Abstract
Context:
Vitamin D deficiency is common among reproductive-aged women and has a role in female reproduction.
Objective:
This study evaluated the role of 1,25-dihydroxyvitamin D3 (vit D3) in ovarian follicular development and steroidogenesis by using a human granulosa cell (GC) model.
Design, Setting, and Participants:
Fifty-four women who underwent in vitro fertilization were enrolled.
Intervention:
Follicular fluid (FF) and mural and cumulus GCs were collected from small and large follicles. In separate experiments, primary cumulus GCs were cultured with or without vit D3 followed by RT-PCR for mRNA expression levels. The effect of recombinant anti-Mullerian hormone (AMH) on nuclear localization of phospho-Smad 1/5/8 was evaluated in the presence or absence of vit D3 by using immunofluorescence. 25-Hydroxyvitamin D levels in FF as well as cell culture media AMH, progesterone, and estradiol (E2) concentrations were determined by ELISA and RIA.
Main Outcome Measures:
The following were measured: 1) mRNA expression levels; 2) 3β-hydroxysteroid dehydrogenase (3β-HSD) enzyme activity; 3) FSH-induced aromatase mRNA and E2 production; and 4) nuclear localization of phospho-Smad 1/5/8.
Results:
In a multivariate analysis, 25 OH-D levels in FF negatively correlated with AMH and AMH receptor (AMHR)-II mRNA levels in cumulus GCs of small follicles. Compared with women with replete 25-hydroxyvitamin D levels in FF, those with insufficient/deficient levels had a 2-fold increase in AMHR-II mRNA levels in cumulus GCs of small follicles (P = .02). Treatment with vit D3 caused a decrease in AMHR-II and FSH receptor mRNA but an increase in 3-βHSD mRNA levels compared with control (P < .05). Vit D3 enhanced 3-βHSD enzyme activity as assessed by increasing progesterone release; however, vit D3 did not affect FSH-induced aromatase mRNA and E2 production, but it decreased the phosphorylation of Smad 1/5/8 and its nuclear localization.
Conclusion:
These data suggest that vit D3 alters AMH signaling and steroidogenesis in human cumulus GCs, possibly reflecting a state of GC luteinization potentiation.
Vitamin D deficiency remains common in both the developed and developing world (1). In the United States, 20%–90% of reproductive-aged women are vitamin D deficient despite prenatal vitamin intake (1). Vitamin D deficiency is associated with obstetrical and reproductive complications including recurrent pregnancy loss, small-for-gestational-age babies, abnormal puberty, and infertility (2–7). Among the many physiological processes influenced by vitamin D, a critical role in reproductive physiology has been suggested (2, 4, 6, 8). Available data to date have correlated circulating vitamin D levels with reproductive success, such as achieving a pregnancy using assisted reproductive technology (2, 8). However, little is known about the mechanisms by which vitamin D deficiency affects reproduction. It was recently shown that serum (2, 9) and follicular fluid (2) levels of 25-hydroxyvitamin D (25 OH-D) were highly positively correlated and that patients who achieved clinical pregnancies after in vitro fertilization (IVF) exhibited significantly higher follicular fluid and serum levels of 25 OH-D (2). Although little is known about the mechanism of this relationship, pregnancy rates after assisted reproductive technology show seasonal variation in accordance with the seasonality of vitamin D (10–12).
Vitamin D receptor is expressed in ovarian tissue including granulosa cells (GCs) (13). We have shown that vitamin D plays an important role in ovulatory dysfunction in mice, likely mediated through anti-Mullerian hormone (AMH) (14). AMH is produced by GCs in the ovaries in which it inhibits the loss of the oocyte pool by slowing down growth followed by death of follicles containing the oocytes (15–17). Additionally, an AMH gene null mutation causes early depletion of the stock of oocyte pool in the mouse ovary (15, 18). The relationship between vitamin D and AMH exists at both the genetic and serum levels (3, 14, 19). The promoter region for the AMH gene contains a vitamin D response element in human prostate cell line (19). Thus, the active form of vitamin D has been shown to up-regulate AMH production in cultured human prostate cell lines (19, 20). In humans, we have demonstrated that serum AMH correlates with serum 25 OH-D levels in late reproductive-aged women (3). Others have shown that there is a well-defined seasonal variation in IVF success (11, 12) corresponding to a seasonal variation in AMH levels, being 18% lower in the winter when vitamin D levels are lowest (10). The extent of seasonal variation in a woman's AMH level correlated with the extent to which her 25 OH-D levels varied (10). Finally, vitamin D supplements were sufficient to block the seasonal changes in both the 25 OH-D and AMH serum levels (10). However, little is known about how vitamin D affects AMH signaling, a pathway important in folliculogenesis, and steroidogenesis in human ovaries. In the present study, we hypothesized that alteration of ovarian function occurring with vitamin D treatment would be reflected by changes in GC function as reflected by follicular differentiation and maturation. Our aim was to investigate the effect of vitamin D treatment in vitro on the regulation of genes responsible for follicular development/differentiation and steroidogenesis.
