Abstract
OBJECTIVE
To investigate the relationship between baseline resting heart rate and incidence of heart failure (HF) and global and regional left ventricular (LV) dysfunction.
BACKGROUND
The association of resting heart rate to HF and LV function is not well described in an asymptomatic multi-ethnic population.
METHODS
Participants in the Multi-Ethnic Study of Atherosclerosis had resting heart rate measured at inclusion. Incident HF was registered (n=176) during follow-up (median 7 years) in those who underwent cardiac MRI (n=5000). Changes in ejection fraction (ΔEF) and peak circumferential strain (Δεcc) were measured as markers of developing global and regional LV dysfunction in 1056 participants imaged at baseline and 5 years later. Time to HF (Cox model) and Δεcc and ΔEF (multiple linear regression models) were adjusted for demographics, traditional cardiovascular risk factors, calcium score, LV end-diastolic volume and mass in addition to resting heart rate.
RESULTS
Cox analysis demonstrated that for 1 bpm increase in resting heart rate there was a 4% greater adjusted relative risk for incident HF (Hazard Ratio: 1.04 (1.02, 1.06 (95% CI); P<0.001). Adjusted multiple regression models demonstrated that resting heart rate was positively associated with deteriorating εcc and decrease in EF, even in analyses when all coronary heart disease events were excluded from the model.
CONCLUSION
Elevated resting heart rate is associated with increased risk for incident HF in asymptomatic participants in MESA. Higher heart rate is related to development of regional and global LV dysfunction independent of subclinical atherosclerosis and coronary heart disease.
Keywords: resting heart rate, heart failure, coronary heart disease, left ventricular dysfunction, myocardial strain, cardiac MRI
Introduction
Resting heart rate is associated with CV events and mortality.(1) Nevertheless, for many years, resting heart rate has not been included among the main CV risk factors, partially because of interdependence with other risk factors.(2) Another reason might have been our incomplete understanding of the mechanisms linking resting heart rate to CV events.
Being related to sympathetic overactivity, atherosclerosis and plaque vulnerability, resting heart rate mediated arterial stress has gained much focus among the potential mechanisms underlying CV disease progression and clinical manifestations.(3) It is well known that elevated resting heart rates are associated with greater mortality from CV disease (CVD) in particular, but also from non-CVD.(1,4) Several prior studies have also reported the association between resting heart rate and LV dysfunction(5) and/or HF in epidemiological studies,(1,6-8) and in patients with CHD.(9,10) On the other hand, few clinical studies have explored the relationships between resting heart rate and LV dysfunction, and/or HF, among asymptomatic individuals without history CVD. Resting heart rate and LV stroke volume are closely regulated for providing adequate cardiac output. During the early phases of LV dysfunction and progression towards HF, subtle reduction of LV function might therefore be accompanied by a compensatory increase in resting heart rate,(5) even before the elevated heart rate is identified as a marker of excessive neuroendocrine activation.(11) In this regard, there are no studies investigating resting heart rate with incident HF and with myocardial dysfunction in a large asymptomatic population of men and women.
Therefore, we hypothesize that 1) resting heart rate may be related to HF independently of hypertension, diabetes and CHD, and 2) an increased resting heart rate might be an early marker of LV dysfunction that precedes traditional indices of LV dysfunction and clinical disease. We hereby explore the relationship between resting heart rate at baseline and incident HF in a large multi-ethnic population of both genders free of CVD at enrolment. We also investigate whether resting heart rate is associated with the development of global and regional LV dysfunction independently of traditional risk markers.
METHODS
Study population
The MESA (Multi-Ethnic Study of Atherosclerosis) study has been described elsewhere.(12) Between 2000 and 2002, 6,814 men and women who identified themselves as white (38%), African-American (28%), Hispanic (22%), or Chinese American (12%) and were 45 to 84 years old were recruited from six U.S. communities in Maryland, Illinois, North Carolina, California, New York, and Minnesota. On entry, all participants underwent an extensive evaluation that consisted of clinical questionnaires, physical examination, resting heart rate from electrocardiogram (ECG), and laboratory tests including fasting plasma glucose, triglycerides, and total-, and high-density lipoprotein (HDL) cholesterol levels.(13) Individuals with a history of CV disease were excluded. The institutional review boards in each of the participating centres approved the study protocol and informed consent was obtained from each participant.
