greater than 2 days of bleeding preceded by at least two bleed-free days (15, 16). We specified premenstrual spotting as 2 or fewer days of bleeding that were preceded and followed by at least 2 recorded bleed-free days. Only 2% of the diary days were missing information on the presence or absence of any menstrual bleeding. If participants reported any menstrual bleeding on a given day, they were instructed to complete a detailed follow-up menstrual flow questionnaire consisting of pictograms that assessed the quantity, size, and observed amount of blood loss for each feminine product used as well as any extraneous blood loss not captured by sanitary protection (17). The total blood loss on each bleeding day was estimated using an algorithm (17, 18).
Covariate assessment
At study enrollment, anthropometric measures including height and weight were obtained using standardized protocols. At the end of the follow-up period, percentage body fat was measured using duel-energy X-ray absorptiometry scans (Holigic Discovery Elite, software version 12.4.1; Hologic Inc) (13). Age, self-identified race, smoking status, and reproductive history were obtained using baseline questionnaires (13). Daily minutes of vigorous exercise were captured via the daily diary. Participants completed a 24-hour dietary recall at the clinic during the four visits corresponding to menstruation, midfollicular phase, ovulation, and midluteal phase from which total energy intake was calculated using the Nutrition Data System for Research (2005, Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota).
Statistical analyses
We restricted analyses to ovulatory cycles in which total cycle length was not missing. Previous work has shown ovulation to occur on the day after the FM peak (ie, urine LH surge) in most cycles (19). Consequently, the day of ovulation was assigned using the day of the urine LH surge from the FM or the day of the serum LH maximum value when FM data were not available, plus one day.
Cycle length was calculated as the number of days between the first day of menstrual bleeding and the onset of the next menses for each cycle. Duration of the follicular phase was calculated as the number of days between the first day of menstrual bleeding and day of ovulation, and luteal length was calculated as the day after ovulation through the day prior to the onset of menses. To ensure that serum samples were compared within the same biologically relevant windows between women, we set the day of ovulation as day 0 and then aligned cycle visits as plus or minus days relative to ovulation.
Our diagnostic criteria for LPD included short luteal phase duration, defined as less than 10 days (clinical LPD) (6, 20, 21), and low luteal progesterone concentrations, defined as maximum luteal level of 5 ng/mL or less (biochemical LPD) (22, 23). We evaluated the number of cycles and women who met clinical and/or biochemical LPD criteria. We additionally assessed the number of cycles and women who had a more liberal definition of low luteal progesterone concentration (defined as maximum luteal level ≤ 10 ng/mL) (23, 24); and a luteal phase duration of less than 9 or less than 11 days (25, 26).
Descriptive statistics were calculated for demographic, lifestyle, and menstrual cycle characteristics for clinical and biochemical LPD, respectively. Generalized linear models were used to determine significant differences between groups. Linear mixed models on the log scale of hormones were used to evaluate the association between clinical and biochemical LPD, respectively, on follicular and luteal E2, LH, and FSH and luteal progesterone concentrations. Random-intercept, repeated-measurement models were used to account for the variation between baseline concentration of hormones in individual women and the correlation between cycles of the same women. Because short luteal phase duration was our primary clinical diagnostic criterion, all luteal hormonal analyses were restricted to 8 days or less after ovulation.
To evaluate integrated hormone concentrations, we calculated the area under the curve (AUC) for each cycle and hormone. We assumed an approximately linear relationship between hormone concentration values between visits and calculated the area under each trapezoid between visits. Mean AUCs were then compared by clinical and biochemical LPD using generalized linear models.
Multivariable models were adjusted for age, race, and percentage body fat. Alternative models that adjusted for parity, smoking, physical activity, and total energy intake did not appreciably alter the estimates (data not shown). All analyses were repeated using a more stringent definition of an ovulatory cycle (ie, luteal progesterone >3 ng/mL). Analyses were conducted in SAS, version 9.3 (SAS Institute).