Materials and Methods
Subjects
Thirty-three infertile women undergoing fresh IVF and intracytoplasmic sperm injection cycles using autologous oocytes at the University of Vermont College of Medicine and Montefiore's Institute for Reproductive Medicine and Health/Albert Einstein College of Medicine between July 2010 and August 2013 were prospectively enrolled in the first experiment. Inclusion criteria consisted of women with normal ovarian reserve defined as day 3 FSH less than 10 mIU/mL and day 3 estradiol (E2) less than 80 pg/mL. Reasons for infertility were male, tubal, unexplained, endometriosis, and uterine factors. Women with polycystic ovarian syndrome as defined by the Rotterdam criteria (21) were excluded from the study. All patients gave informed consent, and the study was approved by the Institutional Review Board of both the University of Vermont College of Medicine (M13–062) and Montefiore Medical Center (MMC 04–08-199E). In these women, we compared AMH and AMH receptor (AMHR)-II mRNA expression levels in both mural and cumulus GCs between those who were replete (≥30 ng/mL) vs those who were deplete and insufficient (<30 ng/mL) of follicular fluid 25 OH-D (see below for details).
Collection of follicular fluid and GCs
Follicle size was estimated immediately at the time of oocyte retrieval by ultrasound. Follicular fluid was obtained from small (SFs; < 14 mm) and large follicles (LFs; ≥ 14 mm) and collected in separate tubes. The size cutoff for SFs and LFs was chosen as previously described (22, 23). We studied SFs and LFs separately to determine differences between mature and immature follicles because AMH gene expression usually declines as a follicle matures (24). LFs were aspirated prior to SF to avoid confusion and contamination. Follicular aspirates included mural GCs and oocytes surrounded by cumulus GCs. The fluid from the first aspirated follicle was used for hormonal protein measurement to avoid blood contamination but was not used for mRNA expression evaluation because of possible contamination with vaginal mucosa cells. After removal of the cumulus-oocyte complex by the embryologist, fluid was pooled separately for all SFs and LFs from each participant in order to isolate mural GCs.
Mural GCs were isolated and concentrated as previously described (23). Briefly, mural GCs were added to 40% PureCeption gradient solution (Cooper Surgical) and then centrifuged to remove red and white blood cells. Cells were then washed with PBS and incubated with CD 45+ tagged magnetic beads (Invitrogen) for 20 minutes at +4°C to remove the remaining white blood cells. The beads were then separated, and the remaining fluid was centrifuged for 5 minutes at 600 × g to collect the mural GCs of SFs and LFs separately.
After identification of the cumulus-oocyte complex in the aspirate, cumulus GCs were mechanically collected by cutting the cumulus cell layer from each oocyte then washed with PBS. Cumulus GCs collected from SFs and LFs were pooled separately.
RNA extraction, reverse transcription, and real-time PCR (RT-PCR)
For each patient, mural and cumulus GCs from either SFs or LFs were pooled. RNA was isolated using Trizol reagent (Invitrogen) and chloroform extraction according to the manufacturer's instructions. RNA quality analysis was assessed by a Nanodrop spectrophotometer and Agilent bioanalyzer. Only samples with a minimum concentration of 10 ng/μL and with an OD 260:280 ratio of 1.8–2.0 were used for the evaluation of AMH, AMHR-II, FSH receptor (FSHR), aromatase (CYP19A1), 3β-hydroxysteroid dehydrogenase (3β-HSD), LH receptor (LHR), P450 side-chain cleavage enzyme (P450scc), steroidogenic acute regulatory protein (StAR), and c-kit ligand mRNA expression levels in GCs was achieved by RT-PCR kinetics using the SYBR Green I chemistry as we described elsewhere (23). The primers used (Supplemental Table 1) were synthesized by Fisher. Glyceraldehyde-3-phosphate dehydrogenase primers were used as a loading control, and the levels of mRNA for each gene relative to glyceraldehyde-3-phosphate dehydrogenase was calculated using the ΔΔcycle threshold method (25).
Cumulus GC culture with 1,25-dihydroxyvitamin D3 (vit D3)
Eight additional women undergoing fresh IVF/intracytoplasmic sperm injection cycles were prospectively enrolled. Their cumulus GCs were collected as described above. We decided to study cumulus GCs in subsequent experiments because of the following: 1) we did not find a significant correlation between follicular fluid 25 OH-D and mRNA levels in the mural compartment, 2) we have previously shown that cumulus and mural GCs responded similarly in cell culture (23), and 3) cumulus GCs reflect the health of the oocyte more than mural GCs do (26, 27). For these studies, all follicles were pooled together because we did not find any difference in AMH or AMHR-II mRNA levels between SFs and LFs (data not shown). Cumulus GCs of the eight participants were briefly treated with 0.614 U/mL hyaluronidase (Sigma), followed by 2× wash with PBS and centrifugation. The pellet of cumulus GCs was washed with fresh medium (DMEM-F12 and 1% fetal bovine serum), counted in a hemocytometer, and cell viability determined using trypan blue dye exclusion.
Cumulus GCs of each participant were then divided in half and cultured in 24-well culture plates (pretreated with poly-L-Lysine for 5 min) for 24 hours. The next day, cells were incubated in fresh culture media with or without vit D3 (50–100 nM; Sigma) for another 24 hours. These concentrations of vit D3 used have been shown to stimulate AMH gene expression in human prostate cancer cell lines (19, 20). Plates were incubated under a humidified atmosphere of 95% air and 5% CO2 at 37°C. At the end of the experiment, RNA extraction and RT-PCR for AMH, AMHR-II, FSHR, 3β-HSD, CYP19A1, LHR, StAR, P450scc, and c-kit ligand mRNA expression were performed as described above.