CV Events during the Follow-up Period
All events in the MESA study were adjudicated by the Morbidity and Mortality Committee composed of cardiologists, epidemiologists and general clinicians who review the reports gathered by a team of trained individuals who interview participants by phone and gather the appropriate records on each of the reported events. MESA participants who developed events were admitted to different hospitals in the community including the centers where they are followed as participants of the MESA Study. A telephone interviewer contacted each participant (or representative) every 6 to 9 months to inquire about all interim hospital admissions, CV outpatient diagnoses, and deaths. Medical records and information were successfully obtained on an estimated 98% of hospitalized CV events and 95% of outpatient CV diagnostic encounters. Two physicians reviewed all records for independent end point classification and assignment of event dates. Reviewers assigned a diagnosis of myocardial infarction based on a combination of symptoms, ECG findings, and cardiac biomarker levels. The definition of angina was adapted from the Women’s Health Initiative criteria. Reports of percutaneous coronary intervention and bypass surgery were obtained from medical records. CHD was defined as myocardial infarction, angina, percutaneous coronary intervention or bypass surgery.(13) COPD status and severity were defined according to the American Thoracic Society/European Respiratory Society COPD criteria(14).
Details regarding the MESA processes and criteria for verifying, classifying, and adjudicating cardiovascular events has been previously reported (13,15,16). A precise definition of each individual outcome and adjudication of clinical event for the MESA study are available online (http://www.mesa-nhlbi.org/). The end point for this sub-study was a composite of probable and definite HF. In addition to clinical HF symptoms or signs, probable HF further required a physician diagnosis of HF and medical treatment for HF. Definite HF also required: 1) pulmonary edema/congestion by chest radiograph; and/or 2) dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction. A supplement has been added detailing the criteria used for adjudication of events in MESA. The risk associated with elevated heart rate was tested by comparing time to HF events with resting heart rate as a continuous variable, as well as analyses of resting heart rate classified into quartiles.
Cardiac MRI at Baseline and Follow-up
Cardiac MRI was performed in 5098 participants and, as an ancillary study protocol, 1793 participants underwent tagged MRI studies; among these, 1115 participants underwent repeated tagged MRI with an identical protocol five years later. The complete cardiac MRI protocol has been previously described elsewhere.(13,17) Change in global systolic LV function was quantified as ejection fraction change (ΔEF) from baseline to follow-up and was calculated by subtracting the baseline from the corresponding follow-up values.
LV Strain Analysis
As a marker of regional myocardial systolic function, myocardial circumferential mid-wall shortening was quantified as circumferential strain (εcc) and analysed by harmonic phase imaging (Diagnosoft, Palo Alto, CA).(18) Average minimal value of εcc from four LV wall segments (septal, anterior, inferior, and lateral) at mid-ventricular level were calculated and change in regional LV function from baseline to follow-up was quantified as Δεcc by subtracting the baseline εcc from the corresponding follow-up value. A positive Δεcc indicates a decline in regional LV function.
Statistical analysis
Continuous variables are presented as means± standard deviation and compared between groups using two-sample t tests. Categorical variables are presented as percent proportions and compared between groups using χ2 tests.
Cox proportional hazards models were constructed for the analysis of the association of risk factors with incident HF by adjusting for the following variables: resting heart rate (continuous or in quartiles), age, ethnicity, gender, current alcohol use, intentional exercise≥600 MET min/wk, college level education, systolic- and diastolic blood pressure, diabetes, BMI, current smoking, HDL cholesterol, triglycerides, current medications (lipid lowering drugs, beta-blocker, and calcium channel blocker [non-dihydropyridines]), LV end-diastolic volume and mass indexed to height, CT coronary calcium score (0 or higher than 0) and baseline LV EF (replaced by baseline εcc in regional LV function analysis). Hazard Ratios were calculated with associated 95% confidence intervals (CI) and reported for one-unit increase in continuous variables or reclassification of categorical variables to a different level. Participants lost to follow-up were censored at the time of the last follow-up and missing values were handled based on an a priori analytic plan, that is, only participants who had missing data on a variable needed for a particular model were excluded from that analysis. In backwards stepwise multivariable linear regression models we investigated Δεcc and ΔEF separately as continuous variables. The same variables were used for the Cox analyses. Statistical analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL).
RESULTS
Subject Characteristics and HF events
Of the 6814 MESA study participants, five participants had pre-baseline events and were excluded, and ECG resting heart rate was available in 6766. Technically adequate data from baseline MRI was available in 5000, and from the follow up cardiac MRI in 942 participants. Baseline characteristics according to resting heart rate quartiles and incident HF are shown in Table 1. The mean age of the participants was 62 years (range 44 to 84 years); 53% of the participants were female, 12% were Chinese-American, 28% were African-American, 22% were Hispanic, and 39% were Caucasian. Participants in the highest resting heart rate (70-130 beats per minute [bpm]) versus lowest quartile (36-56 bpm) were more likely to be female, less likely to be college graduate and to do exercise ≥600 METS per week, more likely to have higher BMI, higher diastolic blood pressure, to be diabetic, to have higher total cholesterol, and triglycerides. At inclusion they had less LV mass indexed to height, lower LV end-diastolic volume index, slightly lower EF and worse regional LV function (less negative εcc).