Results
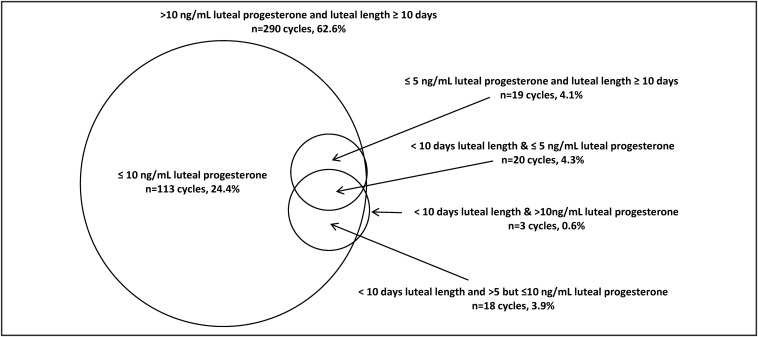
Among the 476 cycles with menstrual cycle length information, there were 13 cycles (2.7%) considered anovulatory, based on luteal progesterone less than 1 ng/mL, and excluded from our analyses. Of the 463 ovulatory cycles, there were 41 cycles with clinical LPD (8.9%), 39 cycles with biochemical LPD (8.4%), and 20 cycles meeting both criteria (4.3%). Although most women experienced either clinical or biochemical LPD for only one cycle, there were eight women who experienced two clinical LPD cycles (3.4%) and five women who experienced two biochemical LPD cycles (2.1%). Nearly all cycles with clinical LPD also had peak luteal progesterone of 10 ng/mL or less (n = 38 cycles, 8.2%), but of the 422 cycles without clinical LPD, approximately one third (n = 132, 31.3%) also had progesterone less than 10.0 ng/mL (Figure 1). The alternative short luteal phase duration cut points showed that 29 cycles among 14 women (6.3%) had a luteal phase less than 9 days and 69 (14.9%) cycles among 39 women had luteal phase less than 11 days.
Figure 1.
Venn diagram outlining number and overlap of cycles with clinical LPD (luteal duration <10 d in length), biochemical LPD (≤5 ng/mL luteal progesterone for all measured levels); and a more liberal definition of biochemical LPD (≤10 ng/mL luteal progesterone for all measured levels). International System of Units (SI) conversion for progesterone: 1 ng/mL = 3.18 nmol/L.
Women with either clinical or biochemical LPD were more likely to be younger, nulliparous, with lower percent body fat, and lighter menses compared with women without LPD (all P ≤ .05) (Table 1). Additionally, women with clinical LPD were more likely to be white (82.9% vs 58.5%, P = .02), with shorter cycle lengths (26.6 vs 29.0 days, P < .001), whereas women with biochemical LPD were less likely to be married (2.6% vs 29.7%, P = .01), more likely to be sexually inactive (66.7% vs 44.8%, P = .03), and have longer cycle lengths (30.4 vs 28.6 d, P = .02). Of the 11 cycles with reported premenstrual spotting, one had clinical LPD (P = 1.0) and none had biochemical LPD (P = .61).
Table 1.