3β-HSD enzyme activity
The effect of vit D3 on progesterone (P4) release by GCs was used as an indicator of 3β-HSD activity. The enzyme 3β-HSD is responsible for the synthesis of P4 from pregnenolone (28). To study the effect of vit D3 on the 3β-HSD activity, cumulus GCs of three participants were briefly treated with 0.614 U/mL hyaluronidase (Sigma) followed by a wash with fresh medium (DMEM-F12 and 1% fetal bovine serum) and centrifugation. The pellet of cumulus GCs was then washed with fresh media and divided in half and then cultured in 24-well culture plates (as described previously) for 24 hours with no other treatment for the cells to attach to the plate. After the first day of culture, the media were collected and recorded as the baseline P4 level. The culture media were then replaced with the addition of pregnenolone (10−7 M) added to the fresh media of each plate and cultured with or without vit D3 (50 nM) for another 24 hours. Because there was no difference in the mRNA expression levels when using 50 or 100 nM of vit D3, the 50 nM dose was used in subsequent experiments. The media were collected for the measurement of P4 concentrations at 24 hours after the addition of the substrate pregnenolone.
FSH-induced aromatase mRNA expression and E2 production
Recombinant FSH induces aromatase mRNA expression and E2 release by GCs (29). GCs isolated from follicles of six additional participants were pooled and cultured as described above. Cells were treated with media alone (controls) or with recombinant FSH (10 IU; urofollitropin; Ferring Pharmaceuticals Inc) in the presence or absence of vit D3 (50 nM). Cells were incubated for 24 hours as described above. The culture media were collected for E2 measurement and cells were lysed for aromatase mRNA quantification by RT-PCR, as described above.
Immunofluorescence for phospho-Smad 1/5/8
AMH signals intracellularly via Smad 1/5/8 (30, 31), and thus, we tested the effect of recombinant AMH on Smad 1/5/8 nuclear localization in cumulus GCs that were treated with vit D3 or media alone (control). Cumulus GCs obtained from additional four participants were cultured on glass cover slides. The cells were cultured in media with or without vit D3 (50 nM) for 24 hours were then serum starved overnight. The next day, GCs were treated with AMH (50 ng/mL) for 45 minutes. Cells were fixed in 3.7% formaldehyde and permeabilized in 0.2% Triton X-100 for 10 minutes at room temperature, and nonspecific binding was inhibited by blocking with BSA in 1% PBS for 1 hour. Fixed GCs were incubated with primary antibody for phospho-Smad 1/5/8 (1:100 dilution; Cell Signaling Technology Inc) overnight at 4°C, washed, and then incubated in a secondary antibody (1:200 dilution; Alexa Fluor 488 goat antirabbit IgG; Invitrogen) for 1–2 hours at room temperature. Slides were mounted with 4′,6′-diamino-2-phenylindole (DAPI) mounting media (Vector Laboratories, Inc) and observed on a Zeiss 510 META laser-scanning confocal microscope. Cellular images were captured using the Plane-NEOFLUAR ×25 immersion objective lens. Using MetaMorph software analysis (Molecular Devices), a threshold of 62 (0–255 scale of intensity) was used to exclude any background produced by DAPI. Grading was done blinded to treatment.
Measurement of 25 OH-D levels, AMH protein, P4, and E2 concentrations
Follicular fluid and cell culture media levels of AMH protein were measured with an ELISA kit (Beckman Coulter, Inc) according to the manufacturer's recommendations, as we previously described (32, 33). Follicular fluid 25 OH-D measurement was performed using liquid chromatography/mass spectroscopy/tandem spectroscopy (3). Progesterone and E2 were quantified in cell culture media by RIA. The interassay coefficients of variation for all assays ranged between 7% and 14%.
Statistics
Data were tested for normality. Comparisons were performed using paired or unpaired t test if the data were normally distributed and Wilcoxon matched-pairs signed rank test or Mann-Whitney test if the data were not normally distributed. Demographic and clinical data were expressed as mean ± SEM. RT-PCR results were expressed as relative number of copies ± SEM. Univariate and multivariate linear regression analyses of follicular fluid 25 OH-D levels with AMH and AMHR-II mRNA levels were performed. Area under the curve (AUC) for total P4 release by GCs was calculated and difference in AUC was compared between vit D3-treated and -untreated GCs. All statistical procedures were run on STATA software (StataCorp). P ≤ .05 will be considered statistically significant.
Results
AMHR-II mRNA expression levels are elevated in women with insufficient follicular fluid 25 OH-D
Eighteen women (54%) had insufficient/deficient follicular fluid 25 OH-D and 15 women (46%) had replete follicular fluid 25 OH-D. Women with insufficient/deficient and replete follicular fluid 25 OH-D did not differ in age, body mass index, or day 3 FSH (Supplemental Table 2). Compared with women with replete follicular fluid 25 OH-D levels, those with insufficient/deficient follicular fluid 25 OH-D levels had a significant 2-fold increase in AMHR-II mRNA levels (P = .02) in the cumulus GCs of their SF (Figure 1). Women with replete and insufficient/deficient follicular fluid 25 OH-D did not have a statistical difference in AMH or AMHR-II mRNA expression levels in the cumulus GCs of LFs and mural GCs (of both SFs and LFs).
Figure 1.