Table 1.
Baseline Characteristics stratified by resting heart rate quartiles and by absence or presence of incident HF events during follow-up.
| Resting Heart Rate Quartiles |
HF Events |
|||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | No HF | HF | |
| (36-56 | (57-62 | (63-69 | (70-130 | (n=4888) | (n=112) | |
| bpm) | bpm) | bpm) | bpm) | |||
| (n=1268) | (n=1311) | (n=1265) | (n=1126) | |||
|
| ||||||
| Demographic characteristics | ||||||
|
| ||||||
| Age, y | 62±10 | 61±10* | 61±10* | 61±10 | 61±10 | 68±8† |
| Female, n (%) | 528 (41·7) | 711 | 719 | 644 | 2583 | 37 (33·0)† |
| (54·2)* | (56·8)* | (57·4)* | (52·8) | |||
| Ethnicity, n (%) |
||||||
| Caucasian | 509 (40·1) | 494 (37·7) | 505 (39·9) | 423 (37·7) | 1907 (39·1) |
48 (42·9) |
| Chinese- American |
141 (11·1) | 199 | 178 (14·1) | 135 (12·0) | 648 (13·3) | 5 (4·5)† |
| African- American |
349 (27·5) | 319 (24·3)* |
314 (24·8) | 296 (26·4) | 1249 (25·6) |
35 (31·3)† |
| Hispanic | 269 (21·2) | 299 (21·2) | 268 (21·2) | 269 (24·0) | 1084 (22·2) |
24 (22·2) |
| College graduate, n (%) |
666 (52·7) | 648 (49·6) | 628 (49·6) | 518 (46·3)* |
2429 (49·8) |
48 (43·2) |
| Exercise ≥600 | 855 | 817 (62·3) | 752 | 607 | 2984 | 65 (58·0) |
| MET-min/wk, n (%) |
(67·43) | (59·5)* | (54·1)* | (61·1) | ||
| BMI, kg/m2 | 26·9±4·3 | 27·6±4·8* | 27·9±5·2* | 28·8±5·4* | 27·7±4·9 | 28·8±5·0† |
| Current smoking, n (%) |
156 (12·3) | 165 (12·6) | 153 (12·1) | 158 (14·1) | 614 (12·6) | 21 (18·8) |
|
| ||||||
| Medical characteristics | ||||||
|
| ||||||
| Resting heart rate, bpm |
52±4 | 60±2* | 66±2* | 76±6* | 63±9 | 66±11† |
| Systolic blood pressure, mmHg |
125·5±23·4 | 124·7±21·3 | 124·9±20·2 | 126·9±19·8 | 125·2±21·2 | 137·8±20·7† |
| Diastolic blood pressure, mmHg |
70·3±10·2 | 71·3±10·4* | 72·3±10·0* | 73·7±10·2* | 71·8±10·3 | 73·3±11·1 |
| Diabetes, n (%) | 93 (7·3) | 114 (8·7) | 146 (11·5)* |
226 (20·1)* |
544 (11·1) | 34 (30·4)† |
| Total cholesterol, mg/dl |
191±33 | 196±35* | 193±36 | 198±37* | 194±35 | 190±35 |
| HDL cholesterol, mg/dl |
51±15 | 52±16 | 51±15 | 51±15 | 51±15 | 49±14 |
| Triglycerides, mg/dl |
120±70 | 125±71 | 136±81* | 146±113* | 131±85 | 135±77 |
| Medication | ||||||
| Lipid lowering medication, n (%) |
202 (15·9) | 192 (14·7) | 207 (16·4) | 183 (16·3) | 766 (15·7) | 27 (24·1)† |
| Beta-blockers, n (%) |
197 (15·6) | 116 (8·9)* | 91 (7·2)* | 47 (4·2)* | 437 (8·9) | 15 (13·4)† |
| Calcium channel blockers, n (%) |
150 (11·8) | 140 (10·7) | 143 (11·3) | 158 (14·1) | 571 (11·7) | 25 (22·3)† |
| ACE inhibitor or ARB, n (%) |
133 (10·5) | 147 (11·2) | 143 (11·3) | 172 (15·3)* |
567 (11·6) | 30 (26·8)† |
|
| ||||||
| Cardiac parameters | ||||||
|
| ||||||
| Coronary Calcium present, n (%) |
648 (51·1) | 601 (45·8) | 606 (47·9) | 559 (49·8) | 2345 (50·0) | 84 (75·0)† |
| LV mass index, g/m |
91·3±20·1 | 85·7±20·1* | 84·6±20·9* | 85·1±21·6* | 86·3±20·3 | 110·2±31·8† |
| LV end- diastolic volume index, ml/m |
80·0±16·3 | 75·7±15·9* | 74·1±16·7* | 72·0±16·9* | 75·3±16·3 | 87·7±27·0† |
| LV ejection fraction, % |
69·3±7·0 | 69·8±7·1 | 68·9±7·3 | 67·9±8·3* | 69·2±7·2 | 63·7±12·2† |
| LV Circumferential Strain, %‡ |
−18·0±2·7 | −18·1±2·3 | −17·8±2·5 | −17·1±3·0* | −17·6±2·6 | −15·9±3·6† |
P<0·05 relative to lowest resting heart rate quartile (Q1).