Characteristics of Women Participating in the BioCycle Study (237 Women, 463 Cycles) by Clinical and Biochemical Diagnostic Criteria for LPD
| Luteal Duration |
P Value | Luteal Progesterone |
P Value | |||
|---|---|---|---|---|---|---|
| <10 d | ≥10 d | ≤5 ng/mLa | >5 ng/mL | |||
| Number of cycles, n, % | 41 (8.9) | 422 (91.1) | na | 39 (8.4) | 424 (91.6) | na |
| Demographic and lifestyle characteristics | ||||||
| Age, y | 24.5 ± 7.6 | 28.1 ± 8.3 | .05 | 23.1 ± 6.8 | 28.2 ± 8.3 | .01 |
| 18–22, n, % | 27 (65.9) | 152 (36.0) | .004 | 31 (79.5) | 148 (34.9) | <.001 |
| >22 to 31, n, % | 7 (17.1) | 123 (29.2) | 3 (7.7) | 127 (30.0) | ||
| >31 to 44, n, % | 7 (17.1) | 147 (34.8) | 5 (12.8) | 149 (35.1) | ||
| Percentage body fat | 27.4 ± 4.9 | 29.8 ± 6.1 | .02 | 27.3 (5.1) | 29.8 ± 6.1 | .01 |
| BMI, n, % | ||||||
| Underweight (<18.5 kg/m2) | 0 (0.0) | (3.3) | .65 | 0 (0.0) | 14 (3.3) | .16 |
| Normal (≥18.5 to <25 kg/m2) | 31 (75.6) | 254 (60.2) | 31 (79.5) | 254 (59.9) | ||
| Overweight (≥25 to <30 kg/m2) | 5 (12.2) | 110 (26.1) | 5 (12.8) | 110 (25.9) | ||
| Obese (≥30 kg/m2) | 5 (12.2) | 44 (10.4) | 3 (7.7) | 46 (10.9) | ||
| Race, n, % | ||||||
| White | 34 (82.9) | 247 (58.5) | .02 | 25 (64.1) | 256 (60.4) | .27 |
| Black | 2 (4.9) | 87 (20.6) | 10 (25.6) | 79 (18.6) | ||
| Asian | 5 (12.2) | 56 (13.3) | 2 (5.1) | 59 (13.9) | ||
| Other | 0 (0.0) | 32 (7.6) | 2 (5.1) | 30 (7.1) | ||
| Completed high school, n, % | 40 (97.6) | 367 (87.0) | .07 | 35 (89.7) | 372 (87.7) | .74 |
| Alcohol consumer, n, % | 32 (78.1) | 299 (70.9) | .54 | 27 (69.2) | 304 (71.7) | .97 |
| Smoking, n, % | ||||||
| Current | 2 (4.9) | 62 (14.7) | .63 | 4 (10.3) | 65 (15.3) | .92 |
| Past | 4 (9.8) | 19 (4.5) | 1 (2.6) | 17 (4.0) | ||
| Married, n, % | 7 (17.1) | 120 (28.4) | .18 | 1 (2.6) | 126 (29.7) | .01 |
| Nulligravid, n, % | 33 (80.5) | 278 (66.4) | .13 | 32 (82.1) | 279 (66.3) | .06 |
| Nulliparous, n, % | 36 (87.8) | 292 (70.0) | .03 | 37 (94.8) | 291 (88.7) | .004 |
| Number of cycles, n, % | 41 (8.9) | 422 (91.1) | na | 39 (8.4) | 424 (91.6) | na |
| Sexual activity, n, %2 | ||||||
| Inactive | 25 (61.0) | 191 (45.3) | .19 | 26 (66.7) | 190 (44.8) | .03 |
| Weekly or less | 7 (17.1) | 122 (28.9) | 7 (18.0) | 122 (28.8) | ||
| Greater than weekly | 9 (22.0) | 109 (25.8) | 6 (15.4) | 112 (26.4) | ||
| Past OC use, n, % | 18 (43.9) | 237 (56.1) | .18 | 17 (43.6) | 238 (56.5) | .19 |
| Total energy, kcal | 1588.7 ± 381.0 | 1621.4 ± 439.0 | .53 | 1562.3 ± 459.6 | 1623.6 ± 431.7 | .47 |
| Vigorous exercise, min/d [median (IQR)] | 12.8 (2.0, 26.9) | 8.0 (0.8, 18.6) | .45 | 11.5 (2.1, 21.1) | 8.0 (0.8, 19.0) | .10 |
| Menstrual cycle characteristics | ||||||
| Cycle length, d | 26.6 ± 3.4 | 29.0 ± 3.6 | <.001 | 30.4 ± 6.5 | 28.6 ± 3.2 | .01 |
| Menses length, d | 7.0 ± 2.0 | 7.0 ± 2.3 | .89 | 7.2 ± 2.5 | 7.0 ± 2.2 | .91 |
| Total amount of blood loss, mL [median (IQR)] | 42.0 (15.5, 70.0) | 58.0 (30.0, 88.0) | <.001 | 36.6 (18.5, 72.5) | 57.9 (28.0, 87.8) | .02 |
| Premenstrual spotting, n, % | 1 (2.4) | 10 (2.4) | 1.0 | 0 (0) | 11 (2.6) | .61 |
Abbreviations: BMI, body mass index; IQR, interquartile range; na, not available; OC, oral contraceptive. Repeated-measures analyses (taking into account multiple cycles from the same woman) used to assess associations between demographic, lifestyle, diet, and menstrual cycle characteristics. Mean ± SD unless otherwise noted.