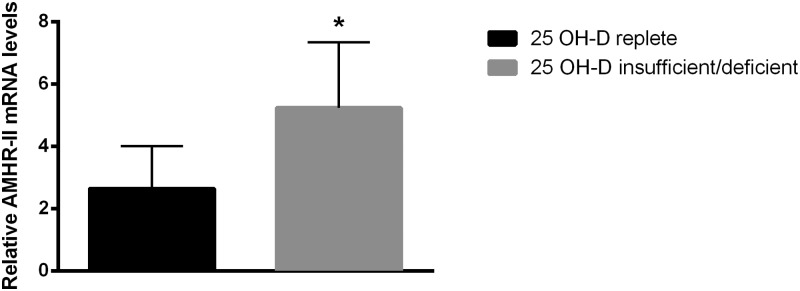
AMHR-II mRNA expression levels in women with replete or insufficient/deficient follicular fluid 25 OH-D. Graph showing mRNA expression of AMHR-II in GCs from women with either replete (n = 15) or insufficient/deficient (n = 18) 25 OH-D levels. Women with insufficient/deficient follicular fluid 25 OH-D levels had a significant 2-fold increase in the AMHR-II mRNA expression levels in cumulus GCs of small follicles when compared with the women with replete follicular fluid 25 OH-D levels. *, P < .05 vitamin D replete vs insufficient/deficient.
In all participants, after adjusting for age, body mass index, and day 3 FSH, there was a negative correlation between follicular fluid 25 OH-D and AMH and AMHR-II mRNA levels in cumulus GCs of SFs (P < .05). Cumulus GCs of LFs trended to be correlated with AMH and AMHR-II (P = .06) (Table 1). There was no correlation between follicular fluid 25 OH-D and AMH or AMHR-II mRNA levels in the GCs of the mural compartment in either SFs or LFs (data not shown).
Table 1.
Correlation Between Follicular Fluid 25 OH-D Concentrations and AMH/AMHR-II mRNA Levels in Cumulus GCs
| Gene | Follicle Size (Cumulus) | RNA Sample Size | R Value | P Value |
|---|---|---|---|---|
| AMH | Large | 15 | −0.50 | .06 |
| Small | 18 | −0.52 | .03 | |
| AMHR-II | Large | 13 | −0.70 | .009 |
| Small | 16 | −0.54 | .03 |
Effect of vit D3 treatment on steroidogenic enzymes in cumulus GCs
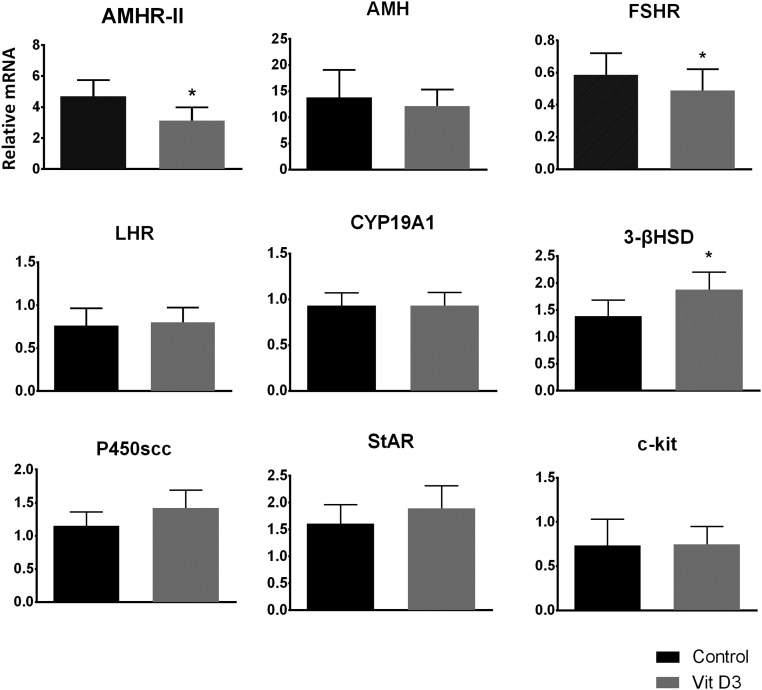
There was no difference in mRNA expression levels in cells treated with 50 or 100 nM of vit D3 (data not shown). Cumulus GCs cultured with vit D3 had a 32% decrease in AMHR-II mRNA levels, 18% decrease in FSHR mRNA levels, and 36% increase in 3β-HSD mRNA levels compared with control (P < .05; Figure 2). Culture with vit D3 did not cause a difference in AMH, CYP19A1, LHR, or c-kit ligand mRNA levels. Although there was an increase in StAR (14%) and P450scc (17%) mRNA levels, it did not reach statistical significance (P = .20; Figure 2). AMH protein levels in the culture media were not different when GCs were cultured with media alone (0.51 ± 0.04 ng/mL) or with vit D3 (0.49 ± 0.03 ng/mL; P = .46).
Figure 2.
Vit D3-induced alterations in AMHR-II, FSHR, and 3β-HSD mRNA expression. Graphs show mRNA levels (n = 8) in cumulus GCs treated with vit D3. Treatment with vit D3 increased 3β-HSD mRNA but caused a decrease in AMHR-II and FSHR mRNA expression levels. There was no effect on other mRNA expression analyzed. *, P < .05 control vs vit D3-treated.