P<005 relative to no HF.
MET=Metabolic equivalent of task, LDL and HDL=Low- and high-density lipoprotein, respectively.
ACE=Angiotensin converting enzyme. ARB=Angiotensin II receptor blocker,
Circumferential myocardial strain (shortening) measured in a subset; Q1: n=227, Q2: n=218, Q3: n=209, Q4: n=168.
HF Events
There were 176 HF events through 9 years of follow-up (median 7 years), of which 140 were definite and 36 were probable. The differences in baseline characteristics between those who developed HF versus those who did not are presented in Table 1. Participants who developed HF were older and more likely to be male. They were less likely to be Chinese-Americans and more likely to be African-Americans. They had higher BMI, higher resting heart rate, and higher systolic blood pressure, lower HDL, higher triglycerides and were more likely to have diabetes and a positive coronary calcium score. Furthermore, they had higher LV mass index and LV end-diastolic volume index and lower LV EF at inclusion. A total of 142 participants were diagnosed with COPD with 33 of them diagnosed with COPD at entry. A total of 27 of them also developed heart failure during follow-up.
Resting Heart Rate and HF Events
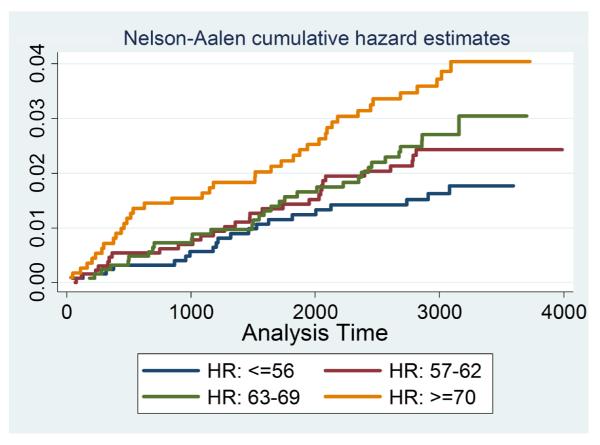
Adjusted analyses of resting heart rate as a continuous variable demonstrated that for every increase in 1 bpm, there was a 4% greater risk for incident HF (Table 2). Adjusted resting heart rates for incident HF relative to a resting heart rate ≤55 bpm are shown in Figure 1, classified by quartile. When compared to a resting heart rate ≤55 bpm (lowest quartile), higher resting heart rate quartiles were associated with greater relative risks for incident HF. Importantly, for the highest resting heart rate quartile we observed a more than threefold greater adjusted relative risk for incident HF (Table 2).
Table 2.
The Relationship of Resting heart rate to CHF Events (Q1 is ref)
|
Cox Models for Incident CHF Events
|
|||
|---|---|---|---|
| Unadjusted | Adjusted* | ||
|
|
|||
| Model | No./No. at Risk | HR (95% CI) | HR (95% CI) |
| Heart Rate, bpm | 112/4963 | 1.02 (1.01, 1.04) | 1.04 (1.02, 1.06) |
| Heart Rate, bpm, in quartiles | |||
| Q1 (<57) | 18/1267 | 1.00 (reference) | 1.00 (reference) |
| Q2 (57-62) | 28/1311 | 1.11 (0.71, 1.74) | 2.62 (1·41, 4.87) |
| Q3 (63-69) | 28/1264 | 1.09 (0.70, 1.71) | 2.57 (1·36, 4.89) |
| Q4 (>69) | 38/1121 | 1.72 (1.13, 2.61) | 3.76 (2·00, 7.07) |
Model includes heart rate, age, ethnicity, gender, alcohol use, intentional exercise and education level as covariates (demographics), systolic- and diastolic blood pressure, diabetes, BMI, smoking status, Total- and HDL cholesterol, triglycerides and current medications (statin, beta-blocker, and calcium channel blocker) (conventional risk factors), and LV end-diastolic volume, LV mass index, CT coronary calcium score (0 or higher than 0) and baseline ejection fraction.
Figure 1. Cumulative Hazard for Incident CHF Events Split by Baseline Resting Heart Rate Groups (Heart Rate ≤56 is reference).