International System of Units (SI) conversion for progesterone: 1 ng/mL = 3.18 nmol/L.
Sexual activity was defined as vaginal intercourse.
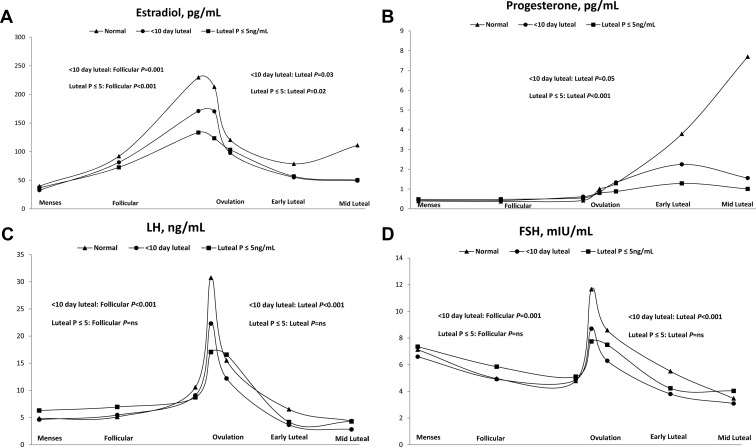
After adjusting for age, race, and percentage body fat, women with clinical LPD had lower log E2 concentrations during the follicular [β = − .26, 95% confidence interval (CI) −0.41, −0.10; P = .001] and luteal (β = −.18, 95% CI −0.33, −0.02; P = .03) phases; lower progesterone during the luteal phase (β = −.26, 95% CI −0.51, 0.00; P = .05); lower LH during the follicular (β = −.26, 95% CI −0.41, −0.11; P < .001) and luteal (β = −.38, 95% CI: −0.53, −0.22; P < .001) phases; and lower FSH during the follicular (β = −.13, 95% CI −0.22, −0.04; P = .001) and luteal (β = −.30, 95% CI −0.42, −0.17; P < .001) phases compared with women without clinical LPD (Figure 2). Similarly, after adjustment, women with biochemical LPD had significantly lower log E2 during the follicular (β = −.44, 95% CI −0.58, −0.29; P < .001) and luteal (β = −.19, 95% CI −0.35, −0.03; P = .02) phases; lower progesterone (as expected due to definition) during the luteal phase (β = −.63, 95% CI −0.89, −0.37; P < .001); but no significant differences in LH or FSH concentrations during either the follicular or luteal phases compared with women without biochemical LPD (Figure 2).
Figure 2.
Geometric mean concentration of reproductive hormones (A, estradiol; B, progesterone; C, LH; D, FSH) among ovulatory women (n = 237 women, 463 cycles) by luteal phase deficiency category (normal, triangle; luteal duration <10 d in length, circle; ≤5 ng/mL luteal progesterone for all measured levels, square). Day of estimated ovulation was set to day 0. The cycles included the following: menses: less than to −8 days; follicular: −7 days to −3 days; ovulation (three visits: day −2, −1, and 0); early luteal: +1 day to +3 days; midluteal: +4 days to +8 days. We restricted analyses to +8 or fewer days after ovulation because the short luteal phase duration was our primary clinical diagnostic criteria, and no woman with a short luteal phase duration came in for a serum sample more than +8 days. P values represent significant differences between reproductive hormone concentrations for clinical (luteal phase duration <10 d) and biochemical (luteal progesterone ≤5 ng/mL) luteal phase deficiency compared with normal cycles after adjusting for age, race, and percentage body fat. ns, nonsignificant. International System of Units (SI) conversion is as follows: estradiol, 1 pg/mL = 3.67 pmol/L; progesterone, 1 ng/mL = 3.18 nmol/L; LH, 1 ng/mL = 4.34 mIU/L; and FSH, 1 mIU/mL = 1 IU/L.