Effect of vit D3 treatment on 3-βHSD enzyme activity
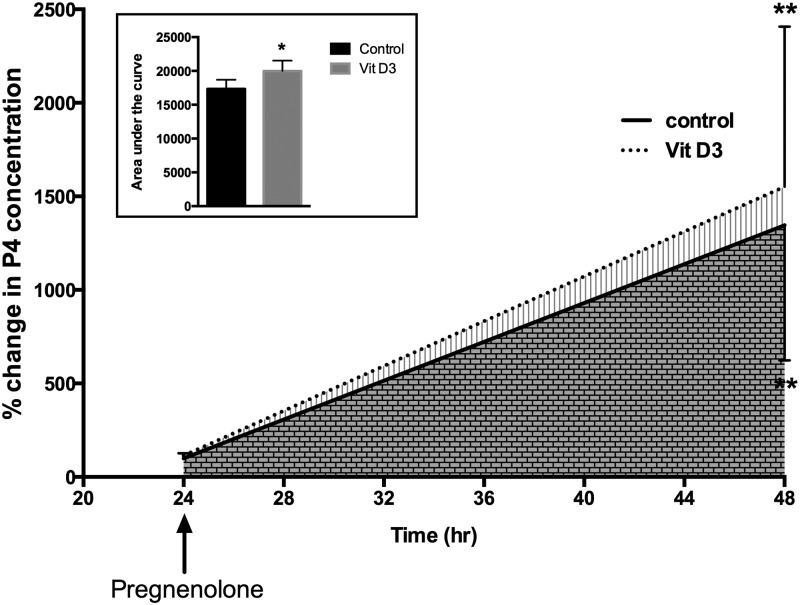
To further study the effect of vit D3 on 3β-HSD activity as reflected by timed cumulus GC P4 production, we cultured cumulus GCs with or without vit D3 when the precursor substrate pregnenolone was provided in excess (Figure 3). The addition of pregnenolone significantly increased P4 release over the 24-hour period in both the vit D3-treated and the vit D3-untreated GCs (P < .001). Treatment with vit D3 increased total P4 release as represented by an increase in AUC by 15% ± 7% at 24 hours when compared with controls (P < .05; Figure 3).
Figure 3.
Vit D3-induced P4 release by cumulus GCs. Graph shows the increase in P4 concentration over time after cumulus GCs (n = 3) were cultured with or without vit D3 (50 nM). Pregnenolone significantly increased P4 release in both vit D3-treated and vit D3-untreated GCs. GCs treated with vit D3 had significantly higher P4 release in the culture media when compared with vit D3-untreated GCs, as determined by AUC (inset). *, P < .05 for AUC between vit D3-treated vs vit D3-untreated. **, P < .001 baseline vs 24 hours after the addition of pregnenolone.
Effect of vit D3 on FSH-induced aromatase mRNA expression and E2 production
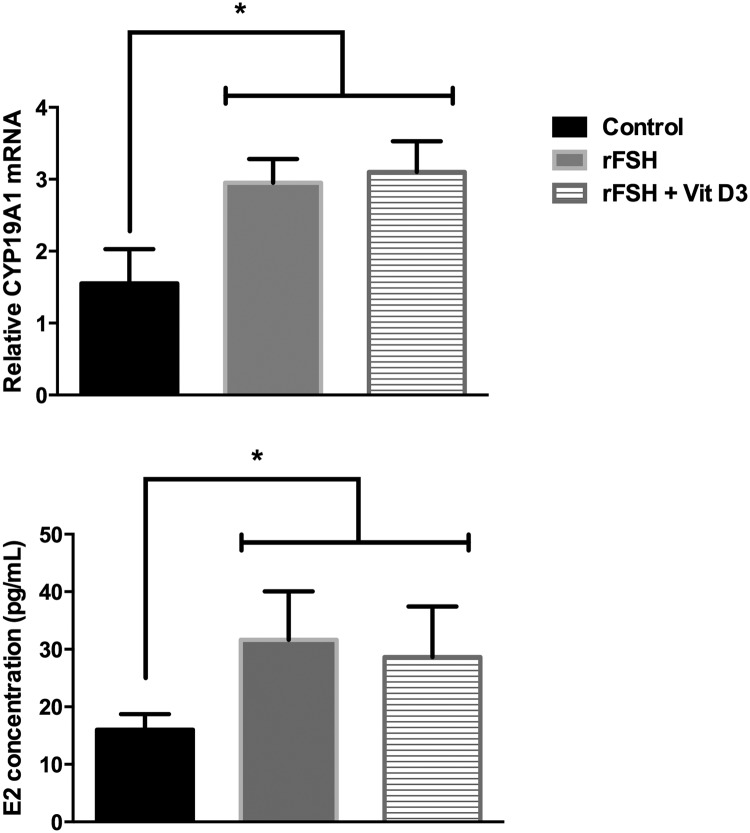
Recombinant FSH stimulated aromatase mRNA expression and increased E2 concentration in culture media by 2-fold in cumulus GCs (P = .03). The addition of vit D3 did not change the FSH-stimulated effect on aromatase mRNA (P = .79) or the FSH-induced E2 concentration (P = .50) (Figure 4).
Figure 4.
Effect of vit D3 on FSH-induced aromatase mRNA expression and estradiol (E2) production. Graph shows the increase in aromatase mRNA expression and E2 concentration after cumulus GCs (n = 6) were cultured with recombinant FSH (rFSH; 10 IU) with or without vit D3. The addition of vit D3 did not alter the FSH-induced aromatase mRNA expression or the FSH-induced E2 concentration in cell culture media. *, P < .05 between controls vs FSH-treated and FSH + vit D3 treated.
Nuclear localization of phospho-Smad 1/5/8 is reduced by vit D3
Nuclear localization of phosphorylated Smad 1/5/8 by vit D3 is shown in Figure 5. There was low staining of phospho-Smad 1/5/8 in the control GCs treated with media alone. Recombinant AMH (50 ng/mL) markedly increased the phosphorylation of Smad 1/5/8 and its localization to the nucleus in GCs compared with controls (P < .0001). When GCs were treated with vit D3 (50 nM) in the presence of recombinant AMH (50 ng/mL), the accumulation of phospho-Smad 1/5/8 in the nucleus was significantly reduced to levels lower than controls (P < .0001).