The heart rate groups are defined as HR: >=56 (blue), HR: 57-62 (red), HR: 63-69 (green) and HR: <=70 (yellow).
The association between resting heart rate as a continuous variable and incident HF was confirmed in adjusted Cox analysis stratified by gender with hazard ratios of 1.07; 95% CI, 1.03 to 1.10; p≤0·001 and 1.03; 95% CI, 1.01 to 1.05; p=0.028, for women and men, respectively. Furthermore, in adjusted analyses stratified by ethnicity we also found increased relative risk for incident HF in Caucasians and African-Americans; with hazard ratios 1.05; 95% CI, 1.01 to 1.08; p=0.005, and 1.04; 95% CI, 1.01 to 1.07; p=0.030, respectively. We did not find significant association between adjusted resting heart rate and HF events in Hispanics and Chinese-Americans; hazard ratio: 1·03; 95% CI, 0·98 to 1·08; p=0·24, and 1·06; 95% CI, 0·92 to 1·22; p=0·43. However, the point estimates reflect a trend similar to that shown in Caucasians and African-Americans.
The participants with HF events versus no HF events were more likely at baseline to have hypertension (76% vs. 44%), diabetes (27% vs. 10%), to develop CHD during follow-up (42% vs. 4%), and have/develop COPD (19% vs. 2%), all of which are established risk factors for incident HF. However, in a sub analysis excluding participants with interim CHD events and COPD, resting heart rate remained associated to incident HF in adjusted Cox models (hazard ratio, 1.04; 95% CI, 1.01 to 1.07, p=0.003). We observed a preserved elevated hazard ratio for incident HF for resting heart rate ≥70 bpm relative to a resting heart rate ≤56 bpm (hazard ratio, 4.55; 95% CI, 1.87 to 11.09, p=0.001). This suggests that the resting heart rate is a predictor for incident HF independently of blood pressure, diabetes, COPD and clinical CHD. In addition, in this study heart rate was not associated with CHD in Cox regression analysis in both univariate and multivariate models.
Resting Heart Rate and Change in Regional and Global LV Function
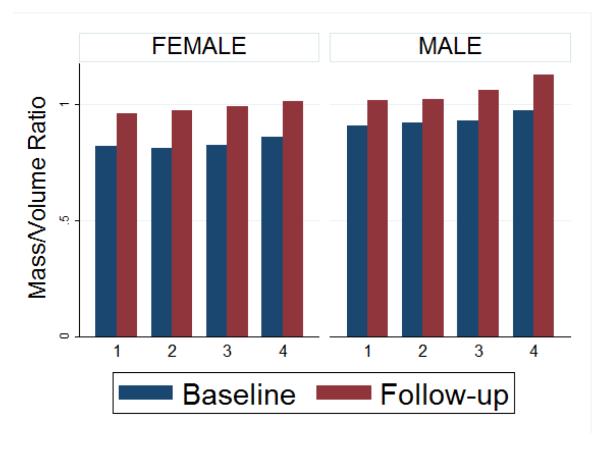
Results of the stepwise, backwards multiple linear regression analysis adjusting for demographics, traditional CV risk factors, markers of subclinical atherosclerosis and LV structure and function at baseline are shown in Table 3 for change in regional LV function, and in Table 4 for change in global systolic LV function. A greater resting heart rate was associated with reduced regional LV function (Δεcc, P<0·001), as well as reduced global LV function (ΔEF, p<0·001). In repeated analyses excluding participants with CHD events, the associations between resting heart rate with Δεcc, as well as with ΔEF, did not change and remained statistically significant, suggesting that increased resting heart rate is an independent predictor for the development of regional as well as global LV dysfunction. Figure 2 shows end-diastolic mass to end-diastolic volume ratios at baseline and follow-up across four quartiles of heart rate for men and women. Mass-volume-ratio is greater in men and is high at high heart rates. The increase in mass-volume ratio was greater at higher heart rates both in univariate analysis (coef=0.003, p<0.001) as well as after adjusting for demographics, traditional CV risk factors, and value at baseline (coef = 0.003, p <0.001).
Table 3.