In addition, women with clinical LPD compared with those without had lower E2 (P < .001), progesterone (P < .001), LH (P = .01), and FSH (P = .03) integrated hormone levels via AUC analysis across the cycle after adjusting for age, race, and percentage body fat (Table 2). Women with biochemical LPD had lower E2 (P = .001) and progesterone (P < .001) but higher LH (P = .04) integrated hormone levels after adjustment compared with women without biochemical LPD. We found similar results for all our analyses when we restricted our sample to ovulatory cycles in which at least one luteal progesterone was greater than 3 ng/mL (n = 443, data not shown).
Table 2.
Area Under the Cycle-Specific Hormone Curve Among Ovulatory Women in the BioCycle Study (n = 237 Women, 463 Cycles) by LPD Category
| Hormone | LPD | Median | Q25 | Q75 | P Valuea |
|---|---|---|---|---|---|
| Luteal duration <10 days | |||||
| Estradiol | Yes | 2008.0 | 1627.5 | 2702.5 | <.001 |
| No | 2970.8 | 2339.0 | 3801.5 | ||
| Progesterone | Yes | 35.2 | 22.0 | 53.9 | <.001 |
| No | 104.4 | 81.2 | 129.3 | ||
| LH | Yes | 189.1 | 146.4 | 276.7 | .01 |
| No | 232.9 | 175.0 | 308.9 | ||
| FSH | Yes | 142.7 | 115.6 | 194.3 | .03 |
| No | 170.8 | 140.4 | 207.0 | ||
| Luteal progesterone ≤5 ng/mL | |||||
| Estradiol | Yes | 1979.6 | 1441.5 | 2824.0 | .001 |
| No | 2958.8 | 2328.5 | 3800.0 | ||
| Progesterone | Yes | 22.6 | 19.2 | 35.2 | <.001 |
| No | 104.4 | 81.2 | 129.1 | ||
| LH | Yes | 272.6 | 182.6 | 377.1 | .04 |
| No | 229.7 | 170.8 | 377.1 | ||
| FSH | Yes | 177.0 | 126.5 | 209.4 | .13 |
| No | 168.3 | 138.3 | 205.3 | ||
Abbreviation: Q, quartile.
Mean area under the curve were compared by LPD status (yes/no) using repeated-measures analyses, adjusting for woman's age, race, and percentage body fat.
Discussion
Our findings suggest that regularly menstruating women do express evidence of subtle menstrual cycle disorders manifested as LPD. We found that short luteal phase duration, defined as less than 10 days, or suboptimal luteal progesterone, defined as less than 5 ng/mL maximum progesterone (primary clinical and biochemical diagnostic criteria) affected 8.9% and 8.4% of ovulatory cycles, respectively, among study participants, with 4.3% of cycles meeting both criteria. Extending the biochemical criterion to maximum serum luteal progesterone less than 10 ng/mL (23) resulted in a greater overlap with clinical LPD but, as a diagnostic criterion alone, would result in a higher false-positive rate of LPD as supported by previous research (2). Although clinical and biochemical LPD share certain characteristics and some of the same associated risk factors, the lack of complete consistency in regard to menstrual cycle characteristics and reproductive hormone concentrations indicates that these two types of LPD may act through different mechanisms.