Figure 5.
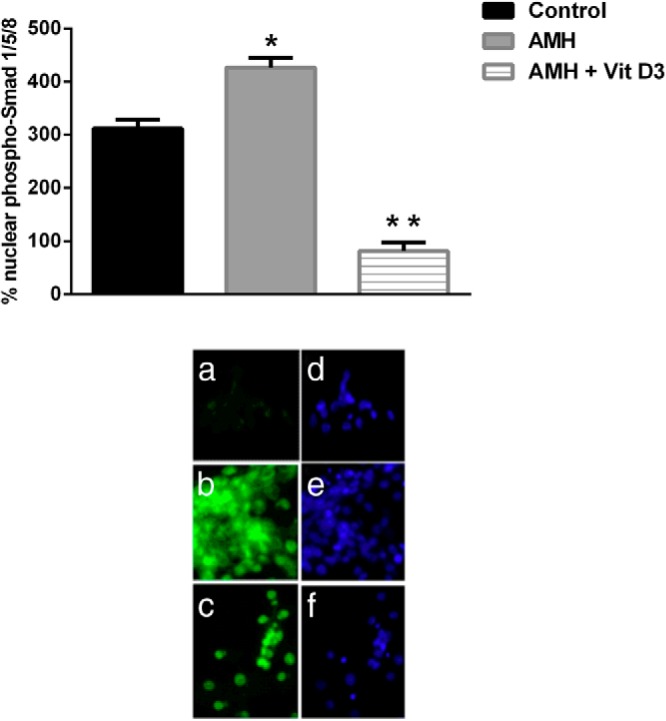
Effect of vit D3 on AMH-induced phospho-Smad 1/5/8 nuclear localization. Immunofluorescence (a, b, and c) and phase contrast (d, e, and f) micrographs of human GCs (n = 4) cultured with media alone (a), recombinant AMH (b), or recombinant AMH with vit D3 (c) is shown. The positive signal is seen in green. Recombinant AMH markedly increased nuclear staining when compared with the controls. When GCs were treated with vit D3 in the presence of recombinant AMH, nuclear staining was significantly reduced. Bars represent mean ± SEM. *, P < .0001 between control vs AMH; **, P < .0001 between control vs AMH + vit D3 treated and between AMH vs AMH + vit D3 treated.
Discussion
The objective of this study was to determine the effect of vit D3 on GC genes involved in follicular development and steroidogenesis. We found that there was a negative correlation between follicular fluid 25 OH-D levels and AMHR-II mRNA expression levels in cumulus GCs of SFs. To further evaluate this relationship, we studied the cause-effect relationship of vit D3 on AMHR-II mRNA and found that vit D3 in vitro significantly decreased AMHR-II and FSHR mRNA expression levels in cumulus GCs. Interestingly, AMHR-II and FSHR are two of the top four genes differentially expressed between cumulus GCs of immature (metaphase I) and mature (metaphase II) oocytes (34). Vit D3 not only lowered the expression of AMHR-II mRNA levels but it also slowed down the effect of recombinant AMH action on cumulus GCs by reducing phospho-Smad 1/5/8 nuclear localization. Lastly, our results indicated that vit D3 induced steroidogenesis by increasing 3β-HSD mRNA expression levels and by increasing P4 release by GCs, suggesting enhancement of 3β-HSD enzyme activity.
Our findings indicated that vit D3 might promote the differentiation and development of GCs. AMH is known to inhibit the primordial to primary follicle transition and to inhibit the rate of the primordial follicle assembly (15, 24, 35). AMH interacts with a highly specific type II receptor (AMHR-II) and signals via Smad 1/5/8 (30, 31). Consistent with its inhibitory role, AMH is usually up-regulated during primordial follicle assembly and down-regulated during the primordial to primary follicle transition (15, 24, 35). By inhibiting AMHR-II expression, vit D3 may counteract the repressive effect of AMH on GC differentiation. The lesser AMH sensitivity allows follicles to reach terminal maturation and ovulation (36). Clinically, follicular development helps determine the reproductive capacity in women and ultimately the response to controlled ovarian hyperstimulation during IVF as well as IVF outcome (ie, number of total and mature oocytes retrieved) (37). The effect of vit D3 on the AMH/AMHR-II system could partly explain the positive observational correlation between vitamin D status and pregnancy outcome after IVF. Unlike what happens in the hen ovaries (38), vit D3 in our study did not seem to down-regulate AMH expression or affect AMH protein release by GCs. Our results were also different from the prostate cell line study in which vit D3 was found to up-regulate AMH expression (19). The discrepancies in the results from the current study may be due to differences in species and/or gender.
The relationship between circulating 25 OH-D levels and IVF outcomes, more specifically clinical pregnancy rates, has been assessed in several studies and the results have been inconsistent (2, 8, 39). Although some studies demonstrated a positive correlation between serum and follicular fluid 25 OH-D levels and clinical pregnancy rates after embryo transfer by IVF (2), other studies have demonstrated an inverse or no correlation (8, 39). A major confounder among these studies is the fact that embryo quality and implantation heavily rely on several other factors such as sperm quality and endometrial environment. Clinical trials are needed to address this subject.