Stepwise Backwards Multiple Linear Regression Analysis for the Change in Regional LV function (Δsεcc) with Resting Heart Rate
|
Change in Regional LV Function (Ascc)
|
||
|---|---|---|
| Overall Analysis | CHD Events Excluded | |
| (R2=0.37) | (R2=0.37) | |
| (N=804) | (N=769) | |
|
|
||
| B* (95% CI) | B* (95% CI) | |
| Heart Rate, bpm | 0.03 (0.01, 0.06) | 0·03 (0.01, 0.06) |
| Age, y | 0.03 (0.01, 0.05) | 0·03 (0.00, 0.05) |
| Current Smoking | 1.12 (0.44, 1.80) | 1·16(0.46, 1.85) |
| Systolic BP, mmHg | −0.02 (−0.03, −0.01) | −0·02 (−0.03, −0.01) |
| Diastolic BP, mmHg | 0.03 (0·00, 0.06) | 0.03 (0.01, 0.06) |
| LV mass, g | 0.01 (0·01, 0.02) | 0.01 (0.01, 0.02) |
| LV εcc, % | −0.82 (−0.90, −0.74) | −0.82 (−0.90, −0.74) |
Regression coefficients are the differences in εcc-change (%) per 1-beat per minute change in resting heart rate. Model includes heart rate, age, ethnicity, gender, alcohol use, intentional exercise and education level as covariates (demographics), systolic- and diastolic blood pressure, diabetes, BMI, smoking status, Total- and HDL cholesterol, triglycerides and current medications (statin, beta-blocker, and calcium channel blocker) (conventional risk factors), and LV end-diastolic volum LV mass index, CT coronary calcium score (0 or higher than 0) and baseline scc. BP- Blood Pressure.
Table 4.
Stepwise Backwards Multiple Linear Regression Analysis for the Change in Global LV function (ΔEF) with Resting Heart Rate
|
Change in Global LV Function (EF)
|
||
|---|---|---|
| Overall Analysis | CHD Events Excluded | |
| (R2=0.17) | (R2=0.18) | |
| (N=954) | (N=890) | |
|
|
||
| B* (95% CI) | B* (95% CI) | |
| Heart Rate, bpm | −0.08 (−0.13, −0.03) | −0.07 (−0.13, −0.02) |
| Age, y | −0.07 (−0.13, −0.02) | 0.04 (−0.09, 0.02) |
| Males compared to Females | −1.6 (−2.73, −0.48) | −1.35 (−2.49, −0.21) |
| Smoking status | −2.5 (−4.18, −0.88) | −2.16 (−3.88, −0.45) |
| LV end-diastole volume index, ml/m | −14.8 (−18.9, −10.7) | −14.1 (−18.3, −9.8) |
| LV mass index, g/m | 3.29 (−0.26, 6.83) | |
| LV ejection fraction (%) | −0.45 (−0.52, −0.38) | −0.47 (−0.55, −0.40) |
Regression coefficients are the differences in EF-changes (%) per 1-beat per minute change in resting heart rate. The same model and abbreviations is used as in Table 3, except that baseline EF is included rather than scc.
Figure 2. End-diastolic mass to end-diastolic volume ratios seen for quartiles of heart rate and categorized by gender at baseline and follow-up.
The quartiles are defined as HR: >=56 (group 1), HR: 57-62 (group 2), HR: 63-69 (group 3) and HR: <=70 (group 4). Average mass/volume ratio at baseline (blue) and follow-up (red).
DISCUSSION
The present study demonstrates that in a large multi-ethnic cohort without symptoms of CV disease at enrolment, elevated heart rate was strongly associated with the development of regional and global LV dysfunction, as well as incident HF. These findings were independent of demographic confounders, established CV risk factors and markers of subclinical atherosclerosis, as well as LV structure and function at inclusion. In Cox models and regression models excluding participants with incident CHD events, resting heart rate remained an important predictor for incident HF, as well as for declining regional- and global LV function. This indicates that resting heart rate is related to incident HF independently of blood pressure, diabetes, COPD and clinical CHD, and that resting heart rate is an important predictor of progressive subclinical LV dysfunction.
To our knowledge few previous studies have addressed the associations of resting heart rate with LV dysfunction and incident HF in a population of asymptomatic individuals at baseline.(7,8) Adjusted hazard ratio for incident HF was of comparable magnitude as in other previous studies that included symptomatic participants at baseline.(8) The majority of previous studies addressing resting heart rate as a CV risk factor have focused on either mortality(6,19) or coronary artery disease events.(20) Consistent with our findings, previous studies have demonstrated an increased risk for incident HF associated with resting heart rates greater than 70 bpm.(9,21) Resting heart rate has often been demonstrated to predict CHD events and hypertension, with or without diabetes, all of which are important contributing etiologies to the development of HF. Several studies have related resting heart rate to longstanding hypertension, CHD or presence and/or development of valvular heart disease.(3) It has been therefore suggested that increasing heart rate may promote CHD which in turn may contribute to incident or progressive HF. The relationship between HF and heart rate, and CHD and heart rate has been explored previously in the SHIFT(22) and BEAUTIFUL studies with heart rate reducing drug ivrabaine. In the SHIFT study(22), in participants on ivrabadine, reduced heart rates were observed in addition to fewer hospital admissions for worsening heart failure and deaths due to heart failure over 28 months of follow-up. In the BEAUTIFUL trial(23), participants on ivabradine did not show a significant reduction in the primary composite endpoints, but a specified subgroup of patients with a heat rate of greater than 70 bpm did show a reduction in secondary endpoints. These two studies along with the present study, suggest that higher heart rate may be directly related to HF.