Among normal cycling women, the prevalence of a luteal phase duration of less than 9 and less than 11 days (relative to ovulation), respectively, have been estimated to be 5.2% (27) and 8.0% (28). We found similar results with 5.1% of women experiencing a luteal phase duration of less than 9 days and 12.4% of women experiencing a luteal phase duration of less than 11 days. Although early studies on LPD report consistency between a short luteal phase duration and suboptimal progesterone levels (29, 30), these studies were small and relied on BBT to identify ovulation and therefore calculate luteal phase duration. In a more recent study conducted among normal cycling women with daily serum hormone assessment, the authors found that a short luteal phase duration is a poor predictor of luteal integrated progesterone levels (24). A larger study (n = 335 cycles), which collected daily serum samples to identify ovulation, showed that up to half of cycles with short luteal phase duration, defined as less than 11 days, had adequate luteal serum progesterone measured +5 to +7 days post-LH surge (28). Likewise, we found that more than half of the cycles with short luteal phase duration had greater than 5 ng/mL peak luteal progesterone levels.
Identification of a short luteal phase (<10 d) via ovulation detection through validated instruments may serve as the least invasive and most accurate tool for assessing short luteal phase duration. Obtaining a serum progesterone level of less than 10 ng/mL approximately 6–8 days after ovulation may provide additional confidence that a cycle can be categorized with LPD. Although any luteal phase progesterone concentration greater than 3 ng/mL provides reliable evidence of ovulation (2), no consistent threshold has been established to identify abnormal corpus luteum function (15), thus supporting the growing body of evidence indicating that serum progesterone alone lacks specificity for diagnosing LPD (9, 15). Moreover, more invasive procedures such as endometrial biopsy have proven to be invalid diagnostic tools for LPD assessment (2, 3).
In the present study, both clinical and biochemical LPD were associated with decreased blood flow at menses. Although a previous study found an association between lighter bleeding and anovulation (28), our finding of lighter bleeding associated with LPD is, to our knowledge, novel. This finding is physiologically plausible because the thickening and maturation of the uterine endometrium throughout the menstrual cycle is highly dependent on estrogen secretion during the follicular phase and on progesterone support in the luteal phase, which were both lower in cycles with LPD (defined either clinically or biochemically) in our study.
In contrast to menstrual flow, we found inconsistent results in regard to LPD criteria and cycle length. In corroboration with previous studies (30, 31), we found shortened luteal duration to be associated with shortened total cycle length. Conversely, we found biochemical LPD to be associated with longer total cycle length. Whether our finding lends credence to the hypothesis that LPD may result from more than one pathological mechanism or is a result of missed peak progesterone assessments is not clear. Polycystic ovary syndrome (PCOS), an independent endocrine disorder but sharing a common pathophysiological profile with LPD (32), has been shown to be associated with longer cycles (>35 d) (33). It has been speculated that LPD may be involved in the infertility observed among women with PCOS, emphasizing the potential overlap of these gynecological disorders (32). However, despite adequate luteal phase visits among biochemical LPD cycles (mean 2.4), there was variability in the days after ovulation that sera were collected, warranting further research to confirm our findings.
We found both follicular and luteal E2 concentrations to be significantly lower in women with clinical and/or biochemical LPD, which is in agreement with two previous studies among regularly menstruating women (26, 28). Only one previous study assessed LH and FSH levels among eumenorrheic women, reporting lower midfollicular FSH in women with luteal phase duration less than 10 days compared with controls but no difference in LH levels (30). In contrast, we found significantly lower LH and FSH across the cycle for women with luteal phase duration less than 10 days compared with cycles of 10 days or longer. Reasons for the discrepant findings may be due to the previous study's small sample size (n = 11 cycles among four women) and use of BBT to assess ovulation. Our finding of reduced follicular LH and FSH for clinical LPD supports the hypothesis that alterations of the hypothalamic-pituitary-ovarian axis impair both folliculogenesis and (indirectly or directly) subsequent corpus luteum function, which may ultimately compromise endometrial maturation and stability. Our findings of higher LH for biochemical LPD via the AUC analysis supports the idea that clinical and biochemical LPD potentially act through different mechanisms. Previous research indicates that ovulatory women with PCOS have increased GnRH pulsatility over the entire cycle, leading to increased LH production yet still have a deficient luteal phase progesterone secretion, suggesting ovarian dysfunction (33).