In our model of human GCs, vit D3 suppressed FSHR expression possibly indicating a more mature follicular state. During a women's follicular phase, the follicle that contains the most number of FSHRs (ie, most sensitive to FSH) emerges as dominant at the time of intercycle FSH rise (40). After the selection, the follicle becomes less dependent on FSH and more dependent on LH; this is followed by terminal maturation and ovulation (40). Similar to AMHR-II, FSHR expression in cumulus GCs has been found to be highest in small immature follicles and gradually diminishes during folliculogenesis (40, 41). Additionally, FSHR is highly expressed in cumulus GCs and is critical for oocyte maturation (42). Its expression decreases along with the progression of maturation of oocytes in vitro and in vivo after human chorionic gonadotropin administration (43). Indeed, Catteau-Jonard et al (22) demonstrated that FSHR together with AMH and AMHR-II are overexpressed in GCs from stimulated follicles of women with polycystic ovarian syndrome, possibly indicating an oocyte maturation defect. It is well documented that there is an indirect interaction and strong positive correlation between AMHR-II and FSHR gene expression (30, 34, 35). The mechanism by which vit D3 affects FSHR is not clear, but it could involve AMH signaling. It is possible that vit D3 alters a common intracellular pathway involved in the regulation of both AMHR-II and FSHR; however, further studies are needed to clarify this complex relationship.
In the current study, consistent with other findings on GCs (44), we evaluated whether vit D3 induced steroidogenic enzymes such as aromatase (CYP19A1), an enzyme critical for E2 production, and did not find any significant effect on aromatase. This result is not surprising because the effect of vit D3 on aromatase has been shown to be dose and tissue specific (45, 46). For instance, calcitriol decreases aromatase expression in breast cancer cells and the breast adipose tissue surrounding these cells, whereas it increases aromatase expression in bone cells (47). Additionally, aromatase transcription is primarily driven by its tissue specific promoters (46). Conversely, vit D3 increased 3β-HSD mRNA levels and 3β-HSD enzyme activity by increasing P4 production and release when GCs were supplemented with the substrate pregnenolone. These results confirm previous findings (13) and suggest that vit D3 enhances key steroidogenic enzymes responsible for P4 synthesis such as 3β-HSD. Although vit D3 increased other enzymes responsible for P4 production such as P450scc and StAR, this effect did not reach statistical significance. Luteinized GCs ultimately form corpus luteum that produces large amounts of P4, which induces endometrial changes such as decidualization to support a pregnancy. Our findings suggest that vit D3 may potentiate GC luteinization as reflected by increased P4 production, thus potentially providing a better endometrial environment.
There are several limitations to our study that should be noted. Sample sizes for gene expression analyses were smaller than the number of participants because we used only high-quality samples with enough extracted RNA for RT-PCR. A second limitation is that we used a luteinized GC model because these cells were collected from women who were hyperstimulated with gonadotropins. However, although this model may not be the best for studying AMH/AMHR-II system, we (23) and others (22) have shown that these GCs are responsive to several hormonal treatments in vitro. Lastly, our study is somewhat limited by the paucity of cumulus GCs such that we were unable to divide these cells from the same patient into more than three groups to perform more dose-response effects for vit D3 in vitro treatment. The reason for this is that the RNA quantity and quality was insufficient for RT-PCR when samples were further divided.
In summary, we found that vit D3 alters AMH signaling and steroidogenesis in human cumulus GCs, reflecting a state of GC luteinization potentiation. Cumulus GCs are unique cells for studying ovarian health because oocytes and cumulus GCs grow and develop in a highly coordinated and mutually dependent manner (48). Indeed, some studies have provided evidence showing that cumulus GCs present potential biomarkers to predict embryo quality and pregnancy outcomes after IVF (48). With the global epidemic of vitamin D insufficiency/deficiency among reproductive-aged women and given our findings, assessment of vitamin D status might be considered in premenopausal women desiring conception. Whether appropriate supplementation of those deemed depleted of vitamin D will translate into improved fertility outcome needs to be determined in future studies.
Acknowledgments
This work was supported by a grant from the American Society for Reproductive Medicine (to Z.M.), grant from Ferring Pharmaceuticals (to Z.M.), the University of Vermont College of Medicine Internal Funds (Internal Grant Program to Z.M.), and National Institutes of Health grant NS045940 (to M.J.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Mullerian hormone
- AMHR
- AMH receptor
- AUC
- area under the curve
- CYP19A1
- aromatase
- E2
- estradiol
- FSHR
- FSH receptor
- GC
- granulosa cell
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- IVF
- in vitro fertilization
- LF
- large follicle
- LHR
- LH receptor
- 25 OH-D
- 25-hydroxyvitamin D
- P4
- progesterone
- P450scc
- P450 side-chain cleavage enzyme
- RT-PCR
- real-time PCR
- SF
- small follicle
- StAR
- steroidogenic acute regulatory protein
- vit D3
- 1,25-dihydroxyvitamin D3.