Resting heart rate as an independent predictor of incident HF
In the current study resting heart rate predicted incident HF independently of hypertension, markers of subclinical atherosclerosis and diabetes, as well as cigarette smoking. Furthermore, this association remained unchanged even after participants with CHD events were excluded. This suggests that resting heart rate is related to pathophysiological processes leading to HF over and above the effects of clinical hypertension, diabetes and atherosclerosis. These processes may include the contributions of preclinical stages of hypertension and diabetes, as well as of other mechanisms such as inflammation(24) as well as pathways similar to tachycardia induced cardiomyopathy among other alternative etiologies.(25) As resting heart rate was not related to CHD events, the association between elevated heart rate and incident HF might be explained by such alternative mechanisms. Undiagnosed atherosclerotic or microvascular coronary artery disease associated or not with diabetes may also represent contributing factors to explain the role of resting heart rate as a predictor of incident HF in the diabetes and glucose intolerance group. A strong relationship has been demonstrated between HF or LV dysfunction in individuals with diabetes,(26) or poor glycemic control.(27)
Another possible mechanism is that low resting heart rate reflects enhanced vagal tone that may protect against arrhythmias including tachycardias (28). Autonomic imbalance has been associated with increased cardiac mortality (29) and arrhythmias (30). The heart-rate profile during exercise and during recovery after exercise has also been demonstrated to be a strong predictor of sudden death in a large cohort of healthy individuals.(2) Low heart rate may also result in increased shear stress between blood flow and arterial endothelium, which is beneficial due to increased production of vasodilating agents such as nitrogen oxide(31). In our study, the quartile with low heart rate has a range of heart rates that is quite low compared to optimal heart rates, but it has to be noted that the number of participants on beta-blockers is similarly high in this group (~14%). Considering the fewer number of events in this category, this further adds to literature describing the benefits of using beta-blockers for heart rate reduction.
In HF patients heart rate reduction by beta-blockade reduces oxygen requirement for non-mechanical work and increases mechanical efficiency, but these benefits are abolished if resting heart rate is kept constant by atrial pacing.(32) Long standing increase in resting heart rate may increase cardiac oxygen requirement and contribute to LV remodelling leading subsequently to LV dysfunction and HF. Therefore, there is increasing evidence supporting the concept that abnormalities in autonomic balance may precede manifestations of HF and may contribute to the early identification of individuals at risk for sudden death.(6)
A high heart rate leads to greater myocardial oxygen consumption and decreased myocardial perfusion, the latter by shortening the duration of diastole. An increased heart rate might be directly related to diastolic heart failure. Women have been observed to be more susceptible to incident HFPEF than men. This might explain the observation that the hazard ratio for heart rate was greater in women as compared to men in this study as has been observed previously in literature for prediction of cardiovascular disease(33).
Regional function and HF
Quantification of myocardial strain from MRI has been demonstrated as an accurate marker of incipient LV dysfunction(15,34). Assessment of regional myocardial strain has also been recently shown to be superior to LVEF for prediction of mortality in patients with suspected cardiac disease.(35) This is the first study to associate regional myocardial dysfunction with resting heart rate in a large population of asymptomatic individuals at baseline. These findings strengthen and add further evidence to the findings of decreased EF in individuals with higher resting heart rate, and support the assumption that higher resting heart rate may promote progressive LV dysfunction and subsequent HF. In addition, higher mass-volume ratio as a measure of concentric LV remodelling(36) was significantly related to higher heart rates, indicating that increased heart rates may promote adverse remodelling.
Limitations
Reliable evaluation of the associations between resting heart rate and CV events other than HF, as well as of ethnicity in relation to resting heart rate and CV events requires additional studies. Moreover, the general applicability of our results may be limited by selection and survival biases. In this regard, because MESA study participants had no known CV disease at baseline, older individuals undergoing MRI in this cohort may represent a healthier sample of the population at large. In addition, the mechanisms by which HF events result from resting heart rate level are not elucidated by these observational data. As stated in the Methods section, change in EF was calculated by subtracting the baseline exam measure from the follow up exam as previously described.(37) In addition, the diagnosis of HF may not be as definitive as for other CV events such as stroke or myocardial infarction. Therefore, in MESA, we required that participants be symptomatic with physician-diagnosed HF documented in the medical records that were adjudicated by physician reviewers. Finally, apparently preserved LV function at rest may be revealed as reduced LV function during physical activity.