The BioCycle Study had several strengths including intensive monitoring of a large number of women with no known reproductive or gynecological disorders over the course of two menstrual cycles. Additionally, we used a validated FM to time clinic visits so that serum samples would be drawn at biologically relevant windows, providing a superior measurement for identifying ovulation compared with BBT. Nevertheless, our study had several limitations including an inability to obtain daily biospecimen measurements or assess ovulation via ultrasound because of cost restrictions. Consequently, we may have incorrectly classified some of the suboptimal luteal progesterone cycles, due to a missed midluteal visit, or some of the short luteal phase duration cycles, due to failure of the FM to correctly identify peak fertility. However, we thoroughly reviewed FM data for the urine LH surge and used serum LH measurements in combination with urine LH measurements to minimize any potential misclassification. Finally, we were not able to assess whether LPD is associated with aneuploid oocytes. However, given the evidence of treatment efficacy for progesterone supplementation in women with three or more pregnancy losses (34), karyotypic abnormalities associated with ovulation are not likely to be the predominant cause of LPD, at least among women with recurrent LPD.
Given our finding that short luteal phase duration of less than 10 days in combination with maximum luteal progesterone of 10 ng/mL or less revealed the best overlap between clinical and biochemical LPD, clinicians and researchers wanting to assess LPD may find that a validated ovulation prediction kit in combination with a well-timed progesterone serum sample (ie, +6 to +8 d after ovulation) is a cost-effective and reasonably accurate means to assess LPD. Because it is well known that implantation occurs within a narrow window of the midluteal phase and that delayed implantation predisposes a woman to infertility and/or pregnancy loss, further population-based research examining whether well-diagnosed LPD is associated with implantation delay is warranted. Additionally, studies looking at treatment efficacy (34, 35) should be reevaluated using improved diagnostic tools. In summary, our findings suggest that women with regular menstrual cycles do express evidence of subtle menstrual cycle disorders manifested as LPD based on luteal length or level of progesterone secretion, potentially through different mechanisms (32). We also found that both types of LPD are highly associated with hormonal differences in both the follicular and luteal phase, suggesting that luteal deficiency may in fact be a manifestation of follicular deficiency affecting all subsequent phases of the menstrual cycle (1, 26). Additional research is needed to assess the clinical implications of both types of LPD.
Acknowledgments
We acknowledge the staff at the University at Buffalo, the BioCycle Study Working Group members, and the BioCycle participants for their assistance and participation.
This work was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Grant HHSN275200403394C).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BBT
- basal body temperature
- CI
- confidence interval
- E2
- estradiol
- FM
- fertility monitor
- LPD
- luteal phase deficiency
- PCOS
- polycystic ovary syndrome.