References
- 1. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 2. Ozkan S, Jindal S, Greenseid K, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merhi Z, Seifer DB, Weedon J, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women's Interagency HIV Study. Fertil Steril. 2012;98:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halhali A, Acker GM, Garabedian M. 1,25-Dihydroxyvitamin D3 induces in vivo the decidualization of rat endometrial cells. J Reprod Fertil. 1991;91:59–64 [DOI] [PubMed] [Google Scholar]
- 5. Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun. 2012;38:J275–J281 [DOI] [PubMed] [Google Scholar]
- 6. Dicken CL, Israel DD, Davis JB, et al. Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod. 2012;87:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod. 2012;27:3015–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, Messinis IE. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol. 2010;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. 2012;27:3321–3327 [DOI] [PubMed] [Google Scholar]
- 10. Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97:2450–2455 [DOI] [PubMed] [Google Scholar]
- 11. Braga DP, Setti A, Figueira Rde C, Iaconelli A, Jr, Borges E., Jr Seasonal variability in the fertilization rate of women undergoing assisted reproduction treatments. Gynecol Endocrinol. 2012;28:549–552 [DOI] [PubMed] [Google Scholar]
- 12. Wood S, Quinn A, Troupe S, Kingsland C, Lewis-Jones I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum Fertil (Cambridge, England). 2006;9:223–229 [DOI] [PubMed] [Google Scholar]
- 13. Parikh G, Varadinova M, Suwandhi P, et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. 2010;42:754–757 [DOI] [PubMed] [Google Scholar]
- 14. Davis J, Merhi Z, Berk TS, Neal-Perry G. Developmental Vitamin D3 deficiency differentially affects ovarian gene expression patterns in adult female mice. Fertil Steril. 2012;98(suppl 3);S32 [Google Scholar]
- 15. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084 [DOI] [PubMed] [Google Scholar]
- 16. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227 [DOI] [PubMed] [Google Scholar]
- 17. Campbell BK, Clinton M, Webb R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology. 2012;153:4533–4543 [DOI] [PubMed] [Google Scholar]
- 18. Visser JA, Durlinger AL, Peters IJ, et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology. 2007;148:2301–2308 [DOI] [PubMed] [Google Scholar]
- 19. Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnan AV, Moreno J, Nonn L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702 [DOI] [PubMed] [Google Scholar]
- 21. Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91:786–789 [DOI] [PubMed] [Google Scholar]
- 22. Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–4461 [DOI] [PubMed] [Google Scholar]
- 23. Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28:1661–1669 [DOI] [PubMed] [Google Scholar]
- 24. Jeppesen JV, Anderson RA, Kelsey TW, et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527 [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-δδC(T)] method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 26. Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99:979–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grondahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2013;100:994–1001 [DOI] [PubMed] [Google Scholar]
- 28. McAllister JM, Mason JI, Byrd W, Trant JM, Waterman MR, Simpson ER. Proliferating human granulosa-lutein cells in long term monolayer culture: expression of aromatase, cholesterol side-chain cleavage, and 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1990;71:26–33 [DOI] [PubMed] [Google Scholar]
- 29. Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364–1370 [DOI] [PubMed] [Google Scholar]
- 30. Dutertre M, Gouedard L, Xavier F, Long WQ, di Clemente N, Picard JY, Rey R. Ovarian granulosa cell tumors express a functional membrane receptor for anti-Mullerian hormone in transgenic mice. Endocrinology. 2001;142:4040–4046 [DOI] [PubMed] [Google Scholar]
- 31. Zhan Y, Fujino A, MacLaughlin DT, et al. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development (Cambridge, England). 2006;133:2359–2369 [DOI] [PubMed] [Google Scholar]
- 32. Merhi Z, Messerlian GM, Minkoff H, et al. Comparison of serum and plasma measurements of Mullerian inhibiting substance. Fertil Steril. 2008;89:1836–1837 [DOI] [PubMed] [Google Scholar]
- 33. Merhi Z, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB. Relationship of bariatric surgery to Mullerian-inhibiting substance levels. Fertil Steril. 2008;90:221–224 [DOI] [PubMed] [Google Scholar]
- 34. Devjak R, Fon Tacer K, Juvan P, Virant Klun I, Rozman D, Vrtacnik Bokal E. Cumulus cells gene expression profiling in terms of oocyte maturity in controlled ovarian hyperstimulation using GnRH agonist or GnRH antagonist. PloS One. 2012;7:e47106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Mullerian hormone (AMH) on ovarian primordial follicle assembly. PloS One. 2011;6:e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baarends WM, Uilenbroek JT, Kramer P, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962 [DOI] [PubMed] [Google Scholar]
- 37. American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98:1407–1415 [DOI] [PubMed] [Google Scholar]
- 38. Wojtusik J, Johnson PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod. 2012;86:91. [DOI] [PubMed] [Google Scholar]
- 39. Aleyasin A, Hosseini MA, Mahdavi A, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. 2011;159:132–137 [DOI] [PubMed] [Google Scholar]
- 40. Macklon NS, Fauser BC. Follicle development during the normal menstrual cycle. Maturitas. 1998;30:181–188 [DOI] [PubMed] [Google Scholar]
- 41. Son WY, Das M, Shalom-Paz E, Holzer H. Mechanisms of follicle selection and development. Minerva Ginecol. 2011;63:89–102 [PubMed] [Google Scholar]
- 42. Kawashima I, Okazaki T, Noma N, Nishibori M, Yamashita Y, Shimada M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21 [DOI] [PubMed] [Google Scholar]
- 43. Salhab M, Tosca L, Cabau C, et al. Kinetics of gene expression and signaling in bovine cumulus cells throughout IVM in different mediums in relation to oocyte developmental competence, cumulus apoptosis and progesterone secretion. Theriogenology. 2011;75:90–104 [DOI] [PubMed] [Google Scholar]
- 44. Yanase T, Mu YM, Nishi Y, et al. Regulation of aromatase by nuclear receptors. J Steroid Biochem Mol Biol. 2001;79:187–192 [DOI] [PubMed] [Google Scholar]
- 45. Lundqvist J, Norlin M, Wikvall K. 1α,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism. Biochim Biophys Acta. 2011;1811:263–270 [DOI] [PubMed] [Google Scholar]
- 46. Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77:1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010;121:343–348 [DOI] [PubMed] [Google Scholar]
- 48. Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16:531–538 [DOI] [PubMed] [Google Scholar]