Conclusion
In an ethnically diverse population free of symptomatic CV disease at baseline, the resting heart rate was strongly associated with incident HF during follow up, and to declining regional- and global LV function. These associations may be mediated through hypertension, diabetes and coronary atherosclerosis. However, our study suggests that the relationships of heart rate with LV dysfunction and incident HF may also be mediated by independent pathways, raising the possibility that heart rate may be an independent risk factor for HF.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating >MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/ (UID: NCT00005487). Anders Opdahl received partial grant support from The Raagholt Foundation, the Norwegian Society of Cardiology, Caroline Musaeus Aarsvold’s grant from The Norwegian Medical Association, Oslo, Norway, and The Unger Vetlesen Trust and The Fulbright Foundation.
ABBREVIATIONS
- CV
cardiovascular
- LV
left ventricle
- HF
heart failure
- CHD
coronary heart disease
- ECG
electrocardiogram
- HDL
high-density lipoprotein
- EF
ejection fraction
- εcc
circumferential strain
- MRI
magnetic resonance imaging
- CI
confidence intervals
- BMI
body mass index
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kannel WB, Kannel C, Paffenbarger RS, Jr., Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 3.Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104:1477–82. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Daviglus ML, Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–62. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 5.Julius S, Randall OS, Esler MD, Kashima T, Ellis C, Bennett J. Altered cardiac responsiveness and regulation in the normal cardiac output type of borderline hypertension. Circ Res. 1975;36:199–207. doi: 10.1161/01.res.36.6.199. [DOI] [PubMed] [Google Scholar]
- 6.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–54. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Cupples A. Epidemiology and risk profile of cardiac failure. Cardiovasc Drugs Ther. 1988;2(Suppl 1):387–95. [PubMed] [Google Scholar]
- 9.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–21. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 10.Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Kronmal RA, Lima JAC, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 15.Choi E-Y, Rosen BD, Fernandes VR, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. European heart journal. 2013 doi: 10.1093/eurheartj/eht133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahrami H, Bluemke DA, Kronmal R, et al. Novel Metabolic Risk Factors for Incident Heart Failure and Their Relationship With Obesity:: The MESA (Multi-Ethnic Study of therosclerosis) Study. Journal of the American College of Cardiology. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes VRS, Edvardsen T, Rosen BD, et al. The influence of left ventricular size and global function on regional myocardial contraction and relaxation in an adult population free of cardiovascular disease: a tagged CMR study of the MESA cohort. Journal of Cardiovascular Magnetic Resonance. 2007;9:921–930. doi: 10.1080/10976640701693824. [DOI] [PubMed] [Google Scholar]
- 18.Garot J, Bluemke DA, Osman NF, et al. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–8. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 19.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Panza JA, Diodati JG, Callahan TS, Epstein SE, Quyyumi AA. Role of increases in heart rate in determining the occurrence and frequency of myocardial ischemia during daily life in patients with stable coronary artery disease. J Am Coll Cardiol. 1992;20:1092–8. doi: 10.1016/0735-1097(92)90363-r. [DOI] [PubMed] [Google Scholar]
- 21.Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST) Eur Heart J. 2008;29:1327–34. doi: 10.1093/eurheartj/ehn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. The Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 23.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. The Lancet. 372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 24.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 25.Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Physiol. 1991;261:H308–18. doi: 10.1152/ajpheart.1991.261.2.H308. [DOI] [PubMed] [Google Scholar]
- 26.Tenenbaum A, Motro M, Fisman EZ, et al. Status of glucose metabolism in patients with heart failure secondary to coronary artery disease. Am J Cardiol. 2002;90:529–32. doi: 10.1016/s0002-9149(02)02529-8. [DOI] [PubMed] [Google Scholar]
- 27.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–73. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 28.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 29.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 30.La Rovere MT, Pinna GD, Hohnloser SH, et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–7. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 31.Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–83. doi: 10.1016/j.jacc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Thackray SD, Ghosh JM, Wright GA, et al. The effect of altering heart rate on ventricular function in patients with heart failure treated with beta-blockers. Am Heart J. 2006;152:713, e9–13. doi: 10.1016/j.ahj.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. American Heart Journal. 2010;159:612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Edvardsen T, Detrano R, Rosen BD, et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:206–11. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]
- 35.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-Related Left Ventricular Remodeling and Associated Risk for Cardiovascular Outcomes: The Multi-Ethnic Study of Atherosclerosis. Circulation: Cardiovascular Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malayeri AA, Johnson WC, Macedo R, Bathon J, Lima JAC, Bluemke DA. Cardiac cine MRI: Quantification of the relationship between fast gradient echo and steady-state free precession for determination of myocardial mass and volumes. Journal of Magnetic Resonance Imaging. 2008;28:60–66. doi: 10.1002/jmri.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.