References
- 1. Brown JB. Types of ovarian activity in women and their significance: the continuum (a reinterpretation of early findings). Hum Reprod Update. 2011;17:141–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Usadi RS, Groll JM, Lessey BA, et al. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93:4058–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fritz MA. The modern infertility evaluation. Clin Obstet Gynecol. 2012;55:692–705 [DOI] [PubMed] [Google Scholar]
- 4. American Society for Reproductive Medicine. The clinical relevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2012. 98:1112–1117 [DOI] [PubMed] [Google Scholar]
- 5. Ginsburg KA. Luteal phase defect. Etiology, diagnosis, and management. Endocrinol Metab Clin North Am. 1992;21:85–104 [PubMed] [Google Scholar]
- 6. Jones HW. Luteal-phase defect: the role of Georgeanna Seegar Jones. Fertil Steril. 2008;90:e5–e7 [DOI] [PubMed] [Google Scholar]
- 7. Murray MJ, Meyer WR, Zaino RJ, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–1343 [DOI] [PubMed] [Google Scholar]
- 8. Coutifaris C, Myers ER, Guzick DS, et al. NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272 [DOI] [PubMed] [Google Scholar]
- 9. Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith ML, Schust DJ. Endocrinology and recurrent early pregnancy loss. Semin Reprod Med. 2011;29:482–490 [DOI] [PubMed] [Google Scholar]
- 11. Barnhart KT. Assisted reproductive technologies and perinatal morbidity: interrogating the association. Fertil Steril. 2013;99:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Semin Reprod Med. 2008;26:423–435 [DOI] [PubMed] [Google Scholar]
- 13. Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fritz MA, Sperhoff L. Female infertility. In: Speroff L, Fritz MA, eds. Clinical Gynecologic Endocrinology and Infertility. 8th ed Philadelphia: Lippincott Williams, Wilkins; 2011:1162–1163 [Google Scholar]
- 15. Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16 [DOI] [PubMed] [Google Scholar]
- 16. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207–225 [DOI] [PubMed] [Google Scholar]
- 17. Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175:536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–131 [DOI] [PubMed] [Google Scholar]
- 19. Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482 [DOI] [PubMed] [Google Scholar]
- 20. Jones G. Some newer aspects of the management of infertility. JAMA. 1949;141:1123–1129 [DOI] [PubMed] [Google Scholar]
- 21. Sonntag B, Ludwig M. An integrated view on the luteal phase: diagnosis and treatment in subfertility. Clin Endocrinol (Oxf). 2012;77:500–507 [DOI] [PubMed] [Google Scholar]
- 22. Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. 1974;44:522–525 [PubMed] [Google Scholar]
- 23. Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102:317–318 [DOI] [PubMed] [Google Scholar]
- 24. Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62:54–62 [DOI] [PubMed] [Google Scholar]
- 25. Downs KA, Gibson M. BBT graph and the luteal phase defect. Fertil Steril. 1983;40:466–468 [DOI] [PubMed] [Google Scholar]
- 26. Hilgers T. Follicular and luteal phase deficiency: advancing concepts and new terminology. In: Hilgers TW, ed. The Medical and Surgical Practice of NaPro Technology. Omaha, NE: Pope Paul VI Institute Press; 2004:425–453 [Google Scholar]
- 27. Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984;91:685–689 [DOI] [PubMed] [Google Scholar]
- 28. Smith KS, Lenton EA, Landgren BM, Cooke ID. Is the short luteal phase a defective luteal phase? Ann NY Academy Sci. 2006;442:387–390 [DOI] [PubMed] [Google Scholar]
- 29. Bayer SR, DeCherney AH. Clinical manifestations and treatment of dysfunctional uterine bleeding. JAMA. 1993;269:1823–1828 [PubMed] [Google Scholar]
- 30. Sherman BM, Korenman SG. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J Clin Endocrinol Metab. 1974;38:89–93 [DOI] [PubMed] [Google Scholar]
- 31. Strott CA, Cargille CM, Ross GT, Lipsett MB. The short luteal phase. J Clin Endocrinol Metab. 1970;30:246–251 [DOI] [PubMed] [Google Scholar]
- 32. Boutzios G, Karalaki M, Zapanti E. Common pathophysiological mechanisms involved in luteal phase deficiency and polycystic ovary syndrome. Impact on fertility. Endocrine. 2013;43:314–317 [DOI] [PubMed] [Google Scholar]
- 33. Zhang HY, Guo CX, Zhu FF, Qu PP, Lin WJ, Xiong J. Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Arch Gynecol Obstet. 2013;287:525–531 [DOI] [PubMed] [Google Scholar]
- 34. Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. 2013;31;10. [DOI] [PubMed] [Google Scholar]
- 35. Guzick DS, Zeleznik A. Efficacy of clomiphene citrate in the treatment of luteal phase deficiency: quantity versus quality of preovulatory follicles. Fertil Steril. 1990;54:206–210 [DOI] [PubMed] [Google Scholar]